Abstract
Although cross-sectional and longitudinal studies report a relationship between osteoporosis and cardiovascular disorders (known as the bone-cardiovascular axis), the benefits of osteoporosis treatment on atherosclerosis are largely unclear. Teriparatide is a bone-forming agent that increases urinary phosphate excretion. Because elevated serum phosphate is associated with the development of atherosclerosis, the purpose of our study was to examine the relationship among lumbar spine bone mineral density (LS-BMD), intima-media thickness at the carotid artery (CA-IMT), and phosphate metabolism in response to daily teriparatide therapy. Osteoporotic patients (n = 28) with low LS-BMD (T-score < −2.5) and/or at least one vertebral fracture were treated with teriparatide (20 μg/day) for 12 months. Metabolic bone markers, LS-BMD, and CA-IMT were measured over the course of treatment. The LS-BMD significantly increased by 0.046 ± 0.038 g/cm2 over the 12-month period (P < 0.001). CA-IMT decreased from 0.701 mm (interquartile range: 0.655–0.774 mm) at baseline to 0.525 mm (0.477–0.670 mm) at 12 months (P < 0.05); however, CA-IMT change was not significantly associated with LS-BMD change. Serum phosphate decreased after 1 month of teriparatide administration, and the change in serum phosphate at 1 months was associated with the change in CA-IMT at 12 months (ρ = 0.431, P = 0.025). Teriparatide improved LS-BMD and CA-IMT, suggesting the existence of the bone–cardiovascular axis. The association between serum phosphate and CA-IMT suggests that the teriparatide decreased CA-IMT in part by reducing serum phosphate, a well-known vascular toxin, in addition to the improvement of bone–cardiovascular axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daily administration of teriparatide, also known as PTH 1–34, is an established therapy for severe osteoporosis because it decreases the risk of fractures and increases vertebral and femoral BMD. Teriparatide increases P1NP, a marker of bone formation, followed by urinary N-telopeptide corrected for creatinine (NTX), a marker of bone resorption [1]. Increase in P1NP after 1 month of teriparatide administration was strongly correlated with increased LS-BMD at 12 months [2], indicating a strong relationship between early change in P1NP and subsequent change in LS-BMD.
Cross-sectional and longitudinal studies show that low BMD is significantly associated with atherosclerosis or cardiovascular calcifications in the general population [3–6] as well as in patients with CKD [7–9]. The connection between osteoporosis and cardiovascular disorders may be due to the similar biological mechanisms behind the pathogenesis between bone and arterial abnormalities, often termed the bone–cardiovascular axis.
Treatment of osteoporosis with bisphosphonates confers beneficial effects on the cardiovascular system. Oral daily risedronate prevents the progression of brachial–ankle pulse wave velocity, and intima-media thickness of the carotid artery (CA-IMT), an early quantitative marker of generalized atherosclerosis [10], whereas these parameters increased significantly in non-treated controls [11]. Additionally, oral risedronate taken weekly for 6 months inhibits the progression of atherosclerosis by improving large and small artery elasticity index and systemic vascular resistance [12]. Furthermore, a population-based matched cohort analysis shows that patients who receive bisphosphonate therapy have a lower risk of acute myocardial infarction during the 2-year follow-up period (hazard ratio = 0.35) [13]. These reports indicate that bisphosphonate has beneficial effects on both bone and cardiovascular systems. However, whether bisphosphonate or simply the general treatment of osteoporosis improves the cardiovascular system is unclear.
Hyperphosphatemia in CKD is associated with increased all-cause mortality as well as cardiovascular morbidity and mortality [14]. Higher serum phosphate (Pi), even within the normal range, is associated with increased risk of cardiovascular disease (CVD), even after adjusting for established CVD risk factors such as age, sex, smoking, high blood pressure, and dyslipidemia [15]. We previously reported that greater serum Pi is a significant and an independent risk factor for increased CA-IMT [16]. Dietary Pi overload accelerates aortic sinus atheroma [17] and attenuates smooth muscle functions [18] in vivo without calcification, suggesting that excessive Pi may accelerate atherogenesis without vascular calcification.
The purpose of this study was to investigate whether teriparatide improves indicators of atherosclerosis, as well as BMD.
Materials and Methods
Subjects
This study included 30 osteoporotic patients with low LS-BMD (T-score < −2.5) and/or at least one vertebral fracture who started daily teriparatide therapy (20 μg/day) from November 2010 to November 2012 at Osaka City University Hospital.
Previous osteoporosis therapies, including oral bisphosphonates (16 cases), selective estrogen receptor modulators (three cases), and alfacalcidol (three cases), were discontinued before the initiation of the teriparatide therapy. DXA confirmed that all participants had at least two measurable lumbar spines in the L2-4 regions.
All participants were capable of visiting the hospital independently. None had a history of unresolved skeletal diseases that affect bone metabolism, current or previous malignant neoplasms, Paget’s disease of bone, skeletal exposure to therapeutic irradiation, symptomatic nephrocalcinosis or urolithiasis, abnormal thyroid and parathyroid functions not corrected by treatments, significantly impaired renal function (eGFR < 45 mL/min/1.73 m2), treatments with heparin or warfarin at any time prior to the initiation, excessive consumption of alcohol, or abuse of drugs.
Subjects were not included if they had a disease or habit that may affect atherosclerosis, such as smoking, or a previous history of coronary or cerebral vascular events. Two were receiving an angiotensin II receptor blocker, and five received both a calcium channel blocker and an angiotensin II receptor blocker. Six participants were receiving statins. The blood pressure (BP) and serum low-density lipoprotein cholesterol (LDL-C) levels were well controlled in these patients (BP < 140/90 mmHg and LDL-C < 140 mg/dL, respectively) and remained stable throughout the study, and their antihypertensive and/or lipid-lowering therapies were not changed throughout the study. One participant dropped out because of a rapid allergic reaction to the teriparatide, and another relocated before completing the study. A total of 28 patients completed at least 12 months of teriparatide therapy.
Bone Mineral Density (BMD) Measurement and Spinal Radiographs
LS-BMD was assessed by DXA using QDR-2000 (Hologic Inc., MA) at baseline, 6, and 12 months. Regions of severe scoliosis and vertebral fracture sites were excluded from LS-BMD calculations. BMD of the total hip was measured in the anterior–posterior projection using DXA. Spinal radiographs were obtained at baseline, 6, and 12 months, or at unscheduled times if patients reported new or worsening symptoms suggestive of clinical vertebral fracture (e.g., back pain). Vertebral fractures were assessed using a semiquantitative technique [19].
Ultrasonographic Measurement of Intima-Media Thickness (IMT)
The CA-IMT was measured at baseline, 6, and 12 months after using an ultrasonic phase-locked echo-tracking system equipped with a high-resolution real-time 13-MHz linear scanner (ProSound SSD 6500; Aloka Corporation, Japan) as previously reported [20]. In brief, approximately 4 cm of the common carotid artery was examined bilaterally in the longitudinal and transverse projections with the images focused on the far wall of the arteries. The CA-IMT was measured in both carotid arteries at the site of the most advanced atherosclerotic lesion that exhibited the greatest distance between the lumen-intimal and the media-adventitia interfaces of the far wall, herein defined as maximum CA-IMT. The intra-observer CV for CA-IMT was 2.8 %. All scans were evaluated by a physician who was unaware of the clinical characteristics of the patients.
Physical and Biochemical Parameters
Blood pressure was determined using a conventional cuff with a mercury sphygmomanometer after the patients had rested for at least 15 min. Serum and second-void urine samples were collected in the morning after an overnight fast before teriparatide administrations. Serum samples were stored at −80 °C until assayed. Serum Ca and urinary Ca levels were determined by the colorimetric method. Serum Pi, creatinine (Cr), alkaline phosphatase (ALP), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), and urinary Pi, and Cr levels were determined by enzymatic methods using an autoanalyzer (Hitachi 7450; Hitachi Co., Japan). Tubular maximum reabsorption of Pi per unit of glomerular filtration rate (TmP/GFR) [21] and estimated GFR (eGFR) by Modification of Diet in Renal Disease equation modified for Japanese patients [22] were calculated as previously described. Serum whole PTH was measured by an immunoradiometric assay (Scantibodies Laboratory, Inc., CA) [23]. Radioimmunoassay was used to measure serum 1,25-(OH)2D (Immunodiagnostic Systems, England) and P1NP (Orion Diagnostica, Finland) [24]. Serum FGF-23 was measured using sandwich enzyme-linked immunosorbent assay kits (Kainos Laboratories, Japan) [25]. Serum whole PTH, 1,25-(OH)2D, P1NP, and FGF-23 were measured concurrently to avoid inter-assay variance.
Statistical Analysis
Data were analyzed using StatView5.0J (Abacus Concepts, Inc., CA). Continuous variables were expressed as the mean ± SD. Median (interquartile range) was used for ALP, whole PTH, 1,25-(OH)2D, P1NP, TG, and CA-IMT because of their skewed distribution. Changes in the time course of serum Pi, TmP/GFR, and LS-BMD were analyzed by repeated measures one-way analysis of variance (ANOVA), whereas changes in the time course of CA-IMT were analyzed using Friedman’s test. Fisher’s protected least significant difference tests were used post hoc to confirm the statistically significant results. Differences between two points were evaluated using Wilcoxon signed-rank tests. Univariate regression analyses were performed using Spearman’s rank correlation test. P values less than 0.05 were considered statistically significant.
Results
Baseline Characteristics of the Participants
The clinical and biochemical profiles of the participants (n = 28) are shown in Tables 1 and 2. All participants had low LS-BMD (T-score < −2.5) and/or at least one vertebral fracture, which often indicates the presence of osteoporosis. The median CA-IMT at baseline was 0.701 mm (interquartile range: 0.655–0.774 mm). Ultrasonography did not find vascular calcifications in the common carotid arteries, and chest or spinal radiographs did not reveal aortic calcifications in any participants, indicating that none of the participants had advanced atherosclerosis. Baseline serum Ca, Pi, ALP, whole PTH, 1,25-(OH)2D, P1NP, and FGF-23 were within normal range.
Changes in Biochemical and Physical Parameters in Response to Teriparatide Therapy
Serum Ca, ALP, 1,25-(OH)2D, and P1NP increased significantly after 12 months of teriparatide therapy. Serum whole PTH, FGF-23, LDL-C, HDL-C, TG, systolic BP, and diastolic BP did not change significantly (Table 2).
Serum Pi and TmP/GFR decreased significantly 1 month after administration of the therapy and remained lower after 12 months (P = 0.008 and 0.029, respectively, Table 3). The change in TmP/GFR between 0 and 12 months was associated with the change in serum Pi (ρ = 0.928, P < 0.001, Fig. 1), but P1NP change was not significantly associated with the change in serum Pi (ρ = 0.187, P = 0.332).
Changes in LS-BMD and CA-IMT in Response to Teriparatide Therapy
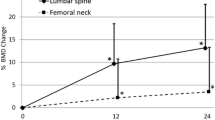
The LS-BMD significantly increased by 0.046 ± 0.038 g/cm2 after 12 months of therapy (P < 0.001, Fig. 2), however, no significant difference was observed in the total hip BMD (P= 0.471). The CA-IMT decreased significantly over the 12-month study period (P < 0.001), and decreased from 0.701 mm (0.655–0.774 mm) at baseline to 0.566 mm (0.509–0.717 mm) after 6 months (P < 0.05 vs. baseline) and 0.525 mm (0.477–0.670 mm) after 12 months (P < 0.05 vs. baseline, Fig. 2). Ultrasonography did not find vascular calcifications in the common carotid arteries in any participants during the study periods. The changes in CA-IMT between 0 and 12 months were not significantly correlated to those in LS-BMD (ρ = 0.033, P = 0.863).
Change in LS-BMD and CA-IMT after 12 months of teriparatide therapy. Teriparatide therapy increased LS-BMD (closed circles) (P < 0.001 by repeated measures one-way ANOVA), and decreased CA-IMT (open squares) (P < 0.001 by Friedman’s test). *P < 0.05 versus baseline by Fisher’s protected least significant difference test. Closed circles denote mean ± SD, whereas open squares denote median with interquartile range. LS-BMD, lumbar spine bone mineral density; CA-IMT, intima-media thickness of the common carotid artery
Relationship between biochemical parameters and LS-BMD and CA-IMT
The changes in P1NP between baseline and 1 month was positively associated with the change in LS-BMD between baseline and 12 months (ρ = 0.599, P = 0.002). The changes in serum Pi and the Ca × Pi product between 0 and 1 month were positively associated with the change in CA-IMT from 0 to 12 months (ρ = 0.431, P = 0.025 and ρ = 0.405, P = 0.035, respectively). None of the other parameters were associated with CA-IMT (Table 4).
Discussion
In the present study, 12 months of teriparatide therapy improved LS-BMD and CA-IMT, suggesting this therapy for osteoporosis also improves atherosclerosis in patients who are at a high risk of sustaining osteoporotic fractures and do not have advanced atherosclerosis. Furthermore, altered Pi metabolism by teriparatide associated with the improvement of atherosclerosis.
The molecular basis for the anabolic effects of teriparatide is still being elucidated. Bone vascularization influences the osteogenic generation of new bone. An angiogenic effect of PTH has been observed in vivo in ovariectomized mice [26]. PTH is osteoanabolic through VEGF-related mechanisms, but did not induce bone angiogenesis, while anti VEGF antibodies blocked the anabolic effects of PTH [27], suggesting that PTH is a potential actor in vascular remodeling by impacting molecular pathways involved in post angiogenesis.
During osteogenesis, a special capillary subtype in the murine skeletal system appears in specific vessel locations and mediates growth of the bone vasculature, generates suitable microenvironments, maintains perivascular osteoprogenitors, and couples angiogenesis to osteogenesis [28]. In addition, endothelial Notch signaling promotes both angiogenesis and osteogenesis in bone [29], indicating that the synthesis of new bone requires crosstalk between bones and the vasculature. Despite the importance of PTH on bone vascularization in osteogenesis, the effect of teriparatide on general systemic vasculature function is limited. Elevated serum PTH levels were associated to higher intra-arterial and calculated central blood pressures in a community-based cohort [30], suggesting that continuous elevation of circulating PTH was one of risk factors for vascular diseases. On the other hand, teriparatide reduces the extent of both aortic and cardiac valve calcification in vivo in diabetic LDL receptor-deficient mice [31]; however, no studies have investigated the beneficial effects of teriparatide on atherosclerosis in human patients.
Transient teriparatide exposure has anabolic effects by enhancing genes associated with bone formation in osteoblasts without inducing osteoclast activity [32]. Teriparatide also has a phosphaturic effect by reducing TmP/GFR as well as PTH [33]. However, this effect is transient, and serum Pi levels are not significantly suppressed in response to osteoporosis treatment [34, 35]. However, combination therapy with teriparatide and raloxifene significantly decreased serum Pi levels in another study [34], suggesting teriparatide combined with other therapies may affect serum Pi levels. In this study, serum Pi levels were significantly lower 1 month after the initiation of teriparatide, although the range of the decrease in serum Pi was wide.
The significant association between the changes in TmP/GFR and the changes in serum Pi suggests that the urinary Pi excretion was the main determinant of serum Pi level. The circulating levels of FGF-23, another phosphaturic hormone, are elevated in patients with primary hyperparathyroidism [36]. PTH is a potent inducer of FGF-23 [37], and elevated circulating FGF-23 levels in response to teriparatide therapy have been reported [38]. However, serum FGF-23 levels did not increase in response to teriparatide, suggesting that the teriparatide directly acts on the nephron to increase urinary Pi excretion. We did not measure circadian changes in FGF-23, so transient rises in FGF-23 induced by teriparatide may have also contributed to hypophosphatemia in addition to the direct effect of teriparatide on Pi excretion.
Epidemiological studies show that serum Pi levels are associated with all-cause and CV-related mortality in the general population [15] as well as in patients with CKD and that Pi binders prevent CV events [14]. The beneficial effects of phosphate binders on vascular calcification (coronary and aortic) were reported in randomized control trial (RCT) in dialysis [39, 40] and pre-dialysis CKD patients [41], indicating excess serum Pi contributes to vascular dysfunction. Although non-calcium-based binders contributed less to the development of vascular calcification than calcium based binders [42], it is still plausible whether non-calcium-containing phosphate binders are superior to calcium-containing phosphate binders, because all-cause and cardiovascular mortalities among these phosphate binders were not significantly different in RCT. [43].
In particular, serum Pi is positively associated with CA-IMT [44], an established early marker of atherosclerosis [45]. Elevated extracellular Pi concentrations give vascular smooth muscle cells an osteoblast-like phenotype, which subsequently accelerates vascular calcification [46]. Dietary Pi overload increases aortic sinus atheroma without calcification in apolipoprotein knockout mice in vivo [17], suggesting that excessive Pi accelerates atherogenesis without vascular calcification. Dietary Pi overload also attenuates the vascular smooth muscle response to physiological and pathological stimuli ex vivo in CKD C57/BL6 mice [18].
The decrement of serum Pi levels was observed after the teriparatide therapy, however, its decrement was only 8 % (from 3.9 ± 0.4 to 3.6 ± 0.5 mg/dL) and within normal ranges. Although the decrement in serum Pi concentrations was relatively small in this study, prior studies reported an elevated risk of adverse cardiovascular outcomes in association with comparably small increases in serum Pi levels [15, 47].
In this study, 12 months of teriparatide therapy increased LS-BMD and decreased CA-IMT, although LS-BMD and CA-IMT were not significantly correlated. The change in P1NP was associated with the change in LS-BMD but not with the change in CA-IMT. In addition, the serum Pi was associated with CA-IMT, but not with LS-BMD. Teriparatide activated bone tissue, resulting in increased P1NP, and also induced phosphaturia in the kidney, resulted in hypophosphatemia. Our results suggest that teriparatide ameliorated both osteoporosis and atherosclerosis. Teriparatide improved CA-IMT in part by reducing serum phosphate, a well-known vascular toxin.
Classical risk factors for atherosclerosis, such as BP, lipid profile, and smoking status, were not changed during the teriparatide therapy. Serum Pi, which was decreased with teriparatide treatment in our study, is a risk factor for vascular calcification [48]. Serum FGF-23 and soluble klotho (data not shown), two other risk factors [48], were not changed during the therapy, and other factors, such as vitamin D, vitamin K, and warfarin administration, were not performed in this study.
The limitation of this study was the study protocol. This study was not a randomized control study, and we did not have appropriate untreated control subjects. However, CA-IMT increases significantly over a 12-month period in untreated patients with postmenopausal osteoporosis [11], suggesting that the decrement in CA-IMT with teriparatide treatment in our study was clinically significant.
In conclusion, daily teriparatide administration improved CA-IMT and LS-BMD, suggesting teriparatide improves both osteoporosis and atherosclerosis. Furthermore, improvements in CA-IMT were associated with decreased serum Pi, a well-known vascular toxin.
References
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH (2011) PINP as an aid for monitoring patients treated with teriparatide. Bone 48:798–803
Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE, Osteoporotic Fractures in Men Study G (2009) Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation 119:2305–2312
Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V (2004) Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89:4246–4253
Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR (2005) Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 20:1912–1920
Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB (2008) Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int 19:1161–1166
Guerin AP, London GM, Marchais SJ, Metivier F (2000) Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transpl 15:1014–1021
London GM, Marchais SJ, Guerin AP, Boutouyrie P, Metivier F, de Vernejoul MC (2008) Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19:1827–1835
Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG (2008) Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23:586–593
Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE (1997) Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam study. Circulation 96:1432–1437
Okamoto K, Inaba M, Furumitsu Y, Ban A, Mori N, Yukioka K, Imanishi Y, Nishizawa Y (2010) Beneficial effect of risedronate on arterial thickening and stiffening with a reciprocal relationship to its effect on bone mass in female osteoporosis patients: a longitudinal study. Life Sci 87:686–691
Luckish A, Cernes R, Boaz M, Gavish D, Matas Z, Fux A, Shargorodsky M (2008) Effect of long-term treatment with risedronate on arterial compliance in osteoporotic patients with cardiovascular risk factors. Bone 43:279–283
Kang JH, Keller JJ, Lin HC (2013) Bisphosphonates reduced the risk of acute myocardial infarction: a 2-year follow-up study. Osteoporos Int 24:271–277
Toussaint ND, Pedagogos E, Tan SJ, Badve SV, Hawley CM, Perkovic V, Elder GJ (2012) Phosphate in early chronic kidney disease: associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 17:433–444
Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS (2007) Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167:879–885
Ishimura E, Taniwaki H, Tabata T, Tsujimoto Y, Jono S, Emoto M, Shoji T, Inaba M, Inoue T, Nishizawa Y (2005) Cross-sectional association of serum phosphate with carotid intima-medial thickness in hemodialysis patients. Am J Kidney Dis 45:859–865
Ellam T, Wilkie M, Chamberlain J, Crossman D, Eastell R, Francis S, Chico TJ (2011) Dietary phosphate modulates atherogenesis and insulin resistance in apolipoprotein E knockout mice–brief report. Arterioscler Thromb Vasc Biol 31:1988–1990
Six I, Maizel J, Barreto FC, Rangrez AY, Dupont S, Slama M, Tribouilloy C, Choukroun G, Maziere JC, Bode-Boeger S, Kielstein JT, Drueke TB, Massy ZA (2012) Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc Res 96:130–139
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis the study of osteoporotic fractures research group. J Bone Miner Res 11:984–996
Yamazaki Y, Emoto M, Morioka T, Kawano N, Lee E, Urata H, Tsuchikura S, Motoyama K, Mori K, Fukumoto S, Shoji T, Nishizawa Y, Inaba M (2013) Clinical impact of the leptin to soluble leptin receptor ratio on subclinical carotid atherosclerosis in patients with type 2 diabetes. J Atheroscler Thromb 20:186–194
Brodehl J, Krause A, Hoyer PF (1988) Assessment of maximal tubular phosphate reabsorption: comparison of direct measurement with the nomogram of Bijvoet. Pediatr Nephrol 2:183–189
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S (2007) Modification of the modification of diet in renal disease (MDRD) study equation for Japan. Am J Kidney Dis 50:927–937
Gao P, Scheibel S, D’Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL (2001) Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 16:605–614
Tahtela R, Turpeinen M, Sorva R, Karonen SL (1997) The aminoterminal propeptide of type I procollagen: evaluation of a commercial radioimmunoassay kit and values in healthy subjects. Clin Biochem 30:35–40
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960
Roche B, Vanden-Bossche A, Malaval L, Normand M, Jannot M, Chaux R, Vico L, Lafage-Proust MH (2014) Parathyroid hormone 1–84 targets bone vascular structure and perfusion in mice: impacts of its administration regimen and of ovariectomy. J Bone Miner Res 29:1608–1618
Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier MT, Thomas M, Laroche N, Malaval L, Langer M, Peter ZA, Peyrin F, Vico L, Lafage-Proust MH (2011) Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res 26:2583–2596
Kusumbe AP, Ramasamy SK, Adams RH (2014) Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507:323–328
Ramasamy SK, Kusumbe AP, Wang L, Adams RH (2014) Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507:376–380
Hagstrom E, Ahlstrom T, Arnlov J, Larsson A, Melhus H, Hellman P, Lind L (2015) Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis 238:420–426
Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA (2003) Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem 278:50195–50202
Dobnig H, Turner RT (1997) The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612
Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocana A, Stewart AF (2003) Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1–36) [hPTHrP-(1–36)] versus hPTH-(1–34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 88:1603–1609
Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH (2005) Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911
Anastasilakis AD, Polyzos SA, Goulis DG, Slavakis A, Efstathiadou Z, Kita M, Koukoulis G, Avramidis A (2008) Endogenous intact PTH is suppressed during Teriparatide (rhPTH 1–34) administration in postmenopausal women with established osteoporosis. Endocr J 55:613–616
Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y (2006) Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol 154:93–99
Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y (2007) Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18:2683–2688
Sridharan M, Cheung J, Moore AE, Frost ML, Fraser WD, Fogelman I, Hampson G (2010) Circulating fibroblast growth factor-23 increases following intermittent parathyroid hormone (1–34) in postmenopausal osteoporosis: association with biomarker of bone formation. Calcif Tissue Int 87:398–405
Chertow GM, Burke SK, Raggi P, Treat to Goal Working G (2002) Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62:245–252
Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P (2005) Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68:1815–1824
Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE (2007) The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72:1255–1261
Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG (2011) Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: a pilot randomized controlled trial. Nephrology (Carlton) 16:290–298
Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK (2007) Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72:1130–1137
Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P (2008) Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 199:424–431
O’Leary DH, Polak JF, Wolfson SK Jr, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA (1991) Use of sonography to evaluate carotid atherosclerosis in the elderly. The cardiovascular health study. CHS Collaborative Research Group. Stroke 22:1155–1163
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87:E10–E17
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol Recurrent Events, Trial I (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112:2627–2633
Leonard O, Spaak J, Goldsmith D (2013) Regression of vascular calcification in chronic kidney disease—feasible or fantasy? A review of the clinical evidence. Br J Clin Pharmacol 76:560–572
Acknowledgments
Maki Yoda contributed to the acquisition, analysis, and interpretation of the data. Yasuo Imanishi and Masaaki Inaba contributed to the conception and design of the study. Yuki Nagata, Masaya Ohara, Koichiro Yoda, Shinsuke Yamada, and Katsuhito Mori contributed to the acquisition of data. Maki Yoda and Koichiro Yoda take responsibility for the integrity of the data analysis. All authors participated in drafting or revising the manuscript and approved the final version of the manuscript for submission.
Conflict of interest
Masaaki Inaba reports grant support and lecture fees from Eli Lilly Japan K.K. Yasuo Imanishi and Katsuhito Mori report lecture fees from Eli Lilly Japan K.K. Maki Yoda, Yuki Nagata, Masaya Ohara, Koichiro Yoda, and Shinsuke Yamada have no conflicts of interest.
Human and Animal Rights and Informed Consent
All participants provided written informed consent before participating in this study, which received institutional ethics committee approval (Osaka City University Graduate School of Medicine, registration number 1775) and was conducted in accordance with the principles of the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoda, M., Imanishi, Y., Nagata, Y. et al. Teriparatide Therapy Reduces Serum Phosphate and Intima-Media Thickness at the Carotid Wall Artery in Patients with Osteoporosis. Calcif Tissue Int 97, 32–39 (2015). https://doi.org/10.1007/s00223-015-0007-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0007-4