Abstract
The aim of the present study was to study the effect of combined therapy of teriparatide (PTH) or strontium ranelate (SR) with whole-body vibration (WBV) on bone healing and muscle properties in an osteopenic rat model. Seventy-two rats (3 months old) were bilaterally ovariectomized (Ovx), and 12 rats were left intact (Non-Ovx). After 8 weeks, bilateral transverse osteotomy was performed at the tibia metaphysis in all rats. Thereafter, Ovx rats were divided into six groups (n = 12): (1) Ovx—no treatment, (2) Ovx + vibration (Vib), (3) SR, (4) SR + Vib, (5) PTH, and (6) PTH + Vib. PTH (40 μg/kg BW sc. 5×/week) and SR (613 mg/kg BW in food daily) were applied on the day of ovariectomy, vibration treatments 5 days later (vertical, 70 Hz, 0.5 mm, 2×/day for 15 min) for up to 6 weeks. In the WBV + SR group, the callus density, trabecular number, and Alp and Oc gene expression were decreased compared to SR alone. In the WBV + PTH group, the cortical and callus widths, biomechanical properties, Opg gene expression, and Opg/Rankl ratio were increased; the cortical and callus densities were decreased compared to PTH alone. A case of non-bridging was found in both vibrated groups. Vibration alone did not change the bone parameters; PTH possessed a stronger effect than SR therapy. In muscles, combined therapies improved the fiber size of Ovx rats. WBV could be applied alone or in combination with anti-osteoporosis drug therapy to improve muscle tissue. However, in patients with fractures, anti-osteoporosis treatments and the application of vibration could have an adverse effect on bone healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and osteoporosis-related fractures as well as bone and muscle loss with age are current problems in human society. The maintenance of bone strength and density with aging is highly dependent on the maintenance of adequate muscle mass and function. The loss of muscle strength leads to physical frailty, which increases the risk of osteoporotic fracture. However, the role of muscle is often underappreciated in osteoporotic studies [63].
There are only a few therapeutic options for the treatment of osteoporosis. Bisphosphonate, estrogen, selective estrogen receptor modulators, denosumab, and a newly investigated drug Odanacatib (an inhibitor of cathepsin K, an enzyme involved in bone resorption), which decelerates bone resorption improving bone mineral density (BMD). The other drugs are parathyroid hormone (PTH, teriparatide) with a bone anabolic effect [14, 23], strontium ranelate (SR), which promotes bone formation and reduces bone resorption [12, 40], and an investigated anabolic treatment which is based on inhibition of sclerostin, a key negative regulator of bone formation [36].
The healing of an osteoporotic fracture is characterized by the impaired mineralization and structure of callus and diminished biomechanical properties [43, 64]. However, the anti-osteoporosis drugs have not yet been prescribed during fracture healing [35].
PTH has been reported to promote fracture healing in a number of experimental studies [2, 29, 44], whereas muscle tissue was not influenced [29]. Recently, the effect of teriparatide on bone healing has been under investigation in a phase 3 clinical trial in severe osteoporotic women (Eli Lilly and Company, Indianapolis, IN, USA).
In animal studies, a positive effect of SR on bone repair has been reported [20, 33, 37, 45]. A few case reports have shown a favorable effect of SR on fracture healing and fracture non-union in patients [1, 56]. The effect on muscle is unknown. SR treatment of osteoporotic patients is now restricted, and it is recommended only for patients who have no alternative treatment [15].
Whole-body vibration (WBV) has recently been gaining interest as a non-pharmacological therapy for osteoporosis [51]. Vibration treatment primarily evokes reflexive muscle contractions, improving movement velocity, muscle force, and power in humans [8]. A meta-analysis of scientific papers for over 60 years showed that WBV ameliorated muscle properties and improved hip bone mineral density in older women [51]. In contrast, the recent clinical study showed no benefit of WBV on BMD of hip and spine in elderly women [34]. However, it is known that mechanical stimulation at the fracture site is essential for bone repair. In experimental studies, WBV improved bone healing [31, 53].
Therefore, a combination therapy of anti-osteoporotic drugs that promotes fracture healing with WBV, which improves both muscle properties and bone healing, may have an additive effect, providing benefit to the musculoskeletal system of the osteoporotic organism.
There are few reports on the combination therapy of WBV with anti-osteoporosis drugs in the literature [11, 39, 55, 60]. The vibration intensified the positive effect of alendronate [11] and estrogen or raloxifene [55] on bone in osteopenic rats. In contrast, WBV did not enhance the effects of PTH in intact adult mice [39] or the effect of estrogen in Ovx mice [60]. In the latest study, the vibration therapy administered as a single or dual treatment with PTH or SR did not improve lumbar vertebrae and femur in Ovx rats [24].
The aim of the present study was to reveal the effect of combination therapy of PTH or SR with WBV on bone healing and muscle properties in an ovariectomy-induced osteopenic rat model.
Materials and Methods
Eighty-four 3-month-old female Sprague–Dawley rats were obtained from Harlan Winkelmann (Borchen, Germany). After a 1-week acclimatization period, 72 rats were bilaterally ovariectomized (Ovx), whereas twelve rats were left intact (Non-Ovx) (Fig. 1). After 8 weeks, bilateral transverse osteotomy was performed at the tibia metaphysis 7 mm distal to the knee surface in all rats [29, 54]. Initially, a 5-hole T-shaped titanium plate (57-05140, Stryker Trauma, Selzach, Switzerland) was fixed at the ventro-medial aspect of the tibia with the aid of two screws (proximally and distally), and the other two holes were drilled but left empty. This procedure allowed the correct repositioning of the osteotomized bone. Thereafter, the distal screw was removed, the plate was moved to the side, and an osteotomy gap of 0.5 mm was generated using a pulsed ultrasound saw (Piezosurgery®, Mectron Medical Technology, Carasco, Italy). Finally, the osteotomized bone ends were fixed by the plate and four screws: two proximal (7 and 6 mm in length) and two distal screws (4 and 5 mm in length).
Schematic flowchart of the experiment. Rats (12 weeks old) were either ovariectomized (Ovx) or left intact; after 8 weeks, they underwent bilateral tibia osteotomy and were divided into groups (n = 12): 1 Non-Ovx, 2 Ovx—no treatments, 3 Ovx + vibration treatment (Vib), 4 SR, 5 SR + Vib, 6 PTH, and 7 PTH + Vib. Treatments with PTH (40 μg/kg BW/day) and SR (613 mg/kg BW) were started on the day of ovariectomy, with vibration 5 days later. Fluorochrome labeling of new bone formation was performed on days 12, 22, 32, and 42 after osteotomy (XO, CG, AC, and TC). Arrows indicate the labeling of the callus which was built during the corresponding time period
Ovariectomy and osteotomy were performed under intraperitoneal ketamine (Medistar, Aschenberg, Germany) and xylazine (Ecuphar GmbH, Greifswald, Germany) anesthesia [100 and 7 mg per kg of body weight (BW), respectively]. During osteotomy, rats were treated subcutaneously with a single dosage of 0.25 mL antibiotics (Veracin®-compositum, Albrecht GmbH, Aulendorf, Germany), 4 mg/kg BW perphenazine (Decentan, Merck, Darmstadt, Germany), and 5 mg/kg BW carprofen (Rimadyl®, Pfizer GmbH, Berlin, Deutschland). Carprofen was given once a day for 2 days post osteotomy.
Rats received a soy-free diet (Ssniff special diet GmbH, Soest, Germany) throughout the experiment. All animals had free access to food and water throughout the experiment. Body weight (BW) and food intake were recorded on a weekly basis.
After osteotomy, Ovx rats were divided into six groups, each of 12 rats: (1) Ovx—no treatments, (2) Ovx + vibration treatment (Vib), (3) SR, (4) SR + Vib, (5) PTH, and (6) PTH + Vib (Fig. 1). The Non-Ovx group was left untreated. Treatments with PTH and SR were started on the day of ovariectomy, whereas vibration treatments 5 days later.
SR (Protelos, Servier, France) was added to the soy-free diet (10.5 g/kg of food) at the established dose [20, 33, 37] by Ssniff special diets GmbH. A daily dosage of SR averaged 217 ± 32 mg/rat in the SR group and 228 ± 31 mg/rat in the SR + Vib group (613 mg/kg BW on average in both groups).
PTH (teriparatide, 1–34 human, Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) was initially dissolved in 1 % acetic acid, diluted with 0.9 % saline, and applied at a daily dosage of 40 μg/kg BW [29], s.c. in 0.2 ml volume 5×/week.
Vertical vibration treatments were started 5 days after osteotomy using the following vibration regime: 70 Hz frequency, 0.5 mm amplitude, twice a day for 15 min for 37 days (7×/week). The rats were vibrated in a plastic cage fixed to the vibration desk with two alternating current engines (Vibra Maschinenfabrik Schultheis GmbH & Co, Offenbach, Germany) [32]. Non-vibrated rats from Non-Ovx and Ovx groups were maintained in the same room during vibration sessions and placed in the vibration apparatus while it was in the off state. The acceleration measured on the cage bottom was 3 g, whereas the transmitted acceleration rate was 0.3 g recorded on the back of the rat. The measurements were done using a handheld vibration monitoring system (SWM 3000, REO Electronic, Berlin, Germany) as described previously [32]. The applied vibration regime has intensified the effect of raloxifene and estrogen, improving bone healing in Ovx rats [55].
To control the changes in bone following ovariectomy, peripheral quantitative computed tomography (pQCT) was performed in vivo in isoflurane-anesthetized rats (five rats per group) using the pQCT device (XCT Research SA, Stratec Medizintechnik GmbH, Pforzheim, Germany). The forth lumbar vertebral body (L4) was scanned at the beginning of the experiment (prior to Ovx), at week 8 after Ovx (prior to osteotomy), and at the end of the experiment (6 weeks after osteotomy). The scan protocol was as follows: 90 mm measurement diameter, 0.2 mm voxel size, 90 s scan time, <0.3 mA anode current, 50 kV high voltage, 180 number of projections, and 1° angle between detectors. The total bone mineral density (BMD, mg/cm3) was assessed with the aid of the XCT-6.20C software (Stratec Medizintechnik GmbH, Pforzheim, Germany) at a threshold of 280 mg/cm3. The tibia could not be analyzed because of the osteosynthesis material (titan plate and screws).
Fluorescent dyes were applied in vivo to label the new bone formation. Xylenol orange (XO, 90 mg/kg BW), calcein green (CG, 10 mg/kg BW), alizarin complexon (AC, 30 mg/kg BW), and tetracycline (TC, 25 mg/kg BW) were applied subcutaneously on days 12, 22, 32, and 42 after osteotomy, respectively. One hour after tetracycline injection (day 42 post osteotomy), CO2-anesthetized rats were decapitated.
Serum was collected for further analyses. Total alkaline phosphatase (Alp) and creatine kinase (Ck) were measured at the Department of Clinical Chemistry, Medical University, Goettingen, Germany. The analyses were done using Architect c16000 analyzer (Abbott, Wiesbaden, Germany). Alp activity was measured by the para-nitrophenyl phosphate method at 404 nm, and creatine kinase was assayed by the N-acetyl-l-cysteine method at 340 nm according to the manufacturer’s instructions (Architect/Aeroset, Abbott). Collagen type 1 cross-linked C-telopeptide, Ctx (RatLaps™ CTX-I EIA, Immunodiagnostic Systems GmbH, Frankfurt am Main, Germany), was measured by enzyme-linked immunosorbent assays (ELISA) intended to use for rat serum.
The musculus soleus (MS), M. gastrocnemius (MG), and M. longissimus (ML) were chosen randomly (left or right) for histological or enzyme analysis. The entire MG and MS were weighed and cut into two parts in the middle across the muscle. One half of the muscle and the block of ML (1 cm3) were immersed in liquid nitrogen and stored at −80 °C until further analyses. One tibia chosen randomly was stored at −20 °C until micro-computed tomographic, biomechanical, and histological analyses. Metaphyseal clips of other tibia containing callus tissue were stored at −80 °C for gene expression analyses. The uterus was extracted and weighed.
Bone Healing Analyses
Tibia stiffness and yield load were determined by a 3-point bending test using a Zwick devise (type 145660 Z020/TND, Zwick/Roell, Ulm, Germany), as described previously [55].
The test was automatically stopped by the software (testXpert, Zwick/Roell) before structural destruction of the callus occurred.
Micro-computed tomographical analysis was performed using an eXplore Locus SP-Scanner (GE Healthcare, Ontario, Canada), as reported earlier [33, 55]. Briefly, the scan protocol was as follows: 72 kVp, 90 μA, 1600 ms exposure time, 360° rotation, 0.029 mm pixel size, and 900 views. The 3D reconstruction was performed with the aid of the MicroView-Program (v2.1.2, GE Healthcare). The 3D measurement area extended 2.5 mm proximally and distally from the osteotomy line. The standard thresholds for the callus and cortical bone were applied for all samples. The following parameters were quantified: total volume (TV), bone volume (BV), bone volume fraction (BV/TV), cortical and callus volumes (Ct.V and Cl.V), total mineral density (Tt.BMD), and callus and cortical densities (Cl.BMD, Ct.BMD) [9].
For histological analyses, 150-µm-thick longitudinal sections of tibia embedded in methyl methacrylate (Merck) were prepared [29]. The time of the incidence of osseous bridging of the osteotomy gap was determined visually (Leica microscope, Leitz DM RXE) by analyzing fluorochrome-labeled callus tissue using at least 10 sections of each tibia. The three central sections were further analyzed quantitatively [29] using the QWin image analysis program (Leica, Bensheim, Germany). The measurement area was similar to that assessed in micro-CT analysis. Additionally, it was divided into three regions of interest: (1) ventral (plate side), (2) dorsal, and (3) endosteal parts of the tibia. The callus area (µm2) was measured according to the fluorescence labeling. The xylenol orange-labeled area was relatively small and, therefore, measured along with the calcein-labeled area. The total callus (Tt.Cl) area was calculated as a sum of the three regions of interest.
The microradiographs of the three central sections were done using the Faxitron Cabinet X-ray system (Hewlett-Packard, Buffalo Grove, IL, USA) and Kodak Industrex film (SR45, 100 NIF, Kodak, Paris, France). Using microradiographs, the cortical width (Ct.Wi) and density (Ct.Dn) distal to the osteotomy line, the periosteal callus width (Cl.Wi) and density (Cl.Dn), the trabecular density (Tb.Dn), the trabecular width (Tb.Wi), and the trabecular number (Tb.N/mm2) were measured with the aid of QWin image analysis program. The density was determined as a percentage of calcified tissue area to the total area of certain bone region [29, 53]. The nomenclature used for the histomorphometry was that used by Dempster et al. [13].
Expression of the following genes was analyzed: alkaline phosphatase (Alp), osteocalcin (Oc), tartrate-resistant acid phosphatase (Trap), receptor activator of nuclear factor κ-B ligand (Rankl), and osteoprotegerin (Opg). Analysis was done by quantitative real-time polymerase chain reaction based on SYBR Green detection using iCycler (CFX96, Bio-Rad Laboratories, Munich, Germany). Frozen samples were homogenized using a micro-dismembrator S (Sartorius, Goettingen, Germany). Total cellular RNA was extracted using the RNeasyTM MiniKit (Qiagen, Hilden, Germany) and then (50 ng) was reverse-transcribed using Superscript™ RNase H-reverse transcriptase (Promega, Mannheim, Germany). Ready-to-use primer pairs were obtained from Qiagen (QuantiTect® Primer Assays, Hilden, Germany). The relative gene expression was calculated using the \( 2^{{-\Delta \Delta C_{\text{t}}}} \) method [38] for each gene of interest (ΔΔC t = (C t treatment group gene − C t reference gene β-2 microglobulin) − (C t Non-Ovx group gene − C t reference gene).
Fifty micrograms of homogenized metaphyseal clip of the tibia was used for the assessment of calcium and strontium contents. The analysis was done using an atomic absorption spectrometer (4100, PerkinElmer, Germany). Samples were prepared according to CEN [10].
Muscle Analyses
Frozen cross sections of muscles (12 µm) were stained using two methods, as described previously [28]. Stained sections were analyzed at a magnification of 10× (microscope Eclipse E 600) using a digital camera (DVC 1310 C, DVC Company) and image analysis program (Lucia G, Laboratory Imaging, Version 4.82).
Muscle capillaries were analyzed using sections fixed in 100 % ethanol/chloroform/glacial acid (16:3:1), incubated in 0.3 % α-amylase (from porcine pancreas), stained in Schiff’s reagent solution (Roth, Karlsruhe, Germany), and treated with a 10 % potassium sulfite solution [3]. The capillaries and fibers found in two randomly selected fields (0.5 mm2 each) within a cross section were counted. The ratio of capillaries to the muscle fibers was calculated using Excel (MS Office 2010).
For the analysis of muscle fibers, sections were fixed in a 1 % paraformaldehyde solution (pH 6.6) containing 1 % CaCl2 and 6 % sucrose and further stained by incubation in a reduced nicotinamide adenine dinucleotide diaphorase (NADH diaphorase) solution (pH 7.4), followed by acidic incubation (pH 4.2) and incubation in adenosine-5′-triphosphate solution (pH 9.4) [26].
The classification of muscle fibers as fast-twitch glycolytic (G), fast-twitch oxidative (O), and slow-twitch O types was done according to Peter et al.’s method [46]. Cross-sectional areas (CSAs) of at least 90 (G) and 90 fast-twitch O along with slow-twitch O fibers were determined within three fields of the cross section (1 mm2 each). In the MS, only O fibers were measured, as the MS mainly consists of these fibers [25].
Muscle enzyme activity was measured using a photometer (LP6; Hach Lange, Duesseldorf, Germany), as described previously [30]. Briefly, muscle samples were powdered using the micro-dismembrator S and then homogenized in ice-cold Chappel–Perry medium (0.1 M of KCl, 0.05 M of Tris, 0.01 M of MgCl2·6H2O, 1 mM of EGTA, pH of 7.5).
Lactate dehydrogenase (LDH) activity was assessed as described previously [30]. Citrate synthase (CS) activity was assayed according to Faloona and Srere’s approach [16]. Complex I activity was assayed according to Hatefi and Stiggall’s method [21]. Protein content was determined using a BCA™ Protein Assay Kit (Pierce, Rockford, IL), a multilabel reader (PerkinElmer Precisely Victor X4), and software version 4.0 (PerkinElmer Life and Analytical Science, Turku, Finland). The activity of the enzymes was calculated relative to the protein content.
Myostatin was measured in MG. Frozen muscle samples were powdered, PBS was added, and then they were used for ELISA analysis according to the manufacturer’s instructions (USCN, Life Science Inc. Wuhan, China). Powdered samples (50 mg) of MG were used for the assessment of calcium and strontium contents [10].
Statistical Analyses
Statistical analyses were conducted using the SAS program (SAS version 9.1; SAS Institute, Cary, NC). One-way ANOVA (F test, P < 0.05) was applied to reveal the impact of the treatments on the respective variables. Differences between individual means were estimated using the t test (P < 0.05) for multiple comparisons (GLM procedure with Lsmeans/pdiff statements). Correlation analysis was performed using GraphPad Prism (Version 5.0, San Diego, CA, USA).
Results
Animal Model
The BW remained at a significantly lower level in the Non-Ovx group compared to all Ovx rats from the second week onward (Fig. 2a). During the last 4 weeks, the BW in the SR group was higher than those in Ovx + Vib and PTH + Vib groups.
a BW (g) and b average food intake (g/rat/day) of ovariectomized (Ovx) or Non-Ovx (week 0) rats treated after osteotomy (week 8) with either PTH or SR and/or vibration for up to 6 weeks (week 14). Data are presented as means and SD, (*) differ versus all other groups, (c) versus Ovx + Vib, (d) versus SR, (e) versus SR + Vib, (f) versus PTH, and (g) versus PTH + Vib (P < 0.05, t test for multiple comparisons)
Food intake in the Non-Ovx group was lower than that in all untreated Ovx rats, reaching a significant level in the SR, SR + Vib, PTH, and PTH + Vib groups at week 3 and in PTH + Vib at week 4 after ovariectomy (Fig. 2b). In the PTH + Vib group, the food intake was observed at a lower level than in the SR + Vib at weeks 10 and 11 after osteotomy.
A drop in BW and food intake was observed after week 8 when osteotomy was performed.
The uterus weight was significantly lower in all Ovx groups than that in the Non-Ovx group [24].
Serum Analyses
The Alp level was higher in all Ovx rats than that in the Non-Ovx rats (Table 1). Vibration treatments significantly increased the Alp level in rats treated with SR. Ctx and Ck levels did not differ significantly among the groups (Table 1).
Bone Analyses
pQCT analyses of L4 made in vivo revealed that at the beginning of the experiment, the total BMD did not differ among the rats. The average total BMD was 500 mg/cm3.
At week 8 after ovariectomy, all Ovx rats had a lower total BMD than Non-Ovx rats (Fig. 3a). At the end of the experiment, the total BMD improved in all rats treated with SR and PTH, irrespective of the vibration treatments (Fig. 3b). In general, the vibration had no effect on bone density in L4.
Results of the pQCT measurements made in vivo. Total BMD a of L4 prior to the treatments (week 8) and b at the end of the experiment (week 14). Means with superscripts differ significantly: aversus Non-Ovx, bversus Ovx, and cversus Ovx + Vib (P < 0.05, t test for multiple comparisons). Dashed line total BMD (500 mg/cm3) at the beginning of the experiment (week 0)
Callus bridging of osteotomized bone ends was detected from week 3 onwards among the groups (Table 2; Fig. 4). In the Non-Ovx, Ovx, Ovx + Vib, and PTH groups, the first callus bridging occurred within 20 and 22 days. In SR-treated rats and PTH + Vib group, it was within 24 and 26 days. The differences were not significant between the groups (P > 0.05). A case of non-bridging of bone was observed in both SR and PTH groups that were exposed to the vibration treatments (Table 2).
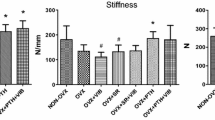
Biomechanical properties were diminished in the Ovx + Vib, SR, SR + Vib, and PTH groups compared to Non-Ovx and PTH + Vib rats (stiffness) (Table 3). In the PTH + Vib group, the yield load did not differ from that in the Non-Ovx, Ovx, and SR groups. No differences in stiffness and yield load between the Non-Ovx and Ovx groups were detected. The standard deviation was very high in biomechanical test results.
The Ovx and Ovx + Vib groups had lower Tt.BMD and BV/TV than the other groups based on micro-CT analysis (Table 3). SR treatments improved Ct.BMD, irrespective of vibration treatments, whereas Cl.BMD was higher in the Non-Ovx, PTH, and PTH + Vib groups than the others. Vibration did not change the bone parameters.
The analysis of microradiographs showed that Ct.Wi increased when PTH was applied in combination with vibration (PTH + Vib) more than that in the PTH, Ovx, Non-Ovx, and SR + Vib groups (ventral aspect, Table 3). In the Ovx-Vib and two SR groups, it was higher than that in Non-Ovx rats. Ct.Dn decreased in vibrated Ovx and PTH rats. SR did not affect Ct.Dn at ventral and dorsal aspects.
Cl.Wi did not change among the groups, whereas Cl.Dn improved after PTH treatments; however, it was at a lower level in the PTH + Vib group (dorsal, endosteal, Table 3). In the Ovx group, vibration decreased the callus density as well (ventral). After SR treatments, Cl.Dn remained at the lower level in Ovx rats and vibration decreased it (endosteal).
Tb.Dn, Tb.Wi, and Tb.N were lower in all Ovx groups than those in the Non-Ovx group, irrespective of treatments (Table 3). In the SR + Vib group, Tb.Dn decreased significantly compared to the SR non-vibrated group. In general, PTH had a stronger anabolic effect than SR on bone parameters (Ct.Wi, Cl.Dn, Tb.Dn, Tb.Wi, Tb.N).
The analysis of fluorochrome-labeled sections revealed the significant effect of treatments during the first 3 weeks of healing (CG and AC) (Fig. 5b, c). An increase in the callus area was observed in Ovx rats after vibrations at the dorsal aspect and by the analysis of the total callus (Fig. 5b, d). At the ventral aspect, no differences were observed (Fig. 5a). A strong anabolic effect of PTH was observed by the assessment of dorsal, endosteal, and total callus areas. SR did not change the callus area compared to the Ovx group. In SR and PTH groups, vibration had less of an effect on the callus areas studied.
Callus area (µm2) measured in fluorescence-labeled sections of the tibia divided into ventral (a), dorsal (b), and endosteal (c) regions in Non-Ovx and Ovx rats treated either with PTH or SR and/or vibration. d Total callus area (µm2). CG calcein green stained area, AC alizarin complexon, TC tetracycline. Means differ significantly: (a) versus Non-Ovx, (b) versus Ovx, (c) versus Ovx + Vib, (d) versus SR, (e) versus SR + Vib, (f) versus PTH, (g) versus PTH + Vib (P < 0.05, t test for multiple comparisons)
Alp mRNA production was significantly higher in almost all groups (excepting SR + Vib) than that in the Non-Ovx group (Table 1). Oc gene expression was enhanced in the SR, PTH, and PTH + Vib groups compared to the Non-Ovx and Ovx groups. Vibration decreased both Alp and Oc gene expression in the SR group. Expressions of the Opg gene and the Opg/Rankl ratio were the highest in the PTH + Vib group, whereas in the SR + Vib group these parameters were lower than those in the Non-Ovx group. Rankl gene expression significantly increased in the Ovx + Vib group; Trap mRNA production did not differ among the groups (Table 1).
Ca content did not change between the groups (Table 1). SR content was 1.07 + 0.26 and 0.95 + 0.22 mg/g sample in the SR and SR + Vib groups, respectively.
Muscle Analyses
In general, MW was higher in all Ovx rats than that in Non-Ovx rats, reaching a significant level in the SR group for MS and in the Ovx and PTH groups for MG (Table 1). After MW was expressed relative to BW, the differences between the groups were not detected. Correlation analysis revealed a significant relationship between MW and BW for both muscles weighed (MS and MG) (Table 4).
In ML, the CSA of glycolytic fibers increased significantly in the SR groups compared to the Non-Ovx, Ovx + Vib, and PTH rats (Table 5). The CSA of oxidative fibers as well as capillary density did not differ between the groups.
In MS, the CSA of oxidative fibers was larger in Ovx, SR, and PTH groups than that in Non-Ovx group (Table 5). After vibration treatments in the PTH + Vib group, the CSA decreased compared to that in the PTH, Ovx, and SR groups. Capillary density did not differ between the groups.
In MG, the CSA of oxidative fibers increased in the Ovx and PTH + Vib groups compared to the Non-Ovx group, whereas after PTH treatment alone a smaller CSA was observed (vs. PTH + Vib and Ovx) (Table 4). The size of glycolytic fibers did not change among the groups. The capillary density was higher in the Ovx, SR, and SR + Vib groups than that in the Non-Ovx group (Table 5). After PTH treatment of Ovx rats, it increased significantly. In vibrated Ovx rats, capillary density decreased to the level of Non-Ovx groups, being significantly different from all Ovx groups. Vibration in the SR and PTH groups did not change the capillary density.
Correlation analysis of CSA and capillary density with BW showed a significant relationship between these variables in MG and MS (Table 4). In ML, a significant relationship was detected between the CSA of glycolytic fibers and BW, whereas other parameters did not correlate significantly.
The analysis of enzymes LDH, CS, and Complex I revealed no significant differences among the treatment groups in all 3 muscles studied (Table 5).
The myostatin level in serum did not change significantly among the groups (Table 1). The Ca content did not differ between the groups (Table 1). SR was not detected in MG.
Discussion
The present study examined the effect of WBV applied in combination with anti-osteoporosis drugs, PTH or SR, on bone healing and muscle tissue in ovariectomy-induced osteopenic rats. Ovariectomy was confirmed by a lower uterus weight in Ovx rats [24], whereas bone loss was confirmed by pQCT analysis of lumbar vertebral bodies.
Bone Healing
The present study showed that the vibration treatments combined with SR therapy decreased the callus density, trabecular number, and expression of Alp and Oc genes, whereas other bone parameters were not changed. When the vibration was applied in PTH-treated rats, the cortical and callus widths were enlarged, biomechanical properties were improved, and Opg gene expression and Opg/Rankl ratio were increased. In the lumbar spine, vibration combined with PTH or SR did not change the bone parameters.
In a previous study, we have shown that the same vibration regime intensified the effects of estradiol and raloxifene, increasing the cortical and cancellous bone volume at the osteotomy site in osteopenic rats [55], which was determined by micro-CT analysis. Although micro-CT is accepted as a modern method for analyses of bone structure and biomechanical tests could provide additional data on bone quality, histological evaluation is indispensable, particularly for the analysis of bone healing.
In the present study, the detailed histological analyses revealed that PTH therapy combined with vibration decreased cortical and callus densities. Moreover, a case of non-bridging was recorded after both combination therapies (PTH + Vib and SR + Vib). Biomechanical properties were improved only when compared with the PTH group and did not differ from impaired Ovx tibia. Biomechanical analysis of the callus produced highly variable results (a coefficient of variation over 40 %). This is a known phenomenon for experimental fracture models [33].
There are few other reports on the impact of combination therapy on bone [11, 39, 60]. WBV and PTH in combination did not enhance the bone anabolic effects of PTH in intact adult mice [39]. The vibration enhanced the positive effect of alendronate on the bone structure and strength of intact tibiae in ovariectomized rats [11]. In estrogen-supplemented Ovx mice, vibration did not improve fracture healing, but rather counteracted the positive effect of estrogen on bone [60]. The vibration regime applied in the present study in combination with PTH or SR did not affect lumbar spine and femur parameters [24].
The hormonal level was shown to influence the response of fracture healing to the vibration. Estrogen-deficient rats were more sensitive to the mechanical stimulations than intact rats [53]. In healthy mice, vibration disturbed fracture healing, whereas in Ovx mice it was improved [60]. It has been suggested that the osteoanabolic effect of vibration in Ovx mice might be mediated by estrogen receptor (ER) α, whereas ERβ might be responsible for impairments of bone healing in Non-Ovx mice [60]. Furthermore, Opg gene, as a target gene of WNT-β-catenin signaling, which is crucial for bone formation and healing, was found to be down-regulated in the impaired bone healing of Ovx mice [4, 60]. In the present study, combined treatment of PTH and vibrations increased the expression of osteoclast regulatory gene Opg and the Opg/Rankl ratio. These findings do not correspond to the changes found in the structure and the biomechanical properties of tibiae in these rats because this may cause an inhibition of osteoclastogenesis and lead to slower healing.
SR + Vib treatments decreased osteoblast activity (Alp and Oc mRNA) at the osteotomy site, whereas serum total Alp activity was enhanced after vibration in SR rats. The differences in the expression of serum markers of bone turnover and mRNA expression of bone genes have been previously reported [29, 31, 33]. First, the measurements are limited to one point in time and the dynamics remain unknown. Second, mRNA expression does not always correspond directly to the level of protein synthesis [41, 59]. Third, total Alp was measured in serum as a commonly used laboratory test, which includes not only bone-specific Alp [48].
When vibration was applied alone, bridging and most of other bone parameters were not affected; solely the callus area and its width were increased at the dorsal aspect of the tibia (the site opposite to the plate fixation). No effect of vibration on bridging has previously been observed in Ovx rats [31]. Periosteal callus formation could be stimulated by micro-movements produced by the vibration treatments, which are known to be essential for sufficient callus formation [52]. Increased periosteal growth of cortical bone has also been reported for Ovx rats [57].
In the lumbar spine, no effect of vibration on BMD was revealed in Ovx rats, which is consistent with previous research [32].
After single SR or PTH therapy, improved total BMD and BV/TV were observed. Cortical BMD was ameliorated in SR-treated Ovx rats, whereas callus BMD and calcified callus areas labeled by fluorochromes were increased after PTH treatments. Similarly to the osteotomy site, in the lumbar vertebral body, improved total BMD was observed after SR and PTH therapy.
Comparing SR and PTH therapy alone, PTH was found to be more potent than SR in improving osteoporotic bone and its healing, which is inconsistent with the findings of Habermann et al. [20]. The differences in these results may be explained by the lower dose of PTH (20 µg/kg BW), another fracture model being used (femoral mid-diaphysis), and the treatment period after fracture (4 weeks) applied in the studies done by Habermann et al. [20].
The different results obtained by the use of PTH and SR may be due to their different effects on bone tissue. Whereas PTH is known to have an anabolic effect on bone tissue, increasing bone formation [23], SR both stimulates new bone formation and reduces bone resorption [40].
In bone, calcium ions are substituted by strontium ions, however at a relatively low rate (less than 1 calcium ions out of 10) [7]. In the present study, no differences in calcium content were detected between the groups, although strontium has been incorporated into the tibia metaphysis in SR-treated groups.
Muscle Tissue
Different effects of vibration were observed in different muscles. Vibration combined with SR decreased the CSA of O and G fibers in ML and MS to the level observed in intact Non-Ovx rats. Combined treatment of PTH and vibration produced similar results in MS. However, in MG the effect on CSA was quite opposite: vibration increased the CSA of O fibers (PTH + Vib group). This may be explained by the different muscle function. While MS is primarily concerned with posture, MG is involved with locomotion [50]. When the vibration treatment was applied combined with anti-osteoporosis drugs (PTH and SR), no effect on capillary density was observed.
After vibration treatment alone, the enhanced capillary density in Ovx rats was reduced in MG. PTH alone increased the capillary density in MG; SR did not change the capillary density and enlarged the CSA of O fibers in ML. Murfee et al. [42] also reported a reduction in the number of vessels per muscle fiber in the M. soleus in adult male mice exposed to WBV (45 Hz, 15 min/day, for 6 weeks). In our previous study, when vibrations were applied at various frequencies (35, 50, 70, or 90 Hz, 15 min/day) for 5 weeks, we did not find capillary density changes [31]. On the other hand, it was also shown that physical exercise as well as WBV treatments (90 Hz, 15 min/day, 2×/day, for 5 weeks) induced vascular remodeling and increased capillary density [47, 53].
Enhanced capillary density after PTH treatments has been previously reported in the MG of Ovx rats during tibia healing [30]. This could be explained, on the one hand, by the PTH action [61] and, on the other hand, by the wound fluid and certain prostaglandins that have been shown to be angiogenic [17].
Correlation analysis showed a significant relationship between BW and CSA and capillary density. An increase in BW as well as partially in food intake was detected in all Ovx rats prior to osteotomy and in rats treated with SR after osteotomy. Increased body weight in Ovx rats is a known effect of hormone depletion [28]. The anti-osteoporosis drug Protelos contained, in addition to SR, aspartame, maltodextrin, and mannitol, which may have an impact on food intake and, as a consequence, on BW as well.
In muscles, calcium plays a crucial role in their function, plasticity, and disease [6]. Both strontium and calcium induce the contractive activation of muscle fibers. The study of the contractile properties of different types of skinned muscle fibers is based on this function of both ions [19, 22]. However, the information on the effect of the anti-osteoporosis drug SR on muscle tissue and SR incorporation into muscle is rare. Wohl et al. [62] measured strontium from soft tissues, including muscle, by X-ray fluorescence (XRF) excitation. However, the XRF measure of strontium from soft tissues was very low compared to the bone strontium measures: 0.02–0.26 for the tibialis anterior muscle and 40–48 for the tibia. We failed to measure strontium by atomic absorption spectrometry in the M. gastrocnemius.
Teriparatide (PTH) induces an increase in calcium concentration in muscle cells that may be destructive for structure and impair the metabolism of muscle cells [5, 58]. However, the effect of continuous and intermittent exposure to PTH is well known [18]. The muscle weight and size of muscle fibers have been reported to be unaffected by intermitted PTH administration in castrated rats as well as in intact male and female rats [29, 30].
The activity of enzymes, the Ca content, and the amount of myostatin in the muscle as well as the Ck level in the blood did not change among the groups, which indicates that muscle metabolism, function, and growth were not affected negatively by the treatments. Previously, it was shown that intermittent PTH treatment had no effect on the activity of muscle metabolic enzymes [29]. CS was found to be significantly enhanced in MS when this vibration frequency (70 Hz) was applied once a day for 5 weeks and muscle samples were collected 1 day after vibration [31]. The CS level depends on the timing of muscle sampling after the last exercise. After acute exercise, the CS level was unchanged in rat muscle, whereas 24 h after exercise, it increased to 30 % [27, 49]. In the present study, samples were collected within 2–3 h after vibration treatments.
The precise mechanism of the vibration action on musculoskeletal tissue and its vascularization has not been elucidated so far. Vibration stimuli evoke muscle contractions, and muscle cells require additional energy and oxygen supply; this causes vascular adaptation at the capillary level [53, 54]. On the other hand, the estrogen level contributes to the muscle sensitivity to the vibration treatments [53, 54]. Additionally, an increase in BW as a consequence of Ovx is correlated with an increase in muscle fiber size as well as in capillary density. In the present study, mechanical stimulation that leads to the decrease of these parameters in Ovx rats to the level observed in healthy rats seems to be favorable for muscle tissue.
Conclusion
The fact that in the vibrated PTH group, the cortical and callus areas and biomechanical properties were improved compared to PTH alone may suggest that vibration has the potential to be used in combination with PTH. However, the decreased cortical and callus densities, enhanced expression of Opg, and the incidence of non-bridging indicate that the applied vibration regime was not optimal for bone healing. Vibration with SR had no advantage for bone healing and instead tended to disturb the healing processes. Vibration applied alone did not change the bone parameters. PTH alone had a stronger effect than single SR therapy on bone healing in Ovx rats.
Although the muscle response to mechanical stimulation depends on the vibration regimes, the findings of the present and previous studies indicate that WBV can modulate muscle structure and, being noninvasive, could be applied therapeutically alone or in combination with anti-osteoporosis drug therapy to improve muscle tissue. Improvements in muscle tissue would lower the fracture risk in osteoporotic patients and facilitate the mobilization of fracture patients. However, in patients with bone fractures, anti-osteoporosis treatments and the application of vibration could have an adverse effect on bone healing. Further studies including the analyses of bone healing at several time points are needed to better understand how combined therapies of different anti-osteoporotic drugs and WBV affect bone healing.
References
Alegre DN, Ribeiro C, Sousa C, Correia J, Silva L, de Almeida L (2012) Possible benefits of strontium ranelate in complicated long bone fractures. Rheumatol Int 32:439–443
Andreassen TT, Fledelius C, Ejersted C, Oxlund H (2001) Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand 72:304–307
Andersen P (1975) Capillary density in skeletal muscle of man. Acta Physiol Scand 95:203–205
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Baczynski R, Massry SG, Magott M, El-Belbessi S, Kohan R, Brautbar N (1985) Effect of parathyroid hormone on energy metabolism of skeletal muscle. Kidney Int 28:722–727
Berchtold MW, Brinkmeier H, Müntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80:1215–1265
Boivin G, Deloffre P, Perrat B, Panczer G, Boudeulle M, Mauras Y, Allain P, Tsouderos Y, Meunier PJ (1996) Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res 11:1302–1311
Bosco C, Colli R, Introini E, Cardinale M, Tsarpela O, Madella A, Tihanyi J, Viru A (1999) Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol 19:183–187
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. JBMR 25:1468–1486
CEN (2002) European committee for standardization. Determination of calcium and magnesium. EN ISO 7980
Chen GX, Zheng S, Qin S, Zhong ZM, Wu XH, Huang ZP, Li W, Ding RT, Yu H, Chen JT (2014) Effect of low-magnitude whole-body vibration combined with alendronate in ovariectomized rats: a random controlled osteoporosis prevention study. PLoS One 9:e96181. doi:10.1371/journal.pone.0096181
Deeks ED, Dhillon S (2010) Strontium ranelate: a review of its use in the treatment of postmenopausal osteoporosis. Drugs 70:733–759
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2012) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. JBMR 28:1–16
Dobnig H, Turner RT (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612
EMA (2014) European Medicine Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/02/news_detail_002031.jsp&mid=WC0b01ac058001d126. Available on-line Sept 2015
Faloona GR, Srere PA (1969) Escherichia coli citrate synthase. Purification and the effect of potassium on some properties. Biochemistry 8:4497–4503
Folkman J, Klagsbrun M (1987) Angiogenic factors. Science 235:442–447
Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM (2003) Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone 33:372–379
Fink RH, Stephenson DG, Williams DA (1986) Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol 373:513–525
Habermann B, Kafchitsas K, Olender G, Augat P, Kurth A (2010) Strontium ranelate enhances callus strength more than PTH 1–34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int 86:82–89
Hatefi Y, Stiggall DL (1978) Preparation and properties of NADH: cytochrome c oxidoreductase (complex I–III). Methods Enzymol 53:5–10
Hill F, Stewart AW, Verrier CS (1996) Age and sensitivity of rat skeletal muscle fibers to calcium and strontium. BAM 6:373–376
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703
Hoffmann DB, Sehmisch S, Hofmann AM, Eimer C, Komrakova M, Saul D, Wassmann M, Stürmer KM, Tezval M (2016) Comparison of parathyroid hormone and strontium ranelate in combination with wholebody vibration in a rat model of osteoporosis. J Bone Miner Metab. doi:10.1007/s00774-016-0736-0
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204
Horak V (1983) A successive histochemical staining for succinate dehydrogenase and “reversed”—ATPase in a single section for the skeletal muscle fibre typing. Histochem Cell Biol 78:545–553
Ji LL, Stratman FW, Lardy HA (1988) Enzymatic down regulation with exercise in rat skeletal muscle. Arch Biochem Biophys 263:137–149
Komrakova M, Werner C, Wicke M, Nguyen BT, Tezval M, Semisch S, Stuermer KM, Stuermer EK (2009) Effect of daidzein, 4-methylbenzylidene camphor or estrogen on gastrocnemius muscle of osteoporotic rats undergoing tibia healing period. J Endocrinol 201:253–262
Komrakova M, Stuermer EK, Werner C, Wicke M, Kolios L, Sehmisch S, Tezval M, Daub F, Martens T, Witzenhausen P, Dullin C, Stuermer KM (2010) Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 47:480–492
Komrakova M, Krischek C, Wicke M, Sehmisch S, Tezval M, Rohrberg M, Brandsch T, Stuermer KM, Stuermer EK (2011) Influence of intermittent administration of parathyroid hormone on muscle tissue and bone healing in orchidectomized rats or controls. J Endocrinol 209:9–19
Komrakova M, Sehmisch S, Tezval M, Ammon J, Lieberwirth P, Sauerhoff C, Trautmann L, Wicke M, Dullin C, Stuermer KM, Stuermer EK (2013) Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int 92:509–520
Komrakova M, Stuermer EK, Tezval M, Stuermer KM, Dullin C, Schmelz U, Doell C, Durkaya-Burchhard N, Fuerst B, Genotte T, Sehmisch S (2014) Evaluation of twelve vibration regimes applied to improve spine properties in ovariectomized rats. Bone Rep. doi:10.1016/j.bonr.2014.12.001
Komrakova M, Weidemann A, Dullin C, Ebert J, Tezval M, Stuermer KM, Sehmisch S (2015) The impact of strontium ranelate on metaphyseal bone healing in ovariectomized rats. Calcif Tissue Int 97:391–401
Kiel DP, Hannan MT, Barton BA, Bouxsein ML, Sisson E, Lang T, Allaire B, Dewkett D, Carroll D, Magaziner J, Shane E, Leary ET, Zimmerman S, Rubin CT (2015) Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized. Placebo-controlled trial. J Bone Miner Res. 30:1319–1328
Larsson S, Fazzalari NL (2014) Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma Surg 134:291–297
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu Q-T, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588
Li YF, Luo E, Feng G, Zhu SS, Li JH, Hu J (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21:1889–1897
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 25:402–408
Lynch MA, Brodt MD, Stephens AL, Civitelli R, Silva MJ (2011) Low-magnitude whole-body vibration does not enhance the anabolic skeletal effects of intermittent PTH in adult mice. J Orthop Res 29:465–472. doi:10.1002/jor.21280
Marie PJ (2006) Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone 38:S10–S14
Meyer RA, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P (2003) Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am 85:1243–1254
Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, Judex S, Skalak TC (2005) High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol 98:2376–2380
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y (2008) Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone 42:90–97
Ozturan KE, Demir B, Yucel I, Cakıcı H, Yilmaz F, Haberal A (2011) Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res 29:138–142
Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE (1972) Metabolic profiles of the three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 11:2627–2633
Prior BM, Lloyd PG, Yang HT, Terjung RL (2003) Exercise-induced vascular remodelling. Exerc Sport Sci Rev 31:26–33
Roudsari JM, Mahjoub S (2012) Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget’s specimens. Caspian J Intern Med 3:478–483
Savard R, Smith LJ, Palmer JE, Greenwood MRS (1987) Site specific effects of acute exercise on muscle and adipose tissue metabolism in sedentary female rats. Physiol Behav 43:65–71
Shorey CD, Everitt AV, Armstrong RA, Manning LA (1993) Morphometric analysis of the muscle fibres of the soleus muscle of the ageing rat: long-term effect of hypophysectomy and food restriction. Gerontology 39:80–92. doi:10.1159/000213518
Slatkovska L, Alibhai SMH, Beyene J, Cheund AM (2010) Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int 21:1969–1980
Stuermer KM, Rack TH, Kauer F (1980) Intravitale Bewegungsmessung bei der Frakturheilung. Hefte zur Unfallheilkunde 212:489–498
Stuermer EK, Komrakova M, Werner C, Wicke M, Kolios L, Sehmisch S, Tezval M, Utesch C, Mangal O, Zimmer S, Dullin C, Stuermer KM (2010) Musculoskeletal response to whole body vibration during fracture healing in healthy and ovariectomized rats. Calcif Tissue Int 87:168–180
Stuermer EK, Sehmisch S, Rack T, Wenda E, Seidlova-Wuttke D, Tezval M, Wuttke W, Frosch KH, Stuermer KM (2010) Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbeck’s Arch Surg 395:163–172. doi:10.1007/s00423-008-0436-x
Stuermer EK, Komrakova M, Sehmisch S, Tezval M, Dullin C, Schaefer N, Hallecker J, Stuermer KM (2014) Whole body vibration during fracture healing intensifies the effects of estradiol and raloxifene in estrogen-deficient rats. Bone 64:187–194
Tarantino U, Celi M, Saturnino L, Scialdoni A, Cerocchi I (2010) Strontium ranelate and bone healing: report of two cases. Clin Cases Miner Bone Metab 7:65–68
Turner RT, Vandersteenhoven JJ, Bell NH (1987) The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. JBMR 2:115–122
Visser M, Deeg DJH, Lips P (2003) Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab 88:5766–5772
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev/Genetics 13:227–232
Wehrle E, Liedert A, Heilmann A, Wehner T, Bindl R, Fischer L, Haffner-Luntzer M, Jakob F, Schinke T, Amling M, Ignatius A (2015) The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis Model Mech. 8:93–104. doi:10.1242/dmm.018622
Wells SA, Stirman JA, Bolman RM (1977) Parathyroid transplantation. World J Surg 1:747–756
Wohl GR, Chettle DR, Pejović-Milić A, Druchok C, Webber CE, Adachi JD, Beattie KA (2013) Accumulation of bone strontium measured by in vivo XRF in rats supplemented with strontium citrate and strontium ranelate. Bone 52:63–69. doi:10.1016/j.bone.2012.09.002
Wolfe RR (2006) The underappreciated role of muscle in health and disease. Am J Clin Nutr 84:475–482
Yingjie H, Ge Z, Yisheng W, Ling Q, Hung WY, Kwoksui L, Fuxing P (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41:631–638
Acknowledgments
This study was supported by the German Research Foundation (DFG, SE 1966/5-1). The authors are grateful to their colleagues R. Castro-Machguth, A. Witt, and R. Wigger for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Komrakova, D. B. Hoffmann, V. Nuehnen, H. Stueber, M. Wassmann, M. Wicke, M. Tezval, K. M. Stuermer, and S. Sehmisch declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animal study protocol was approved by the local regional government (33.9-42502-04-12/0854, Oldendurg, Germany) in accordance with German animal protection laws prior to performing the study.
Additional information
Prof. M. Wicke—deceased.
Rights and permissions
About this article
Cite this article
Komrakova, M., Hoffmann, D.B., Nuehnen, V. et al. The Effect of Vibration Treatments Combined with Teriparatide or Strontium Ranelate on Bone Healing and Muscle in Ovariectomized Rats. Calcif Tissue Int 99, 408–422 (2016). https://doi.org/10.1007/s00223-016-0156-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0156-0