Abstract
Cerebellar strokes induce coordination disorders that can affect activities of daily living. Evidence-based neurorehabilitation programs are founded on motor learning principles. The cerebellum is a key neural structure in motor learning. It is unknown whether and how well chronic cerebellar stroke individuals (CCSIs) can learn to coordinate their upper limbs through bimanual motor skill learning. The aim was to determine whether CCSIs could achieve bimanual skill learning through a serious game with the REAplan® robot and to compare CCSIs with healthy individuals (HIs). Over three consecutive days, sixteen CCSIs and eighteen HIs were trained on an asymmetric bimanual coordination task (“CIRCUIT” game) with the REAplan® robot, allowing quantification of speed, accuracy and coordination. The primary outcomes were the bimanual speed/accuracy trade-off (BiSAT) and bimanual coordination factor (BiCo). They were also evaluated on a bimanual REACHING task on Days 1 and 3. Correlation analyses between the robotic outcomes and clinical scale scores were computed. Throughout the sessions, BiSAT and BiCo improved during the CIRCUIT task in both HIs and CCSIs. On Day 3, HIs and CCSIs showed generalization of BiSAT, BiCo and transferred to the REACHING task. There was no significant between-group difference in progression. Four CCSIs and two HIs were categorized as “poor learners” according to BiSAT and/or BiCo. Increasing age correlated with reduced BiSAT but not BiCo progression. Over three days of training, HIs and CCSIs improved, retained, generalized and transferred a coordinated bimanual skill. There was no between-group difference, suggesting plastic compensation in CCSIs. Clinical trial NCT04642599 approved the 24th of November 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum is a key neural hub that hosts 70–80% of intracranial neurons and is extensively connected with telencephalic cortical areas and subcortical structures (Azevedo et al. 2009; Herculano-Houzel 2009). Acute focal damage to the cerebellum, such as a stroke, can induce ataxia, stance and gait instability, dysarthria, nystagmus and vertigo, depending on the location and extent of the damage (Jung and Roh 2022). There are 12.2 million new occurrences of stroke each year, making it the leading cause of acquired motor disability in adults worldwide (World Stroke Organisation (WSO) 2022). Populations are growing and living to an older age, therefore, the number of stroke survivors living with long-term impairment is expected to increase (Wafa et al. 2020).
The cerebellum is damaged in 2–10% of stroke victims (Jung and Roh 2022). Most patients recover relatively well after a cerebellar stroke, possibly because of the structural cerebellar reserve, i.e., the capacity of the cerebellum to compensate efficiently after limited damage through the plastic recruitment of non-injured cerebellar areas (Kelly et al. 2001; Manto 2022; Mitoma et al. 2021; Sadeghihassanabadi et al. 2022). However, up to 40% of patients with damage to critical parts of the cerebellum such as the anterior cerebellar lobe (e.g., lobules IV and V) and a part of the posterior lobe (e.g., lobules VI and VIII), are left with coordination impairments and ataxia, which limits their independence in activities of daily living and quality of life (Jung and Roh 2022; Mitoma and Manto 2016; Sadeghihassanabadi et al. 2022; Schmahmann et al. 2009b; Tohgi et al. 1993; Ye et al. 2010). Limb ataxia refers to an impairment of fine temporospatial motor control of movements and joints coordination in the absence of a significant force impairment (Barboi 2000). Whereas ataxia is predominantly unilateral after a stroke, it may impact bimanual activities of daily living as well since most activities of daily living require the coordinated use of both hands.
The cerebellum is crucial for executing smooth actions, and cerebellar lesions or degeneration result in ataxia because the cerebellum is hypothesized to be the neural substrate of forward models (Hardwick et al. 2013a; Mitoma et al. 2021). A forward model is believed to continuously predict the sensory consequences of our own actions based on a copy of efference (a motor intention) issued by the motor/premotor cortex along with the motor command sent to the spinal cord. If the sensory prediction matches the sensory feedback, there is no need for correction, either on-line or in subsequent occurrences. If there is a mismatch between the sensory prediction and the actual sensory feedback, a sensory prediction error is generated. Sensory prediction errors can be used to correct both on-line movements, ensuring smooth action performance and goal completion, and future movements through this error-based correction system (Welniarz et al. 2021).
This finding could explain why the cerebellum plays a crucial role in motor learning (Hardwick et al. 2013a; Shadmehr et al. 2010). Motor learning is broadly defined as the ability to acquire or refine motor patterns—i.e., movements—through experience (Krakauer et al. 2019). According to functional brain imaging studies involving healthy individuals (HIs), the cerebellum is consistently activated during both adaptation learning (the capacity to compensate for a perturbation disturbing an overlearned movement such as reaching) and motor skill learning tasks (Baldassarre et al. 2021; Doyon and Benali 2005; Tracy et al. 2001; Tseng et al. 2007). Motor skill learning is the capacity to elaborate, learn and retain new sensorimotor skills that become habitual or quasi-automatized once mastered (Krakauer and Mazzoni 2011). Virtually every voluntary movement we make has been learned and can be considered a skill that improves through practice-dependent training, leading to a greater speed of execution and reduced variability (Krakauer & Carmichael 2017). Functional brain imaging studies of motor skill learning revealed strong cerebellar activation during the first stage of learning (acquisition), consistent with the involvement of the cerebellum in error-based learning and the rapid correction of (new) movements; decreased activation during the intermediate stage (consolidation); and minimal activation during the later stage (retention) (Baldassarre et al. 2021; Dahms et al. 2020; Nezafat et al. 2001). A quantitative meta-analysis by Hardwick and collaborators on motor learning involving the upper limb showed consistent activation in cerebellar lobule VI during motor sequence learning and in multiple foci, including the vermis (lobules I–IV) and bilateral cerebellar hemispheres (lobules V–VI), during the learning of new movement kinematics/dynamics (Hardwick et al. 2013b). Thus, the cerebellum is adaptively recruited in partly overlapping networks during both adaptation learning and motor skill learning. Interestingly, brain imaging studies have also demonstrated the involvement of the cerebellum in bimanual coordination and bimanual motor skill learning, with activation centred around the anterior lobe likely involved in the spatial/sequential control of movement and activation in the posterior lobe (lobules V, VI and VIII) for temporal control (Rémy et al. 2008; van Dun et al. 2022). Since the majority of activities of daily living (e.g., dressing, using a fork and a knife, driving a car or crafting an object) (Vega-Gonzalez et al. 2007) are bimanual skills requiring fine coordination that must be learned, bimanual motor skill learning is crucial for both the normal acquisition of activities of daily living and rehabilitation.
In chronic cerebellar stroke individuals (CCSIs, > 6 months post-stroke (Langhorne et al. 2011)), with stable impairments, focal cerebellar damage does not prevent unimanual motor sequence consolidation but impairs training-induced improvements in performance in both hands in implicit motor sequence learning tasks (Hermsdorf et al. 2020). Although the cerebellum is critical for motor learning and interlimb/bimanual coordination (Debaere et al. 2004; Rémy et al. 2008; Serrien and Wiesendanger 2000), we found only two studies exploring the consequences of chronic cerebellar stroke on bimanual motor skill learning. Dirnberger et al. reported that CCSIs can achieve bimanual visuomotor sequence learning through serial repetitive training tasks (SRTT) variants, although their motor performance was less accurate than those of HIs (Dirnberger et al. 2010, 2013). However, CCSIs showed higher-order impairments not observed in HIs, such as interference with another motor sequence or an impairment in the use of perceptual information (Dirnberger et al. 2010, 2013; Schmahmann et al. 2009a; Serrien and Wiesendanger 2000). The aim of our study was to determine whether CCSIs could achieve bimanual motor skill learning requiring asymmetrical bimanual coordination by training with a serious game implemented on a robotic device. The hypotheses were that CCSIs would (1) be able to learn, improve, retain, generalize and transfer a new complex bimanual coordination skill; (2) perform worse than HIs; and (3) exhibit bimanual motor skill learning impairments correlated with their scores on clinical scales.
Materials and methods
Participants
Sixteen CCSIs and twenty HIs were recruited for this nonblinded prospective cohort study from January 2021 to February 2023 at the CHU UCL Namur (Godinne site), Belgium. For CCSIs, the inclusion criteria were as follows: (1) 18–90 years of age; (2) unique chronic unilateral cerebellar stroke (> 6 months); and (3) ability to participate for three consecutive days. The exclusion criteria were as follows: (1) inability to move the affected arm; (2) inability to understand or execute commands; (3) drug/alcohol abuse; and (4) cognitive, psychiatric, or serious health disorders that could interfere with the study. HIs were considered eligible if they met the following criteria: (1) 18–90 years of age, (2) no neurological conditions, (3) no drug/alcohol abuse and (4) no psychiatric conditions.
Data collection
Clinical assessments
Since cerebellar stroke can induce various impairments, several clinical scales have been used in CCSIs, focusing on motor aspects. Ataxia was assessed using the Modified International Cooperative Ataxia Rating Scale (MICARS) (Schmahmann et al. 2009a). The 120-point MICARS evaluates posture and gait disturbances, kinetic functions, speech and oculomotor disorders. A higher score indicates worse ataxia (motor normality is indicated by a score ≤ 4) (Schmahmann et al. 2009b). The Fugl Meyer Assessment for Upper Extremity (FMA-UE) was used to evaluate motor impairment in the affected upper limb (Fugl-Meyer et al. 1975). A normal motor score is 66/66. Unilateral gross hand dexterity was assessed with the box and block test (BBT) (Mathiowetz et al. 1985) for each hand. A higher score (mean of three trials) indicates better performance. The normal mean score (± SD) for right handed females and males are respectively 78.5 ± 10.4 and 76.8 ± 11.5 blocs. The ABILHAND questionnaire for chronic stroke individuals was used to measure the patients’ manual ability to manage bimanual activities of daily living, including 23 items with three response levels (impossible/difficult/easy). Scores range from − 6.017 (worse) to + 6.017 (better) logits for performance (Penta et al. 2001). A brief cognitive impairment screening was carried out with the French version of the Montreal Cognitive Assessment test (MoCA; cut-off score for cognitive impairment < 26/30) (Nasreddine et al. 2005). In addition, short-term visuospatial working memory was assessed with the Corsi Block-Tapping Task (forward and backward) (Corsi 1973). A span of up to 5–6 blocks is standard for HIs (Kessels et al. 2000).
Study design
Assessments and intervention (training) took place over three consecutive days (Supplementary Fig. 1). On Day 1, CCSIs performed the MICARS (Sections 1–2), the dexterity tasks (see below), the BBT and two tasks with the bimanual robot (REACHING followed by training with the CIRCUIT). On Day 2, CCSIs performed the MICARS (Sections 3–4), MoCA, FMA-UE, Corsi and were subsequently trained with the CIRCUIT on the robot. Day 3 consisted of one questionnaire (ABILHAND) and performing three tasks on the robot (training with the CIRCUIT, followed by assessment with REACHING and NEWCIRCUIT). The HIs followed the same protocol except that they were not evaluated with clinical scales other than the BBT.
REAplan® robot
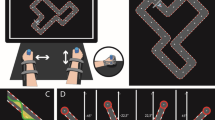
The REAplan® (AXINESIS, Wavre, Belgium) is a neurorehabilitation robot with a distal effector that allows the arm to move in the horizontal plane. The movement kinematics and forces were quantified at 80 Hz through position and force sensors. The participants were trained and evaluated on the bimanual version of the REAplan®, which has been used previously in chronic stroke patients and HIs (Gerardin et al. 2022; Lefebvre et al. 2012; Riga et al. 2022). The participants were seated in front of the immersive REAplan® screen with the elbows and knees flexed at 90°. The participants were instructed to coordinate their hands to move a common cursor with the help of handles fixed on forearm rest gutters in the horizontal plane. In line with Lissajous feedbacks, the common cursor provided real-time feedback, augmented by the direct vision of the upper limbs (Kovacs et al. 2009). One handle controlled the lateral X-axis (left–right) displacement of the common cursor, and the other controlled the sagittal Y-axis (front-back, Fig. 1a). Virtual walls constrained the movements of each hand along their respective axes. On Day 1, a range of motion calibration was carried out to adapt the range of motion to the subject’s comfort zone. Which hand (left/right) controlled each axis (X/Y) on the REAplan® was randomized and balanced. An R 4.2.3 (The R Foundation for Statistical Computing, Austria, Vienna, 2019) routine with minimization was used to randomize each participant (details in Supplementary Materials).
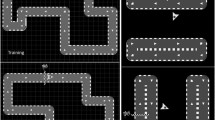
REAplan® setup and tasks (Gerardin et al. 2022). a Bimanual configuration controlling the common cursor displayed on the REAplan® screen and the CIRCUIT task used for training. b The NEWCIRCUIT task was used to assess generalization (90° rotation of the trained CIRCUIT). c Calculation of error (yellow): distance between the ideal path (defined as the centre of the track) and the actual common cursor position (green line). d Positions of the four targets used in the bimanual REACHING task
The training task was the CIRCUIT. Learning a new bimanual control policy is necessary to perform this bimanual cooperative serious game, which consists of completing as many laps as possible (speed constraint) with the bimanually controlled cursor while remaining within the track of a complex circuit (accuracy constraint) during a 1-min block (Fig. 1) (De Laet et al. 2022; Doost et al. 2019; Yeganeh Doost et al. 2017). There was a 30-s rest period between each block, while the score for the block (reflecting the bimanual speed/accuracy trade-off (BiSAT); see the Kinematic data processing section) and the high score were displayed. The participants trained on 20 blocks each day. After completing the training on Day 3, a NEWCIRCUIT was displayed to assess the ability of the participants to generalize bimanual motor skill learning (Fig. 1b). The NEWCIRCUIT track was identical to the track used for training except that it was rotated by 90°.
The bimanual REACHING task consisted of fast reaching movements from the home position towards an eccentric target with the common bimanual cursor (Fig. 1d). Four target positions were pseudorandomly displayed (4 times/target, 16 trials in total). The subjects were instructed to reach the target as fast as possible with straight movement, remain within the track and then return to the target home position. The participant had to keep the cursor within the eccentric target or home target during 300 ms to validate the trial. A period of 100 ms elapsed between home target validation and the display of the new eccentric target. Using the vertical median Y-axis on the screen as a reference, the eccentric target positions were ± 45° (requiring symmetric bimanual movements as in the CIRCUIT task) and ± 25° (requiring asymmetric bimanual movements). The bimanual REACHING task was used to evaluate potential transfer of training-induced BiSAT and BiCO improvements.
Dextrain® manipulandum
The Dextrain® manipulandum (DEXTRAIN, Paris, France) was used to quantify different aspects of manual dexterity. It is composed of five pistons connected to five sensors simultaneously monitoring the forces applied by each finger (Térémetz et al. 2015). The participant sat on a chair in front of a computer where visuomotor tasks were displayed, the forearm in pronation resting on a triangular cushion and the manipulandum was in the hand with fingers secured on pistons through flat magnets taped to the pulp of fingers (Supplementary Information). Only the ipsilesional (CCSIs) or nondominant (HIs) hand was assessed.
For the Multi-Finger Tapping task (MFTapping), which is used to assess finger accuracy and coactivation, one pair of columns by finger was displayed on the screen (one as a target and one for real-time force feedback). The participants were instructed to press as soon as possible on the piston corresponding to the target finger during the given period (1.5 s) and to release after this period. In total, the subject performed 20 trials in a pseudorandomized order (4 trials/fingers) with a rest of 3 s after 5 trials. Two versions of the MFTapping were completed: an MFTapping monofinger (MFTapping-mono) pressing one finger at a time; and an MFTapping plurifingers (MFTapping-pluri) pressing two fingers simultaneously. MFTapping-pluri required the participant to press their thumb with one of the other four fingers or to press their index finger with the middle finger (4 trials/finger pairs, total: 20 trials). The task duration was 1.29 min. Before performing the MFTapping task, a familiarization task (easier MFTapping-mono, one trial/finger, 30 s) was provided.
In the second task, the Finger Force Tracking (FFTracking) was used to assess fine finger force control. Through slight finger flexion/extension, the subject controlled a red cursor displayed on the screen (real-time feedback). The participants were instructed to follow a white path as closely as possible, moving up and down in a fixed sequential order, scrolling from right to left. The patients were subjected to the same four cycles (1.33 min) once with the thumb and then once with the index finger (details about the tasks are provided in the Supplementary Information).
Kinematic data processing
MATLAB (2021, MathWorks Inc, Natick, MA, United States) routines were used for kinematic analysis. For REAplan®, the raw data were resampled in 3-s intervals (20 intervals for one 1-min block). The primary outcomes were the bimanual speed/accuracy trade-off (BiSAT) and the bimanual coordination factor (BiCo).
-
1.
BiSAT quantified the training-induced improvement in arbitrary units (a.u.).
$$BiSAT= \frac{speed (\frac{{\text{cm}}}{{\text{s}}})}{error ({\text{cm}})}\times C \, (Where \, C=1 {\text{cms}})$$
The speed is the norm of the velocity vector of the common cursor’s position. The error was quantified by the distance between the ideal path (the centre of the track) and the real cursor’s trajectory. A larger BiSAT (in a.u.) indicates better performance.
Based on data sampled at 80Hz, the average speed and error were computed for each 3-s intervals to compute the BiSAT average used for statistical analysis.
-
2.
BiCo is a mathematical measure of the phase coherence between the velocities of both hands, where |V| is the velocity for displacement of the hand in the X- or Y-axis. The numerator is the minimum value between |\(\frac{{V}_{x}}{{\text{cos }}\alpha }\)| and |\(\frac{{V}_{y}}{{\text{sin }}\alpha }\)|. The denominator \(\sqrt{{\left(\frac{{V}_{x}}{{\text{cos }}\alpha }\right)}^{2}+{\left(\frac{{V}_{y}}{{\text{sin }}\alpha }\right)}^{2}}\) represents the bimanual velocity.
$$BiCo=\frac{\mathit{min}\left(\left|\frac{{V}_{x}}{{\text{cos }}\alpha }\right|,\left|\frac{{V}_{y}}{{\text{sin }}\alpha }\right|\right)}{\sqrt{{\left(\frac{{V}_{x}}{{\text{cos }}\alpha }\right)}^{2}+{\left(\frac{{V}_{y}}{{\text{sin }}\alpha }\right)}^{2}}}$$
If the speeds of both hands along the X and Y-axes are identical (perfect synchronization) BiCo is equal to 1. Based on data sampled at 80 Hz, the average BiCo were computed for each 3-s intervals then averaged for the 1-min block to be used for statistical analysis.
On the Dextrain® manipulandum, to validate a trial with the MFTapping task, the target finger had to press on its piston within the given time interval (1.5 s) with a force > 0.5 N (for MFTapping-pluri = the first finger to press must be one of the two targeted fingers). Two outcomes were computed: press error (PError) and the coactivation index (Coact). An error was counted if force was applied by any unwanted finger (> 0.5 N) before the target finger. PError was the sum of the errors across the 20 trials. Coact was the number of coactivated fingers across the 20 trials. Coactivation was defined as when any nontarget finger pressed the piston (> 0.5 N) after the target finger pressed within the given time interval (1.5 s).
The FFTracking outcome was the root mean square error (RMSE), computed as a proxy of the speed/accuracy trade-off. The difference between the applied force (y: observed values) and the target force (x: predicted values) over the four cycles was computed for the thumb and index finger. A smaller RMSE reflects a higher accuracy of finger force control.
Statistical analysis
On each day (D1, D2 and D3), performances during the first (C1) and last (C20) blocks of CIRCUIT training were calculated, as well during the first (NC1) and last (NC3) blocks of the NEWCIRCUIT on D3. BASELINE was defined as the performance at D1C1. The overall progression according to the CIRCUIT (TOTAL) was computed as the difference between D3C20 and D1C1 (Supplementary Fig. 3). GENERALIZATION was defined as the progression between the first block of NEWCIRCUIT (D3NC1) and D1C1. NEWCIRCUIT progression (NC TOTAL) was the difference between D3NC3 and D3NC1. Overnight (ON) losses on D2-D1 and D3-D1 were computed as the difference between the last block of the previous day and the first block of the current day (ON1 and ON2). The loss due to circuit change (Circuit Change) was the difference between D3NC1 and the last block of the CIRCUIT at D3 (D3C20). For REACHING, BiSAT and BiCo were computed as BASELINE and DELTA (D3–D1).
Baseline characteristics are described as the mean and standard deviation (mean ± SD) for continuous measures and percentages for categorical measures. Welch two-sample t-test and two-sample test for equality of proportions without continuity correction were used for continuous and binary outcomes, respectively.
Spearman’s correlation coefficients were computed between the baseline clinical scales (MICARS, BBT, MoCA, Corsi block, age, ABILHAND, MFTapping and FFTracking) and robotic outcomes (CIRCUIT: BiSAT TOTAL and GENERALIZATION, BiCo TOTAL and GENENERALIZATION; REACHING: BASELINE and DELTA for BiSAT and BiCo) for CCSIs, with |r| ≥ 0.7considered to indicate a strong correlation (Schober et al. 2018).
In an exploratory analysis, the differences in the overall progression of BiSAT and BiCo with the circuit in CCSIs and HIs were adjusted for age and randomization group with a linear regression model (Schneider et al. 2010).
Statistical analysis were performed using R 4.3.0 (The R Foundation for Statistical Computing, Austria, Vienna, 2019) and the gtsummary (for tables) and ggplot2 (for graphics) packages. A Welch two sample t-test was used to compare descriptive statistics of progression between groups. The alpha threshold was set at 5%.
Results
Subjects
Between January 2021 and February 2023, 298 stroke patients were screened for eligibility. Thirty-two were CCSIs and met the inclusion criteria. Sixteen CCSIs and twenty HIs agreed to participate in this study. One CCSI dropped out on D3 for personal convenience. Two HIs were excluded from the REAplan® analysis due to technical issues. One HI and one CCSI could not use the Dextrain® manipulandum (flow chart and TREND checklist are shown in Supplementary Figs. 4–6). The characteristics of each group at baseline are given in Supplementary Table 1. There was a greater proportion of women in the HI group than in the CCSI group (65% in HIs vs. 19% in CCSIs, difference [95% CI] = − 46% [− 80 to − 12%], P = 0.02). On the Dextrain® MFTapping-mono task, the CCSIs made significantly more errors (26.9 ± 17.5 for HIs vs. 43.7 ± 17.8 for CCSIs, difference [95% CI] = 17 [4 to 30], P = 0.01; Table 1) and had less coactivations (18.4 ± 7.1 vs. 31.3 ± 11.3, difference [95% CI] = -13 [− 19 to − 6.4], P < 0.001) than the HIs. The other Dextrain® outcomes were not significantly different. The stroke lesion locations are shown in Supplementary Fig. 7.
Bimanual motor skill learning: CIRCUIT
The BiSAT BASELINE did not differ between groups (7.4 ± 2.0 a.u. for HIs vs. 8.0 ± 3.4 a.u. for CCSIs, difference [95% CI] 0.63 [− 1.4 to 2.7], P = 0.53; Tables 2 and 3). For both HIs and CCSIs, the mean BiSAT curves showed continuous progression over the three days (TOTAL: 21.4 ± 9.7, [95% CI] = [17 to 26] in HIs and 20.0 ± 15.3, [95% CI] = [12 to 28] in CCSIs Table 3), interspersed by ONs (ON1: − 5.3 ± 4.7, [95% CI] = [− 7.7 to − 3] in HIs and − 3.9 ± 3.7, [95% CI] = [− 5.9 to − 1.9] in CCSIs; ON2: − 7.5 ± 6.3, [95% CI] = [− 11 to − 4.3] in HIs and − 4.9 ± 5.9, [95% CI] [− 8.2 to − 1.7] in CCSIs; Fig. 2). The TOTAL BiSAT progression did not differ among the three training days between groups nor ONs (TOTAL: difference [95% CI] = − 1.4 [− 11 to 8], P = 0.76; ON1: difference [95% CI] = 1.4 [− 1.6 to 4.3], P = 0.34; ON2: difference [95% CI] = 2.5 [− 1.8 to 6.9], P = 0.24). Upon visual analysis, three CCSIs (1, 12, 14) displayed almost flat BiSAT curves; therefore, they were categorized as “poor learners”. Unexpectedly, a similar flat BiSAT curve pattern was observed for two HIs (5, 17), who were also categorized as "poor learners”.
BiSAT and BiCo progressions. a.u.: arbitrary units. The grey lines represent individual performances, and the bold lines represent group mean performances. Training blocks 1 to 60 correspond to the CIRCUIT task through the three training days (20/day), and blocks 61 to 63 correspond to the NEWCIRCUIT task. There were slight overnight losses between D1-D2 and D2-D3 and a larger drop on D3 when the NEWCIRCUIT was introduced (blocks 61 to 63)
The BiCo BASELINE did not significantly differ between groups (0.29 ± 0.03 a.u. for HIs vs. 0.27 ± 0.08 a.u. in CCSIs, difference [95% CI] = − 0.01 [− 0.06 to 0.03], P = 0.52; Tables 2 and 3). There was a significant BiCo progression over the three training days in HIs and CCSIs (TOTAL: 0.19 ± 0.07, [95% CI] = [0.16 to 0.23] in HIs and 0.19 ± 0.07, [95% CI] = [0.15 to 0.23] in CCSIs) and significant overnight losses (ON1: − 0.07 ± 0.06, [95% CI] = [− 0.1 to − 0.04] in HIs and − 0.04 ± 0.05, [95% CI] = [− 0.07 to − 0.01] in CCSIs; ON2: − 0.03 ± 0.05, [95% CI] = [− 0.06 to − 0.01] in HIs and − 0.03 ± 0.04, [95% CI] = [− 0.06 to − 0.01] in CCSIs). There was no significant between-group difference in BiCo TOTAL (difference [95% CI] = − 0.01 [− 0.06 to 0.05], P = 0.84), ON1 (difference [95% CI] = 0.03 [− 0.01 to 0.07], P = 0.13) or ON2 (difference [95% CI] = 0 [− 0.03 to 0.03], P = 0.95). In both groups, BiCo improved continuously over D1-D2 and tended to plateau on D3, although the trend was more clear in HIs than in CCSIs (Fig. 2). On visual inspection, three CCSIs (1, 2, 12) and two HIs (5, 17) were categorized as “poor learners” because their BiCo curves were almost flat or very hectic. The two HIs and two of these CCSIs (1, 12) were also “poor learners” according to BiSAT; thus, they were globally and consistently poor at learning bimanual coordination and performing the task efficiently (Supplementary Fig. 8, Supplementary Tables 2 and 3). Interestingly, despite being a “poor learner” according to BiCo, CCSI 2 achieved a large improvement in BiSAT, whereas CCSI 14 showed the opposite pattern (almost no improvement in BiSAT but large improvement in BiCo).
Generalization with NEWCIRCUIT
After training on D3, the introduction of the NEWCIRCUIT induced a significant decrease in BiSAT (Circuit Change: HIs − 7.3 ± 5.5, [95% CI] = [− 10 to − 4.6] and CCSIs, − 8.5 ± 8.8, [95% CI]=[− 13 to − 3.6]; Table 2; blocks 61 to 63 in Fig. 2), with no between-group difference (difference [95% CI]= − 1.2 [− 6.6 to 4.3], P = 0.66; Table 3). In contrast for BiCo, the Circuit Change induced a significant decrease in HIs (− 0.02 ± 0.02, [95% CI] = [− 0.03 to − 0.01]), but not in CCSIs (− 0.02 ± 0.04, [95% CI] = [− 0.04 to 0.00]). However, there was no significant between-group difference (difference [95% CI] = 0.00 [− 0.02 to 0.02], P = 0.88). Despite these decreases, the improvement in BiSAT and BiCo on NC1 largely remained compared to BASELINE performances (GENERALIZATION—BiSAT: in HIs 14.1 ± 5.7, [95% CI] = [11 to 17] and in CCSIs 11.5 ± 8.1, [95% CI] = [7 to 16]; GENERALIZATION—BiCo: in HIs 0.18 ± 0.06, [95% CI] = [0.15 to 0.21] and in CCSIs 0.18 ± 0.07, [95% CI] = [0.14 to 0.21]), demonstrating a large generalization. There was no significant between-group difference (BiSAT difference [95% CI] = − 2.6 [− 7.7 to 2.6], P = 0.31; BiCo difference [95% CI] = 0 [ − 0.05 to 0.04], P = 0.87). Furthermore, BiSAT significantly improved over the three NEWCIRCUIT blocks in both HIs and CCSIs (NC TOTAL in HIs 2.4 ± 4.2, [95% CI] = [0.29 to 4.5] and in CCSIs 2.6 ± 3.8, [95% CI] = [0.51 to 4.7]), without significant between-group differences ([95% CI] = 0.23 [− 2.6 to 3.1], P = 0.87). In contrast, BiCo did not significantly improve (NC TOTAL in HIs 0.01 ± 0.01, [95% CI] = [0.00 to 0.01] and in CCSIs 0.00 ± 0.02, [95% CI] = [− 0.01 to 0.01]), again without significant between-group differences ([95% CI] = 0 [− 0.02 to 0.01], P = 0.50).
Transfer on the REACHING task
The bimanual coordination improvements observed in the CIRCUIT were transferred to the REACHING task in both HIs and CCSIs (DELTA BiSAT: in HIs 7.2 ± 2.7, [95% CI] = [5.9 to 8.5] and in CCSIs 6.3 ± 3.9, [95% CI] [4.2 to 8.5]; DELTA BiCo: in HIs 0.09 ± 0.05, [95% CI] = [0.07 to 0.11] and in CCSIs 0.1 ± 0.05, [95% CI] = [0.07 to 0.12]; Tables 2). There was no significant between-group BASELINE difference for BiSAT or BiCo (BiSAT 6.7 ± 1.6 in HIs vs. 7 ± 2.4 in CCSIs, difference [95% CI] = 0.27 [- 1.2 to 1.7], P = 0.71; BiCo 0.29 ± 0.04 in HIs vs. 0.27 ± 0.06 in CCSIs, difference [95% CI] = − 0.02 [− 0.06 to 0.02], P = 0.23; Table 3) or D3-D1 DELTA (BiSAT difference [95% CI] = − 0.86 [− 3.3 to 1.6], P = 0.47; BiCo, difference [95% CI] = 0.01 [− 0.03 to 0.04], P = 0.77).
Correlations between clinical scale scores and age in CCSIs
The correlation coefficients computed between the robotic and clinical outcomes are displayed in Fig. 3; only the correlations considered to be strong (|r| ≥ 0.7, (Schober et al. 2018)) are highlighted here. For BiSAT TOTAL, a positive correlation was found with the MoCA score (r = 0.72, [95% CI] = [0.26 to 0.91], P = 0.003; Supplementary Table 4), BBT of the ipsilesional (r = 0.7, [95% CI] = [0.23 to 0.9], P = 0.005) and contralesional hands (r = 0.71, [95% CI] = [0.24 to 0.91], P = 0.003). A negative correlation was found between age and both BiSAT TOTAL (r = − 0.85, [95% CI] = [− 0.96 to − 0.54], P < 0.001) and BiSAT GENERALIZATION (r = − 0.71, [95% CI] = [− 0.91 to − 0.25], P = 0.003). REACHING BiSAT DELTA was correlated with BBT of the ipsilesional (r = 0.82, [95% CI] = [0.47 to 0.95], P < 0.001) and contralesional hands (r = 0.73, [95% CI] [0.29 to 0.92], P = 0.002). Neither BiCo (TOTAL or GENERALIZATION) nor REACHING BiSAT DELTA were strongly correlated with the other outcomes. In addition, a correlation analysis between BiSAT and BiCo progressions were computed and no strong correlation were highlighted (rs = 0.07 [− 0.28; 0.41], P = 0.41; Supplementary Fig. 8).
Correlation analysis between robotic outcomes and baseline characteristics. A darker blue colour indicates a greater positive correlation. A darker red colour indicates a greater negative correlation. Pale colours correspond to weaker correlations or no correlation. Only Spearman’s correlation coefficients with |r|≥ 0.7 were considered strong. Acronyms are detailed in Table 1. RMSE is related to Finger Force Tracking task. Coact or PError are related to the Multi Finger Force Tapping task
Exploratory analysis
A multivariable linear regression model was used to evaluate the progression of BiSAT and BiCo TOTAL by three variables: group (HI or CCSI), individual age (years) and randomization group (which hand controlled the X or Y displacement of the common bimanual cursor; see Supplementary Fig. 9 and Table 5).
The adjusted difference in the BiSAT TOTAL progression between groups was 2.2 a.u. ([95% CI] = [− 3.8 to 8.3], P = 0.45). There was a significant effect of age (slope [95% CI] = − 0.96 a.u./year [− 1.3 to − 0.64], P < 0.001). In other words, for each additional year older than 50 years, a participant lost 0.96 a.u. of BiSAT TOTAL progression. There was no significant effect of randomization (slope [95% CI] = 2.9 [− 3.2 to 8.9], P = 0.34) on BiSAT TOTAL progression.
In contrast, the model analysis with BiCo TOTAL progression showed no significant effect of age (slope [95% CI] = − 0.001 [− 0.004 to 0.001], P = 0.33), randomization (slope [95% CI] = 0.03 [− 0.02 to 0.08], P = 0.26) or group (slope [95% CI] = 0.01 [− 0.05 to 0.06], P = 0.79).
Discussion
Using the bimanual version of the REAplan® robot, 16 CCSIs and 18 HIs trained over three consecutive days on a new skill requiring the development of asymmetrical bimanual coordination. Both groups improved during the training sessions, retained improved bimanual coordination and skill performance from day to day, and showed evidence of generalization and transfer of these improvements at the end of the third day. There was no significant between-group difference, suggesting that efficient compensatory plasticity occurs in the chronic phase after a cerebellar stroke. Strikingly, 4 CCSIs and 2 HIs showed little or virtually no bimanual improvement, and the only factor correlated with such poor learning was age.
Bimanual motor skill learning
Over three days of training on the bimanual CIRCUIT task, HIs achieved bimanual motor skill learning, as quantified through BiSAT and BiCo. Confirming our first hypothesis, CCSIs also achieved bimanual motor skill learning. Previous studies have shown that whereas various aspects of unimanual motor sequence performance may be impaired in CCSIs, overall, they could achieve motor sequence learning, consolidation and/or retention with keypress SRTT or sequential visuomotor tracking tasks (Boyd and Winstein 2004; Gómez-Beldarrain et al. 1998; Hermsdorf et al. 2020; Molinari et al. 1997). Interestingly, impaired unimanual motor performance was observed both with the ipsilesional and contralesional hands without learning prevention. Similarly, when learning a new bimanual keypress SRTT, CCSIs improved during training despite slower or poorer motor performances than HIs (Dirnberger et al. 2010, 2013). The new information provided by our study is that retention from day to day and accrual of bimanual motor skill learning were demonstrated on a continuous task requiring the development of a new asymmetrical bimanual coordination in CCSIs, as well as generalization and transfer of improved bimanual performance.
The lack of significant between-group differences in BiSAT and BiCo did not confirm our second hypothesis that CCSIs would achieve poorer bimanual motor skill learning than HIs. This lack of difference between HIs and CCSIs could be due to functional reorganization supported by the structural cerebellar reserve through neuroplasticity occurring in non-injured cerebellar areas, possibly in lobules IV and VI for motor control compensation (Sadeghihassanabadi et al. 2022). Functional compensation in CCSIs could also be provided by extracerebellar areas (Mitoma et al. 2021; Sadeghihassanabadi et al. 2022). For example, in a small sample of individuals with focal cerebellar stroke or surgical lesions, functional connectivity between remote cortical areas (including interhemispheric connections) was increased, suggesting widespread extracerebellar reorganization (De Vico Fallani et al. 2017). Alternatively, the lack of between-group bimanual motor skill learning differences could be explained by the lack of severe upper limb ataxia in our CCSI cohort (median MICARS score 10/120). Thus, potentially large initial stroke-induced impairments (ataxia and/or bimanual incoordination) may have been compensated for by the time of the experiment (mean time since stroke onset: 6.7 years ± 5.08 (SD)).
A plateau trend was observed at D3 for BiCo in both groups, suggesting that the coordination between each hand’s velocity tended towards a stable level, without reaching the theoretical maximal BiCo of 1. A similar observation was made in HIs and chronic patients who underwent a single supratentorial stroke training on the same bimanual CIRCUIT with the REAplan®, suggesting that perhaps longer training should be provided to further enhance bimanual coordination (Gerardin et al. 2022). Interestingly, whereas the BiCo group curves showed a clear trend towards plateauing on D3, the BiSAT group curves of both HIs and CCSIs continued to improve through D3 with a slope similar to that observed over D1 and D2. Thus, whereas the coordination of hand speeds—quantified by the BiCo reflecting the bimanual control policy—seemed to approach a ceiling on D3, there was still room for improvement in the execution of the sequential aspects of the task, as reflected by BiSAT. Learning the control policy refers to establishing the new bimanual coordination pattern needed to move the common cursor through synchronized movements in the context of the interactions between the hands and the REAplan® robot (i.e., learning the task rules). Learning the sequential aspect of the task refers to acquiring and improving the -cooperative- bimanual sequence needed to move the common cursor as fast and accurately as possible, as quantified by BiSAT. As previously suggested, learning the new bimanual coordination control policy could predominate early, whereas circuit-specific skill improvements (i.e., speed and/or accuracy of the bimanual motor sequence) occur later (Buchanan and Wang 2012; Ronsse et al. 2011; Yeganeh Doost et al. 2017).
Strikingly, the BiSAT curves remained virtually flat over the three training days in three CCSIs and two HIs, suggesting that they were “poor learners”. The exploratory analysis demonstrated that increasing age negatively impacted the BiSAT TOTAL progression (i.e., bimanual motor skill learning) but not the BiCo TOTAL progression (i.e., bimanual coordination improvement). In HIs, age-related cerebellar atrophy and changes in cerebellar inhibition of the primary motor cortex (M1) might be linked to age-related loss of Purkinje cells and/or decline of cerebellar white matter integrity (Andersen et al. 2003; Bernard and Seidler 2013; Mooney et al. 2022; Rurak et al. 2022; Tang et al. 2001; Vandevoorde and Orban de Xivry 2019). Therefore, age-related cerebellar atrophy and/or cerebellar-M1 interactions could have reduced the ability to achieve bimanual motor skill learning in our older HIs and CCSIs. Interestingly, age did not negatively correlate with bimanual coordination enhancement through training, suggesting a negative effect of age specifically on the capacity to enhance BiSAT in the CIRCUIT task. Furthermore, BiSAT and BiCo progression were uncoupled in the opposite way in CCSI 2 and 14. This uncoupling is consistent with the hypothesis that bimanual motor skill learning and bimanual coordination enhancement could rely on partly distinct processes or strategies (Yeganeh Doost et al. 2017).
Correlations between age and clinical scale scores in CCSIs
Our third hypothesis was that poorer bimanual motor skill learning would correlate with larger deficits in baseline clinical scale scores in CCSIs. The correlation analyses confirmed that increasing age negatively impacted the ability to achieve bimanual motor skill learning and to generalize the bimanual skill in CCSIs; these findings were consistent with the exploratory analyses. Conversely, preserved cognitive function indicated by a higher MoCA score correlated with greater overall bimanual motor skill learning. This finding is in line with the negative influence of poststroke cognitive impairment on motor control recovery (Everard et al. 2020; McDonald et al. 2019; Mullick et al. 2015; Rinne et al. 2018). Notably, there was no strong correlation with Corsi scores, suggesting that short-term visuospatial working memory was not critical for improving performance on the bimanual CIRCUIT task in CCSIs. There was also a strong correlation between the BBT score of both the ipsilesional and contralesional upper limb and (i) bimanual motor skill learning and (ii) REACHING progression. More surprisingly, there was a lack of strong correlations between the MICARS score and BiSAT TOTAL or BiCo TOTAL progression (r = - 0.5 and - 0.29, respectively), whereas the BiSAT REACHING DELTA showed a moderate negative correlation with the MICARS score (r = - 0.67). In CCSIs, mild to moderate ataxia did not impair the capacity to learn a new skill requiring asymmetrical bimanual coordination. It is possible that other correlations could emerge with the inclusion of severely ataxic CCSIs.
Another surprising finding was that, at baseline, the CCSIs had less finger coactivation and more press errors than the HIs on one of the fine dexterity tasks assessed with the Dextrain® manipulandum. Mild chronic cerebellar strokes outside a critical zone might explain the lack of dexterity impairment in our CCSI sample, possibly due to functional compensation by structural cerebellar reserve or extracerebellar mechanisms.
Limitations
Utilizing a sample of 16 CCSIs and 18 HIs, the power for a medium, large and very large effect sizes is 29%, 62% and 92% (characterized by Cohen’s d values of 0.5, 0.8, and 1.2 respectively) showing a poor power for the current study. Indeed, the CCSI sample size was small but comparable to that of motor learning studies in CCSIs (Dirnberger et al. 2013; Hermsdorf et al. 2020), consistent with the small proportion of cerebellar strokes. Recruitment was also limited by the COVID-19 pandemic. However, we suggest that the small number of subjects might have been partly compensated for by the fine-grained analysis of bimanual motor skill learning, computed with the continuous robotic outcomes specifically designed for this purpose. Nevertheless, recruiting more CCSIs, especially patients with more severe ataxia, would allow us to broaden the current observations. The REAplan® robot allowed the HIs and CCSIs to train on this asymmetric bimanual coordination task and to achieve bimanual motor skill learning and bimanual coordination in a closed kinetic chain i.e., the upper limbs were supported by forearm rest gutters in the horizontal plane which allowed easier movement control. Perhaps ataxia would have caused more severe impairment in a bimanual task performed in an open kinetic chain – i.e. without support of the upper limbs. Studying the acute phase of stroke would also be interesting for clarifying the compensatory mechanisms involved—larger bimanual motor skill learning impairments could theoretically be observed in patients with acute cerebellar stroke than in those with chronic stroke. Identifying “poor learners” in studies with larger samples using clinical or imaging biomarkers could lead to tailored neurorehabilitation approaches with specific interventions designed to help “poor learners”.
Conclusion
By training with a serious game on the bimanual REAplan® robot, both HIs and CCSIs were able to perform, improve, retain, generalize and transfer complex motor skills requiring learning a new bimanual coordination control policy in a closed kinetic chain. The lack of between-group differences in all the robotic outcomes suggested plastic compensation at the chronic stage after a cerebellar stroke, possibly through functional and/or structural cerebellar reserve. Interestingly, “poor learners” were observed in both CCSIs and HIs in whom ageing reduced the capacity to achieve bimanual motor skill learning. These findings suggest that bimanual motor skill learning could be used to enhance bimanual motor control in chronic cerebellar stroke patients with mild to moderate ataxia.
Data availability
The datasets are available through request to the corresponding author.
References
Andersen BB, Gundersen HJG, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466(3):356–365. https://doi.org/10.1002/cne.10884
Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Jacob Filho W, Lent R, Herculano-Houzel S (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513(5):532–541. https://doi.org/10.1002/cne.21974
Baldassarre A, Filardi MS, Spadone S, Penna SD, Committeri G (2021) Distinct connectivity profiles predict different in-time processes of motor skill learning. Neuroimage 238:118239. https://doi.org/10.1016/j.neuroimage.2021.118239
Barboi AC (2000) Cerebellar ataxia. Arch Neurol 57(10):1525–1527. https://doi.org/10.1001/archneur.57.10.1525
Bernard JA, Seidler RD (2013) Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum (london, England) 12(5):721–737. https://doi.org/10.1007/s12311-013-0481-z
Boyd LA, Winstein CJ (2004) Cerebellar stroke impairs temporal but not spatial. Neurorehabil Neural Repair 18(3):134–143. https://doi.org/10.1177/0888439004269072
Buchanan JJ, Wang C (2012) Overcoming the guidance effect in motor skill learning: feedback all the time can be beneficial. Exp Brain Res 219(2):305–320. https://doi.org/10.1007/s00221-012-3092-x
Corsi PM (1973) Human memory and the medial temporal region of the brain, vol 34. ProQuest Information & Learning, Ann Arbor, p 891
Dahms C, Brodoehl S, Witte OW, Klingner CM (2020) The importance of different learning stages for motor sequence learning after stroke. Hum Brain Map 41(1):270–286. https://doi.org/10.1002/hbm.24793
De Laet C, Herman B, Riga A, Bihin B, Regnier M, Leeuwerck M, Raymackers J-M, Vandermeeren Y (2022) Bimanual motor skill learning after stroke: combining robotics and anodal tDCS over the undamaged hemisphere: an exploratory study. Front Neurol 13:882225. https://doi.org/10.3389/fneur.2022.882225
De Vico Fallani F, Clausi S, Leggio M, Chavez M, Valencia M, Maglione AG, Babiloni F, Cincotti F, Mattia D, Molinari M (2017) Interhemispheric connectivity characterizes cortical reorganization in motor-related networks after cerebellar lesions. Cerebellum (london, England) 16(2):358–375. https://doi.org/10.1007/s12311-016-0811-z
Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004) Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21(4):1416–1427. https://doi.org/10.1016/j.neuroimage.2003.12.011
Dirnberger G, Novak J, Nasel C, Zehnter M (2010) Separating coordinative and executive dysfunction in cerebellar patients during motor skill acquisition. Neuropsychologia 48(5):1200–1208. https://doi.org/10.1016/j.neuropsychologia.2009.12.016
Dirnberger G, Novak J, Nasel C (2013) Perceptual sequence learning is more severely impaired than motor sequence learning in patients with chronic cerebellar stroke. J Cogn Neurosci 25(12):2207–2215. https://doi.org/10.1162/jocn_a_00444
Doost MY, Orban de Xivry J-J, Herman B, Vanthournhout L, Riga A, Bihin B, Jamart J, Laloux P, Raymackers J-M, Vandermeeren Y (2019) Learning a bimanual cooperative skill in chronic stroke under noninvasive brain stimulation: a randomized controlled trial. Neurorehabil Neural Repair 33(6):486–498. https://doi.org/10.1177/1545968319847963
Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15(2):161–167. https://doi.org/10.1016/j.conb.2005.03.004
Everard GJ, Ajana K, Dehem SB, Stoquart GG, Edwards MG, Lejeune TM (2020) Is cognition considered in post-stroke upper limb robot-assisted therapy trials ? A brief systematic review. Int J Rehabil Res Internationale Zeitschrift Fur Rehabilitationsforschung. Revue Internationale De Recherches De Readaptation 43(3):195–198. https://doi.org/10.1097/MRR.0000000000000420
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S (1975) The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7(1):13–31
Gerardin E, Bontemps D, Babuin N-T, Herman B, Denis A, Bihin B, Regnier M, Leeuwerck M, Deltombe T, Riga A, Vandermeeren Y (2022) Bimanual motor skill learning with robotics in chronic stroke: comparison between minimally impaired and moderately impaired patients, and healthy individuals. J Neuroeng Rehabil 19(1):28. https://doi.org/10.1186/s12984-022-01009-3
Gómez-Beldarrain M, García-Moncó JC, Rubio B, Pascual-Leone A (1998) Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Exp Brain Res 120(1):25–30. https://doi.org/10.1007/s002210050374
Hardwick RM, Dagioglou M, Miall RC (2013a) State estimation and the cerebellum. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N (eds) Handbook of the cerebellum and cerebellar disorders. Springer, Dordrecht, pp 1297–1313. https://doi.org/10.1007/978-94-007-1333-8_57
Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013b) A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67:283–297. https://doi.org/10.1016/j.neuroimage.2012.11.020
Herculano-Houzel S (2009) The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci 3:31. https://doi.org/10.3389/neuro.09.031.2009
Hermsdorf F, Fricke C, Stockert A, Classen J, Rumpf J-J (2020) Motor performance but neither motor learning nor motor consolidation are impaired in chronic cerebellar stroke patients. Cerebellum (london, England) 19(2):275–285. https://doi.org/10.1007/s12311-019-01097-3
Jung K-H, Roh J-K (2022) Cerebellar stroke. In: Manto MU, Gruol DL, Schmahmann JD, Koibuchi N, Sillitoe RV (eds) Handbook of the cerebellum and cerebellar disorders. Springer International Publishing, Cham, pp 2229–2255. https://doi.org/10.1007/978-3-030-23810-0_90
Kelly PJ, Stein J, Shafqat S, Eskey C, Doherty D, Chang Y, Kurina A, Furie KL (2001) Functional recovery after rehabilitation for cerebellar stroke. Stroke 32(2):530–534. https://doi.org/10.1161/01.str.32.2.530
Kessels RP, van Zandvoort MJ, Postma A, Kappelle LJ, de Haan EH (2000) The corsi block-tapping task: standardization and normative data. Appl Neuropsychol 7(4):252–258. https://doi.org/10.1207/S15324826AN0704_8
Kovacs AJ, Buchanan JJ, Shea CH (2009) Using scanning trials to assess intrinsic coordination dynamics. Neurosci Lett 455(3):162–167. https://doi.org/10.1016/j.neulet.2009.02.046
Krakauer JW, Carmichael ST (2017) Broken movement: the neurobiology of motor recovery after stroke. The MIT Press, Cambridge
Krakauer JW, Mazzoni P (2011) Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21(4):636–644. https://doi.org/10.1016/j.conb.2011.06.012
Krakauer JW, Hadjiosif AM, Xu J, Wong AL, Haith AM (2019) Motor learning. Compr Physiol 9(2):613–663. https://doi.org/10.1002/cphy.c170043
Langhorne P, Bernhardt J, Kwakkel G (2011) Stroke rehabilitation. Lancet (london, England) 377(9778):1693–1702. https://doi.org/10.1016/S0140-6736(11)60325-5
Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y (2012) Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front Hum Neurosci 6:343. https://doi.org/10.3389/fnhum.2012.00343
Manto M (2022) The underpinnings of cerebellar ataxias. Clin Neurophysiol Pract 7:372–387. https://doi.org/10.1016/j.cnp.2022.11.002
Mathiowetz V, Volland G, Kashman N, Weber K (1985) Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 39(6):386–391. https://doi.org/10.5014/ajot.39.6.386
McDonald MW, Black SE, Copland DA, Corbett D, Dijkhuizen RM, Farr TD, Jeffers MS, Kalaria RN, Karayanidis F, Leff AP, Nithianantharajah J, Pendlebury S, Quinn TJ, Clarkson AN, O’Sullivan MJ (2019) Cognition in stroke rehabilitation and recovery research: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Int J Stroke 14(8):774–782. https://doi.org/10.1177/1747493019873600
Mitoma H, Manto M (2016) The physiological basis of therapies for cerebellar ataxias. Ther Adv Neurol Disord 9(5):396–413. https://doi.org/10.1177/1756285616648940
Mitoma H, Kakei S, Yamaguchi K, Manto M (2021) Physiology of cerebellar reserve : redundancy and plasticity of a modular machine. Int J Mol Sci 22(9):4777. https://doi.org/10.3390/ijms22094777
Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L (1997) Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain J Neurol 120(Pt 10):1753–1762. https://doi.org/10.1093/brain/120.10.1753
Mooney RA, Ni Z, Shirota Y, Chen R, Ugawa Y, Celnik PA (2022) Age-related strengthening of cerebello-cortical motor circuits. Neurobiol Aging 118:9–12. https://doi.org/10.1016/j.neurobiolaging.2022.04.016
Mullick AA, Subramanian SK, Levin MF (2015) Emerging evidence of the association between cognitive deficits and arm motor recovery after stroke: a meta-analysis. Restor Neurol Neurosci 33(3):389–403. https://doi.org/10.3233/RNN-150510
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Nezafat R, Shadmehr R, Holcomb HH (2001) Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res 140(1):66–76. https://doi.org/10.1007/s002210100787
Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL (2001) The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke 32(7):1627–1634. https://doi.org/10.1161/01.str.32.7.1627
Rémy F, Wenderoth N, Lipkens K, Swinnen SP (2008) Acquisition of a new bimanual coordination pattern modulates the cerebral activations elicited by an intrinsic pattern: an fMRI study. Cortex J Devoted Study Nerv Syst Behav 44(5):482–493. https://doi.org/10.1016/j.cortex.2007.07.004
Riga A, Gathy E, Ghinet M, De Laet C, Bihin B, Regnier M, Leeuwerck M, De Coene B, Dricot L, Herman B, Edwards MG, Vandermeeren Y (2022) Evidence of motor skill learning in acute stroke patients without lesions to the thalamus and internal capsule. Stroke 53(7):2361–2368. https://doi.org/10.1161/STROKEAHA.121.035494
Rinne P, Hassan M, Fernandes C, Han E, Hennessy E, Waldman A, Sharma P, Soto D, Leech R, Malhotra PA, Bentley P (2018) Motor dexterity and strength depend upon integrity of the attention-control system. Proc Natl Acad Sci USA 115(3):E536–E545. https://doi.org/10.1073/pnas.1715617115
Ronsse R, Puttemans V, Coxon JP, Goble DJ, Wagemans J, Wenderoth N, Swinnen SP (2011) Motor learning with augmented feedback: modality-dependent behavioral and neural consequences. Cereb Cortex 21(6):1283–1294. https://doi.org/10.1093/cercor/bhq209
Rurak BK, Rodrigues JP, Power BD, Drummond PD, Vallence AM (2022) Reduced cerebellar brain inhibition measured using dual-site TMS in older than in younger adults. Cerebellum (london, England) 21(1):23–38. https://doi.org/10.1007/s12311-021-01267-2
Sadeghihassanabadi F, Frey BM, Backhaus W, Choe C-U, Zittel S, Schön G, Bönstrup M, Cheng B, Thomalla G, Gerloff C, Schulz R (2022) Structural cerebellar reserve positively influences outcome after severe stroke. Brain Commun 4(6):fcaa203. https://doi.org/10.1093/braincomms/fcac203
Schmahmann JD, Gardner R, MacMore J, Vangel MG (2009a) Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord 24(12):1820–1828. https://doi.org/10.1002/mds.22681
Schmahmann JD, Macmore J, Vangel M (2009b) Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience 162(3):852–861. https://doi.org/10.1016/j.neuroscience.2009.06.023
Schneider A, Hommel G, Blettner M (2010) Linear regression analysis: part 14 of a series on evaluation of scientific publications. Deutsches Arzteblatt Int 107(44):776–782. https://doi.org/10.3238/arztebl.2010.0776
Schober P, Boer C, Schwarte LA (2018) Correlation coefficients: appropriate use and interpretation. Anesth Analg 126(5):1763–1768. https://doi.org/10.1213/ANE.0000000000002864
Serrien DJ, Wiesendanger M (2000) Temporal control of a bimanual task in patients with cerebellar dysfunction. Neuropsychologia 38(5):558–565. https://doi.org/10.1016/s0028-3932(99)00116-5
Shadmehr R, Smith MA, Krakauer JW (2010) Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33:89–108. https://doi.org/10.1146/annurev-neuro-060909-153135
Tang Y, Whitman GT, Lopez I, Baloh RW (2001) Brain volume changes on longitudinal magnetic resonance imaging in normal older people. J Neuroimaging 11(4):393–400. https://doi.org/10.1111/j.1552-6569.2001.tb00068.x
Térémetz M, Colle F, Hamdoun S, Maier MA, Lindberg PG (2015) A novel method for the quantification of key components of manual dexterity after stroke. J Neuroeng Rehabil 12:64. https://doi.org/10.1186/s12984-015-0054-0
Tohgi H, Takahashi S, Chiba K, Hirata Y (1993) Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke 24(11):1697–1701. https://doi.org/10.1161/01.str.24.11.1697
Tracy JI, Faro SS, Mohammed F, Pinus A, Christensen H, Burkland D (2001) A comparison of “Early” and “Late” stage brain activation during brief practice of a simple motor task. Brain Res Cogn Brain Res 10(3):303–316. https://doi.org/10.1016/s0926-6410(00)00045-8
Tseng Y-W, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98(1):54–62. https://doi.org/10.1152/jn.00266.2007
van Dun K, Brinkmann P, Depestele S, Verstraelen S, Meesen R (2022) Cerebellar activation during simple and complex bimanual coordination: an activation likelihood estimation (ALE) meta-analysis. Cerebellum (london, England) 21(6):987–1013. https://doi.org/10.1007/s12311-021-01261-8
Vandevoorde K, Orban de Xivry J-J (2019) Internal model recalibration does not deteriorate with age while motor adaptation does. Neurobiol Aging 80:138–153. https://doi.org/10.1016/j.neurobiolaging.2019.03.020
Vega-Gonzalez A, Bain BJ, Dall PM, Granat MH (2007) Continuous monitoring of upper-limb activity in a free-living environment: a validation study. Med Biol Eng Compu 45(10):947–956. https://doi.org/10.1007/s11517-007-0233-7
Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y (2020) Burden of stroke in Europe : thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke 51(8):2418–2427. https://doi.org/10.1161/STROKEAHA.120.029606
Welniarz Q, Worbe Y, Gallea C (2021) The forward model: a unifying theory for the role of the cerebellum in motor control and sense of agency. Front Syst Neurosci 15:644059. https://doi.org/10.3389/fnsys.2021.644059
World Stroke Organisation (WSO) (2022) Global Stroke Fact Sheet 2022
Ye BS, Kim YD, Nam HS, Lee HS, Nam CM, Heo JH (2010) Clinical manifestations of cerebellar infarction according to specific lobular involvement. Cerebellum (london, England) 9(4):571–579. https://doi.org/10.1007/s12311-010-0200-y
Yeganeh Doost M, Orban de Xivry J-J, Bihin B, Vandermeeren Y (2017) Two processes in early bimanual motor skill learning. Front Hum Neurosci 11:618. https://doi.org/10.3389/fnhum.2017.00618
Acknowledgements
We warmly thank the persons with a stroke and the control subjects who kindly accepted to participate in this experiment. We are grateful to Adrien Denis for the creation of the serious game’s software on the REAplan®, the neuropsychology team of the CHU UCL Namur (site Godinne) for their help about cognitive assessments and interpretation, Dr André Peeters from the Cliniques universitaires Saint-Luc (UCLouvain, Brussels) and the physical therapy team of the CHU UCL Namur (site Godinne) who helped with the recruitment of participants.
Funding
The work of Estelle Gathy was supported by the following grants: Fédération Wallonie-Bruxelles—Action de Recherche Concertée (ARC, 20/25-105) 2020–2025 UCLouvain. The work of Eloïse Gerardin was supported by the following grants: PDR-FNRS grant T.0239.19 and Fondation Mont-Godinne grants 2020 and 2021. The work of Yves Vandermeeren was supported by the following grants: Fonds de la Recherche Scientifique—FNRS 1.R.506.16, 1.R.506.18, 1.R.506.20F and 1S00722F, PDR-FNRS grant T.0239.19, Fonds Spécial de Recherche (FSR) grant from the UCLouvain (2018), and Fondation Mont-Godinne grants (2019–2023).
Author information
Authors and Affiliations
Contributions
Study design: YV, EGa and EGe. Recruitment of patients: EGa, NC, EGe, YV and ML. Randomization: EGa and NC. Data collection: EGa and NC. Creation of Matlab routine: BH, JL and EGe. Data analysis: BB. Interpretation of the results and statistics: BB, YV and EGa. Writing manuscript: EGa and YV. All authors critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Non-financial interests: YV is advisory member of the Scientific Committee of the Dextrain company (Igny, France). The other authors have no financial interest or competing interest in the subject matter or materials discussed in this article.
Ethical approval
The study was approved by the local ethics committee (B039201421432) and was in accordance with the Declaration of Helsinki.
Informed consent
All participants gave their written informed consent.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gathy, E., Cadiat, N., Gerardin, E. et al. Bimanual coordinated motor skill learning in patients with a chronic cerebellar stroke. Exp Brain Res 242, 1517–1531 (2024). https://doi.org/10.1007/s00221-024-06830-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-024-06830-x