Abstract
Vascular calcification (VC) is highly prevalent and represents a major cardiovascular risk factor in chronic kidney disease (CKD) patients. High phosphate (HP) levels are strongly associated with VC in this population. Secreted frizzled-related protein 5 (SFRP5), one of the inhibitors of the Wnt pathway, is a known anti-inflammatory adipokine with a positive effect on metabolic and cardiovascular diseases, in addition to its anticancer potency. However, the role of SFRP5 in the pathophysiology of VC is unclear. This work aimed to study the mechanism of action of SFRP5 on the progression of HP-induced VC, which resembles the CKD-related VC, through its direct effect on vascular smooth muscle cells (VSMCs) in vitro. Addition of SFRP5 significantly inhibited HP-induced calcification of VSMCs as determined by Alizarin red staining and calcium content. The inhibitory effect of SFRP5 on calcification of VSMCs was due to the suppression of HP-induced expression of calcification and osteoblastic markers. In addition, SFRP5 abrogated HP-induced activation of the Wnt/ß-catenin pathway, which plays a key role in the pathogenesis of VC. The specificity of SFRP5 for the inhibition of calcification of VSMCs was confirmed by using a neutralizing antibody to SFRP5. Our results suggest that SFRP5 inhibits HP-induced calcification of VSMCs by inhibiting the expression of calcification and osteoblastic markers, as well as the Wnt/ß-catenin pathway. Our study may indicate that SFRP5 is a potential therapeutic agent in calcification of VSMCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has become a worldwide public health problem, with increasing prevalence, high associated costs, and poor outcomes [1]. Vascular calcification (VC) is one of the independent risk factors that substantially contribute to cardiovascular events and it increases the mortality of CKD patients [2, 3]. In these patients, both intimal and medial calcification occurs, but medial arterial calcification is the most common. Medial arterial calcification has been recognized as a regulated process similar in many ways to bone mineralization [4]. The mineral and bone disorder associated with CKD, particularly the increased levels of serum phosphate (Pi), plays a central role in the induction of medial VC [5]. Some clinical studies indicated that hyperphosphatemia appears to be related to mortality in cardiovascular events in CKD patients, making it one of the most important risk factors [6, 7]. Hyperphosphatemia has been shown in both in vivo and in vitro experiments to be closely associated with calcification by inducing osteogenic differentiation and other steps of the calcification process [8, 9]. However, the molecular mechanism of high phosphorus-induced VC has not been fully elucidated.
The Wnt/ß-catenin signalling complex, known as the canonical signalling complex of the Wnt pathway, has been shown to participate in the pathogenesis of VC [10]. In the canonical Wnt pathway, the inactivation of a destruction complex that targets ß-catenin for proteasomal degradation results in the translocation of ß-catenin to the nucleus to regulate the expression of its target genes [11]. The activation of ß-catenin signalling modulates the proliferation and differentiation of osteoblasts [12]. Additionally, high Pi levels can activate the Wnt/ß-catenin pathway, which subsequently stimulates the expression of calcification and osteoblast markers, including bone morphogenetic protein-2 (BMP-2), runt-related transcription factor 2 (Runx2) and alkaline phosphatase (ALP) [9]. In vascular smooth muscle cells (VSMCs) cultured in high phosphorous medium, the inhibition of excessively activated Wnt/ß-catenin pathway can negatively regulate VC [13, 14]. An in vivo study indicated that ß-catenin was involved in CKD rat aortic calcification induced by high Pi, while knockdown expression of ß-catenin reduced VC [9].
Secreted frizzled-related protein 5 (SFRP5) is a newly identified anti-inflammatory secreted adipokine belonging to the SFRP family [15]. It is well known that SFRPs are inhibitors of the Wnt pathway, and SFRP5 can inhibit either canonical or non-canonical signalling depending on the cell type [16, 17]. Plasma SFRP5 levels are significantly decreased in patients with obesity, insulin resistance, diabetes, major contributing components of metabolic syndrome, and other disease conditions such as atherosclerosis and autoimmune disorders [18]. SFRP5 falls under the category of being a good adipokine, showing a positive influence on some cardiovascular diseases and anticancer potency. Although the functions of SFRP5 in cardiovascular disease are the subject of active investigation, and SFRP1-4 are altered in the progression of VC [19], the role of SFRP5 in VC has not been studied. Here, we investigated the role of SFRP5 in cardiovascular disease, particularly in calcification of VSMCs, and we have shown for the first time that SFRP5 attenuates high phosphate (HP)-induced calcification of VSMCs.
Materials and Methods
Cell Culture
Human aortic smooth muscle cells (VSMCs) were obtained from ScienCell Research Laboratories (San Diego, CA, USA). VSMCs were cultured in smooth muscle cell medium containing 2 % FBS, 1 % smooth muscle cell growth supplement, 1 % penicillin/streptomycin solution (all reagents from ScienCell) at 37 °C in a humidified atmosphere with 5 % CO2. Cells were used from the third to sixth passage. The medium was changed every 2–3 days.
Experimental Design
After reaching 80 % confluence, VSMCs were incubated for 9 days in a high phosphate medium that contained NaH2PO4 salts to obtain a final phosphate concentration of 3 mM. Depending on the experiments, high phosphate medium was supplemented with recombinant human SFRP5 (50 ng/mL, R&D Systems, Minneapolis, MN, USA) or SFRP5 plus neutralizing anti-SFRP5 (1 µg/mL, R&D Systems) in growth medium. The control IgG (1 µg/mL, R&D Systems) was added as the additional control of anti-SFRP5. The medium was replaced with fresh medium every 48 h.
Quantification of Calcification
After the incubation period, cells were decalcified by 24-h incubation in HCl(0.6 mol/L). The calcium content in the supernatant was determined by the phenolsulphonephthalein method (QuantiChrom™ Calcium Assay Kit, Bioassay System, Hayward, CA, USA). Cells were washed three times with PBS (Solarbio, Beijing, China) and solubilized in 0.1 mol/L NaOH/0.1 % SDS. Cell protein content was measured by BCA protein assay kit (Bioss, Beijing, China). The calcium content was normalized for total protein.
Alkaline Phosphatase (ALP) Activity Assay
After the indicated treatments, VSMCs were washed three times with PBS and protein lysates were prepared with a lysis buffer (10 mM Tris–HCl, pH 7.5, 0.1 % TritonX-100). ALP activity was determined using an Alkaline Phosphatase Assay Kit (Beyotime, Jiangsu, China). ALP activity was normalized to total protein concentration, as assessed by the BCA assay (Bioss).
Alizarin Red Staining
The deposition of calcium in cells was detected using an Alizarin red staining kit according to the manufacturer’s instructions (GenMed, Shanghai, China).
Western Blot Analysis
Total protein was extracted from VSMCs using a lysis buffer (50mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 % Triton X-100, 1 % sodium deoxycholate, 0.1 % SDS, 1 % PMSF). Nuclear–cytoplasmic fractionation was conducted using a NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Protein concentration was determined using a BCA protein assay kit (Bioss). Samples were mixed with an equal amount of 5X SDS loading buffer (125 mM Tris-HCl, 4 % SDS, 20 % glycerol, 100 mM DTT, and 0.2 % bromophenol blue) and heated at 99 °C for 10 min. Subsequently, equal amounts of protein from each sample were separated by 8–12 % SDS-PAGE gel and transferred onto a nitrocellulose membrane. After blocking with 5 % milk for 2 h at room temperature, the membranes were incubated with primary antibody overnight at 4 °C, and then with secondary antibodies at room temperature for 60 min. Primary antibodies and dilutions were as follows: anti-GAPDH (1:5000, Abcam), anti-BMP-2 (1:1000, Abcam), anti-Runx2 (1:100, Santa Cruz), anti-ß-catenin (1:1000, Abcam), anti-phospho-ß-catenin (Ser675; 1:1000, Cell Signaling Technology), anti-Histone H3 (1:1000, Cell Signaling Technology). The blots were visualized with an enhanced chemiluminescence kit (Millipore) in Image Lab system (Bio-Rad).
Quantitative Real-Time PCR
Total RNA was isolated from cells using Trizol reagent (Invitrogen), and cDNA synthesis was performed using the First-Strand cDNA synthesis kit (Promega). The primers used for PCR amplification are shown in Table 1. Quantitative RT-PCR was performed in duplicate with Power SYBR Green PCR Master Mix (Applied Biosystems, ABI) according to the manufacturer’s protocol on an ABI 7500 sequence detection system. The mRNA levels of target genes were calculated after normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Confocal Microscopy
VSMCs were seeded on coverslips, and received the different experimental treatments for 24 h after reaching 80 % confluence. Then, they were rinsed in PBS, fixed and permeated in cold 4 % paraformaldehyde for 30 min. The specimens were subsequently washed in PBS (3 × 5 min) and incubated for 15 min with Triton X-100. After being washed with PBS (3 × 5 min), they were blocked with 1 % BSA solution at 37 °C for 30 min. Then, the specimens were incubated with anti-ß-catenin antibody (1:100; Abcam) at 4 °C overnight. After being washed with PBS (3 × 5 min), specimens were incubated for 1 h with fluorescein isothiocyanate–conjugated affinipure goat anti-rabbit IgG (1:100; Proteintech). After a final wash with PBS (3 × 5 min), the specimens were counterstained with DAPI for nuclear stain. Cells were mounted on slides to examine fluorescence using a LEICA TCS SP5 confocal microscope. Image J software (National Institutes of Health, Bethesda, MD, USA) was used to analyse confocal immunofluorescence staining. Mander’s coefficient M2 plugin (DAPI vs. green) was used to analyse nuclear translocation of beta-catenin. Mander’s coefficient M2 is the percentage of above-background pixels in blue (DAPI) that overlap above-background pixels in green colour (ß-catenin).
Luciferase Activity Assay
ß-catenin-mediated transcription was evaluated using a TOP/FOP-Flash luciferase assay. Cells were grown to 60–70 % confluence in 24-well plates and then transiently transfected with a TOP-Flash reporter plasmid containing multiple TCF/LEF consensus sites (Promega) and a Renilla control plasmid (internal control). A FOP-Flash reporter plasmid containing a mutated TCF/LEF binding site (Promega) was also used as a negative control. After transfection for 6 h, the cells were treated with high phosphate (HP; 3 mM), HP + SFRP5 (50 ng/mL), both of which were added the control IgG, or HP + SFRP5 + anti-SFRP5 (1 µg/mL) medium for 72 h. The luciferase activity was determined using a luciferase assay system (Promega). Renilla activity was used to normalize transfection efficiency.
Statistical Analysis
Each experiment was performed at least three times in quadruplicate. The results are expressed as the mean ± standard error of the mean (SEM). The differences between control and the experimental groups were determined by using one-way ANOVA and Student’s Newman–Keuls test for post hoc comparisons. All statistical analyses were performed using SPSS software (version 18.0). P < 0.05 was considered statistically significant.
Results
SFRP5 Prevents High Phosphate-Induced Calcification in Vascular Smooth Muscle Cells
Incubation of VSMCs in a HP (3 mM) medium for 14 days induced calcification compared with control cells maintained in a medium with 0.9 mM Pi (P < 0.05) (Fig. 1a). In our preliminary study, 50 ng/mL of SFRP5 treatment was most effective in the inhibition of VSMC calcification without cytotoxic effects (data not shown). Therefore, most experiments were performed with an SFRP5 concentration of 50 ng/mL. The addition of SFRP5 to cells in HP medium (HP + SFRP5) decreased the degree of calcification observed with HP alone (P < 0.05) and inhibitory effect of SFRP5 on calcification of VSMCs was restored to the control IgG level by treatment with anti-SFRP5 (Fig. 1a).
The effect of SFRP5 on calcification of VSMCs induced by high a concentration of phosphorus (HP) in vitro. VSMCs were cultured with 3 mM Pi in the presence or absence of SFRP5 as indicated in Fig. 1 for 14 days with media changes every 2 days. a Calcium content was normalized to the cellular protein concentration. Bars are the mean ± SEM of four different experiments. *P < 0.05 compared with the individual controls, b calcification of VSMCs was determined by Alizarin red staining. Representative result of one of three different experiments
We also performed Alizarin red staining, a histological technique broadly used in vitro to further demonstrate VC in cellular models of calcification. As shown in Fig. 1b, cells cultured with HP exhibited significantly higher levels of red staining than controls. The addition of SFRP5 resulted in decreased staining; this effect was reversed by anti-SFRP5 treatment.
SFRP5 Inhibits High Phosphate-Induced Gene Expression of Calcification and Osteoblast Markers
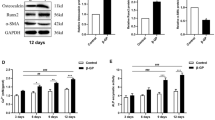
Calcification of VSMCs is associated with an increased expression of calcification and osteoblast markers, including BMP-2, Runx2, and ALP. In the present study, the mRNA expression of BMP-2 and Runx2 was substantially increased in HP-treated calcified VSMCs compared with the uncalcified control (Fig. 2a). The increase in the mRNA levels of BMP-2 and Runx2 was inhibited by SFRP5, and this effect was neutralized by anti-SFRP5 treatment. Moreover, the effects of SFRP5 on BMP-2 and Runx2 protein expression in HP-calcified VSMCs, as determined by western blot analysis, were consistent with the mRNA expression data (Fig. 2b). Moreover, we also assessed the activity of alkaline phosphatase (ALP). As depicted in Fig. 2c, ALP activity was increased by HP, as compared with controls. However, addition of SFRP5 to HP cells induced a decrease of ALP activity to achieve control levels, which was neutralized by anti-SFRP5 treatment. These data suggest that SFRP5 is capable of inhibiting HP-induced VC by suppressing the expression of calcification and osteoblast markers.
SFRP5 inhibited HP-induced osteoblast-like transformation in VSMCs. VSMCs were treated with 3 mM Pi in the presence or absence of SFRP5 (50 ng/mL) as indicated for 6 days with media changes every 3 days. Control group with normal Pi was also included. a The relative mRNA expression levels of BMP-2 and Runx2 detected by qRT-PCR. b Protein expression of BMP-2 and Runx2 was determined by western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. c Alkaline phosphatase (ALP) activity of whole cell extracts from VSMCs. Bars are the mean ± SEM of four different experiments. *P < 0.05 compared with the individual controls
Protective Effect of SFRP5 on Calcification Induced by High Phosphate is Mediated Through the Inhibition of the Canonical Wnt/ß-Catenin Signalling Pathway
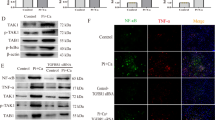
We next explored the involvement of the canonical Wnt/ß-catenin signalling pathway in our experimental model. In Pi-induced calcification of VSMCs, the Wnt/ß-catenin signalling pathway has been shown to be activated, and SFRP5 is the inhibitor of Wnt signalling pathway. First, we assessed the presence of ß-catenin in the nucleus by immunofluorescence using confocal microscopy (Fig. 3a). Control cells showed nuclear exclusion of ß-catenin, whereas cells cultured with HP presented a markedly increased nuclear presence of ß-catenin. Treatment with SFRP5 prevented nuclear translocation of ß-catenin. This effect was no longer observed after the addition of anti-SFRP5 neutralizing antibodies. Quantification of nuclear ß-catenin staining intensity (see methods) confirmed the differences in the levels of nuclear ß-catenin fluorescence between groups (Fig. 3b).
Modulation of canonical Wnt/ß-catenin pathway activation by SFRP5 during calcification of VSMCs. VSMCs were treated with 3 mM Pi in the presence or absence of SFRP5 (50 ng/ml) and/or anti-SFRP5 (1 µg/ml) for 24 h. Cells incubated in normal phosphate medium were used as controls. a Intracellular localization of ß-catenin was visualized by immunofluorescence using confocal microscopy as described in Materials and Methods. For each treatment, ß-catenin staining (green immunofluorescence) is shown on the left; in the middle, the same sample is shown counterstained with DAPI (blue) nuclear stain; the merged image is shown on the right. Images are representative of three different experiments. b Quantification of nuclear ß-catenin staining was performed by finding the Mander’s coefficient (M2 plugin: DAPI vs. Green). Bars are the mean ± SEM of three experiments in quadruplicate. *P < 0.05 compared with the individual controls. c The nuclear localization of ß-catenin was assessed by western blotting of nuclear–cytoplasmic extracts as described in the “Materials and Methods” section. The cytoplasmic level of phospho-ß-catenin (Ser675) was normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The histone H3 was used as internal control intranuclear ß-catenin. Quantification was performed by measurement of the integrated OD (optical density). Images are representative of three different experiments. Data are mean ± SEM of the three independent experiments. *P < 0.05 compared with the individual controls. d ß-catenin-dependent gene transcription as determined by TOP/FOP-Flash luciferase assay. Relative luciferase activity was normalized to Renilla activity. Data are presented as mean ± SEM of three different experiments. *P < 0.05 compared with the individual controls (Color figure online)
Then, we evaluated the translocation of ß-catenin into the nucleus by western blotting. As shown in Fig. 3c, incubation of cells in the HP medium resulted in increased levels of nuclear ß-catenin (P < 0.05) and the phosphorylation of ß-catenin at the Ser675 residue (p-Ser675 ß-catenin) was restricted in cytoplasm lysate (P < 0.05). The addition of SFRP5 to HP cells produced a significant decrease of nuclear ß-catenin levels (P < 0.05) and increase of p-Ser675 ß-catenin (P < 0.05), both of which were restored by the addition of anti-SFRP5 (P < 0.05).
Thereafter, we directly evaluated the ß-catenin-dependent transcriptional activation by a nucleofection of a TCF/LEF reporter and dual-luciferase reporter assays. As shown in Fig. 3d, the TCF/LEF luciferase activity, representing Wnt/ß-catenin pathway activation, had an increase of approximately twofold after HP treatment, as compared with controls (P < 0.05). However, addition of SFRP5 to HP cells greatly abolished this increase (P < 0.05).
To further confirm the involvement of Wnt/ß-catenin signalling, we also evaluated the expression of Wnt ligand and direct transcriptional target genes of Wnt/ß-catenin. As compared with controls, the expression of Wnt3a, c-Myc and Cyclin D1 genes were upregulated by the treatment of VSMCs with HP (Fig. 4). This upregulation was suppressed by SFRP5, and this effect was neutralized by anti-SFRP5 (P < 0.05).
SFRP5 attenuated the HP-induced activation of the Wnt/ß-catenin pathway. VSMCs were treated with 3 mM Pi in the presence or absence of SFRP (50 ng/ml) and/or anti-SFRP5 (1 µg/ml) for 24 h. Expression of ß-catenin nuclear-transcriptional target genes a Wnt3a b c-Myc and c cyclin D1. Bars are the mean ± SEM of four different experiments. *P < 0.05 compared with the individual controls
Discussion
Many researchers have investigated the molecular mechanism of the anti-inflammatory activity of SFRP5 in metabolic syndrome-related diseases. However, the role of SFRP5 in cardiovascular diseases has not been as thoroughly studied. Miyoshiet al. [20] reported that serum SFRP5 levels are significantly lower in coronary artery disease(CAD) patients than those in the non-CAD subjects, suggesting that low SFRP5 levels may contribute to CAD. In addition, it has been shown that SFRP5 is upregulated in Ang II induced cardiomyocyte hypertrophy and the addition of SFRP5 can downregulate the expression of BNP and TNF-α in hypertrophic cardiomyocytes [21]. Interestingly, these cardiovascular diseases are frequently accompanied by VC. Therefore, we examined the role of SFRP5 in the pathophysiology of cardiovascular disease, particularly in VC. VC is often detected in patients with osteoporosis, atherosclerosis, and CKD, and is highly correlated with cardiovascular morbidity and mortality. In this study, we investigated the role of SFRP5 through a series of in vitro experiments with a model of HP-induced calcification in VSMCs, which resembles CKD-related VC. The results showed that SFRP5 actually prevented the HP-induced osteogenic differentiation and calcification, which was mediated by the inhibition of the Wnt/ß-catenin signalling pathway.
Using Alizarin red staining and calcium content analysis, we found increased VSMC calcification in HP-treated cells as compared with controls. These results were consistent with the finding that HP promotes calcification of VSMCs [9]. Moreover, we found that SFRP5 treatment attenuates the HP-induced calcification of VSMCs, and we confirmed the specificity of this effect with a neutralizing antibody to SFRP5, suggesting that SFRP5 may suppress HP-induced VSMC calcification by inhibiting calcium deposition.
VC is a multifaceted active, cell-mediated process including transformation of VSMCs to osteoblast-like cells [22]. BMP-2, a member of the transforming growth factor-ß super family, is expressed and secreted from various cell types, including VSMCs, endothelial cells and osteoblasts, and it promotes the calcification of adjacent cells in a paracrine manner [23]. BMP-2 induces VC and accelerates phosphate uptake [24], and its downstream effects are achieved by upregulating Runx2 and ALP expression [10]. Runx2, a transcription factor, controls the expression of calcification and osteoblastic markers, such as the pro-calcifying enzyme ALP. In our model, calcification was associated with the upregulation of osteogenic factors as previously reported [13, 14]. We found that HP increased the expression of these molecules in VSMCs and that this effect was specifically reduced by SFRP5 treatment, indicating that the attenuation of VC by SFRP5 occurs through the inhibition of the osteogenic programme.
Signalling pathways were evaluated to explore, in a greater detail, putative cellular mechanisms underlying the regulation of calcification of VSMCs calcification by SFRP5. The canonical Wnt/ß-catenin pathway, a key mediator of osteogenic differentiation, has been shown to be activated in VSMCs during the development of VC in vivo and in vitro [9, 25]. In the present study, we examined whether the Wnt/ß-catenin pathway is regulated by SFRP5 in HP-induced, calcified VSMCs. Previously, we showed that HP induced nuclear translocation of ß-catenin in our cellular model, with subsequent ß-catenin-dependent TCF/LEF promoter activation. Furthermore, HP induced an increase in the expression of the Wnt3a gene and the accumulation of cytosolic ß-catenin by downregulating the phosphorylation of ß-catenin, shown for the first time to occur at Ser675. The activation of Wnt/ß-catenin ß-catenin signalling was also demonstrated by the enhanced expression of its transcriptional target genes c-Myc and cyclin D1. In contrast, the preventive effect of SFRP5 on HP-induced calcification of VSMCs was associated with the inhibition of Wnt/ß-catenin signalling. Thus, all of the aforementioned effects elicited by HP were counteracted by coincubation with SFRP5. Accordingly, nuclear translocation of ß-catenin, TCF/LEF promoter activation, and measured expression of target genes such as Wnt3a were inhibited, whereas the cytoplasmic level of phosphorylation of ß-catenin was increased to reach levels similar to those of controls. Although we did not explore the influence of the non-canonical pathway on the inhibition of HP-induced calcification of VSMCs by SFRP5, it is worth noting that the specificity of the inhibiting effect of SFRP5 on the Wnt/ß-catenin pathway was demonstrated by using a neutralizing antibody to SFRP5. Therefore, from our results, it can be concluded that the upregulation of the canonical Wnt/ß-catenin pathway is involved in the induction of calcification of VSMCs by HP, while the downregulation of this pathway is related to the attenuation of calcification elicited by SFRP5. This is coincident with the inhibition of the Wnt/ß-catenin signalling that we observed with the prevention of calcium deposition by the potentially anticalcifying molecules, such as paricalcitol, magnesium, and Interleukin-24 in a similar experimental model [13, 14, 25, 26].
Although the inhibiting effects of SFRP5 are mostly involved in non-canonical Wnt signalling by antagonizing Wnt5a in metabolic syndrome-related diseases [18, 27], the results of this study highlight a novel protective effect of SFRP5 on calcification of VSMCs through the inhibition of the canonical Wnt/ß-catenin pathway. However, the cellular and signalling actions of SFRP5 seem to depend on the type of tissue as well as its inflammatory and metabolic state [28]. The canonical Wnt signalling ligand, Wnt3a, which contributes to calcification of VSMCs [29], was found to have an increased gene level in HP-induced calcified VSMCs. Jian et al. [10] reported that Wnt3a was downstream of BMP-induced arterial calcification, but our results showed the increased expression of both BMP-2 and Wnt3a could be reversed by treatment with SFRP5. It has been suggested that the inhibition of Wnt signalling by SFRP5 is correlated with Wnt3a [30]. We hypothesize that Wnt3a is one of the Wnt ligands antagonized by SFRP5 to attenuate the HP-induced calcification of VSMCs. However, Wnt3a is not the only ligand altered in the calcification and osteogenic process. Conversely, SFRP5 is not solely a Wnt-binding protein, but can antagonize the activity of Wnt, bind to Fz receptors and interfere with BMP signalling [31, 32]. We did not elucidate every component in the attenuation of HP-induced calcification of VSMCs by SFRP5 through the inhibition of Wnt/ß-catenin pathway, and these components should continue to be examined in future studies.
In summary, our results have shown for the first time that SFRP5 attenuates HP-induced calcification of VSMCs, and that this effect correlated with the suppression of the expression of calcification and osteoblast markers (BMP-2, Runx2, and ALP) through the inhibition of the Wnt/ß-catenin signalling pathway. The present study may help elucidate the role of SFRP5 in the pathophysiology of VC.
References
Eknoyan G, Lameire N, Barsoum R et al (2004) The burden of kidney disease: improving global outcomes. Kidney Int 66:1310–1314
Briet M, Burns KD (2012) Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. ClinSci 123:399–416
Liabeuf S, Desjardins L, Diouf M et al (2015) The addition of vascular calcification scores to traditional risk factors improves cardiovascular risk assessment in patients with chronic kidney disease. PLoS ONE 10:e0131707
Covic A, Kanbay M, Voroneanu L et al (2010) Vascular calcification in chronic kidney disease. ClinSci 119:111–121
Moe SM, Chen NX (2008) Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 19:213–216
Palit S, Kendrick J (2014) Vascular calcification in chronic kidney disease: role of disordered mineral metabolism. Curr Pharm Des 20:5829–5833
Palmer SC, Hayen A, Macaskill P et al (2011) Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305:1119–1127
El-Abbadi MM, Pai AS et al (2009) Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 75:1297–1307
Yao L, Sun YT, Sun W et al (2015) High phosphorus level leads to aortic calcification via β-catenin in chronic kidney disease. Am J Nephrol 41:28–36
Shao JS, Aly ZA, Lai CF et al (2007) Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 1117:40–50
Gough NR (2012) Focus issue: Wnt and β-catenin signaling in development and disease. Sci Signal 5:eg2
Johnson ML, Kamel MA (2007) The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19:213–216
Martínez-Moreno JM, Muñoz-Castañeda JR, Herencia C et al (2012) In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/β-catenin activation. Am J Physiol Renal Physiol 303:F1136–F1144
Montes de Oca A, Guerrero F, Martinez-Moreno JM (2014) Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenice transformation of vascular smooth muscle cells. PLoS ONE 9(2):e89525
Ouchi N, Higuchi A, Ohashi K et al (2010) Sfrp5 is an anti-inflammatory adipokine thatmodulates metabolicdysfunction in obesity. Science 329:454–457
Li Y, Rankin SA, Sinner D et al (2008) Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev 22:3050–3063
Wang R, Hong J, Lin R et al (2014) SFRP5 acts as a mature adipocyte marker but not as a regulator in adipogenesis. J Mol Endocrinol 53:405–415
Jaikanth C, Gurumurthy P, Indhumathi T et al (2014) Emergence of sfrp5 as a pleiotropic adipocytokine and its association with wnt signaling. Minerva Endocrinol July 8[Epub ahead of print]
Román-García P, Carrillo-López N, Fernández-Martín JL et al (2010) High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 46:121–128
Miyoshi T, Doi M, Usui S et al (2014) Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis 233:454–459
Jin X, Guo B, Yan J et al (2015) Angiotensin IIincreases secreted frizzled-related protein 5(sFRP5) expression through AT1 receptor/Rho/Rock1/JNK signaling in cardiomyocytes. Mol Cell Biochem 408:215–222
Giachelli CM (2009) The emerging role of phosphate in vascular calcification. Kidney Int 75:890–897
Rong S, Zhao X, Jin X et al (2014) Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol Biochem 34:2049–2060
Li X, Yang HY, Giachelli CM (2008) BMP-2 promotes phosphate uptake, phenotypicmodulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 199:271–277
Herencia C, Rodríguez-Ortiz ME, Muñoz-Castañeda JR et al (2015) Angiotensin II prevents calcification in vascular smooth muscle cells by enhancing magnesium influx. Eur J Clin Invest 45:1129–1144
Lee KM, Kang HA, Park M et al (2012) Interleukin-24 attenuates β-glycerophosphate-induced calcification of vascular smooth muscle cells by inhibiting apoptosis, the expression of calcification and osteoblastic markers, and the Wnt/β-catenin pathway. Biochem Biophys Res Commun 428:50–55
Chatani N, Kamada Y, Kizu T et al (2015) Secreted frizzled-related protein 5 (Sfrp5) decreases hepatic stellate cell activation and liver fibrosis. Liver Int 35:2017–2026
Carstensen M, Wiza C, Röhrig K et al (2014) Effect of Sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PLoS ONE 9:e85906
Mikhaylova L, Malmquist J, Nurminskaya M et al (2007) Regulation of in vitro vascular calcification by BMP4, VEGF and Wnt3a. Calcif Tissue Int 81:372–381
Mori H, Prestwich TC, Reid MA et al (2012) Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest 122:2405–2416
Bovolenta P, Esteve P, Ruiz JM et al (2008) Beyond Wnt inhibition: new functions of secretedFrizzled-related proteins in development and disease. J Cell Sci 121:737–746
Stuckenholz C, Lu L, Thakur PC et al (2013) Sfrp5 modulates both Wnt and BMP signaling andregulates gastrointestinal organogensis in the Zebrafish, Daniorerio. PLoS One 8:e62470
Acknowledgments
This study received financial support from Beijing Municipal Science and Technology Commission Funds (D131100004713001) and National Natural Science Foundation of China (81300607).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Deng, D., Diao, Z., Han, X. et al. Secreted Frizzled-Related Protein 5 Attenuates High Phosphate-Induced Calcification in Vascular Smooth Muscle Cells by Inhibiting the Wnt/ß-Catenin Pathway. Calcif Tissue Int 99, 66–75 (2016). https://doi.org/10.1007/s00223-016-0117-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0117-7