Abstract
Vestibular contributions to linear motion (i.e., translation) perception mediated by the otoliths have yet to be fully characterized. To quantify the maximal extent that non-vestibular cues can contribute to translation perception, we assessed vestibular perceptual thresholds in two patients with complete bilateral vestibular ablation to compare to our data in 12 young (< 40 years), healthy controls. Vestibular thresholds were assessed for naso-occipital (“x-translation”), inter-aural (“y-translation”), and superior-inferior (“z-translation”) translations in three body orientations (upright, supine, side-lying). Overall, in our patients with bilateral complete vestibular loss, thresholds were elevated ~ 2–45 times relative to healthy controls. No systematic differences in vestibular perceptual thresholds were noted between motions that differed only with respect to their orientation relative to the head (i.e., otoliths) in patients with bilateral vestibular loss. In addition, bilateral loss patients tended to show a larger impairment in the perception of earth-vertical translations (i.e., motion parallel to gravity) relative to earth-horizontal translations, which suggests increased contribution of the vestibular system for earth-vertical motions. However, differences were also noted between the two patients. Finally, with the exception of side-lying x-translations, no consistent effects of body orientation in our bilateral loss patients were seen independent from those resulting from changes in the plane of translation relative to gravity. Overall, our data confirm predominant vestibular contributions to whole-body direction-recognition translation tasks and provide fundamental insights into vestibular contributions to translation motion perception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vestibular perceptual thresholds, quantified using whole-body direction-recognition tasks, represent the smallest magnitude of passive self-motion which can be reliably perceived (Merfeld 2011). Vestibular perceptual thresholds are sensitive to peripheral (Agrawal et al. 2013; Bremova et al. 2016; Priesol et al. 2014; Van Stiphout et al. 2021) and central vestibular disorders (King et al. 2019; Lewis et al. 2011a, b), thus, have been proposed to have potential future clinical applications. However, widespread implementation of vestibular thresholds is contingent upon the ability for thresholds to specifically target vestibular function (Kobel et al. 2021b). Previous research efforts have focused on quantifying the maximal extent that non-vestibular afferent cues (e.g., tactile, somatosensory, somatograviception) can contribute to vestibular thresholds through assessing these passive self-motion direction-recognition tasks in patients with complete bilateral surgical ablation of vestibular function. These earlier efforts identified predominant vestibular contributions to self-motion perceptual thresholds, as thresholds were elevated by ~ 1.5–85 times in the complete absence of peripheral vestibular input (Kobel et al. 2023; Valko et al. 2012). These measures are therefore referred to as “vestibular thresholds” due to the predominant vestibular sensory contributions, despite potential non-vestibular sensory contributions. However, past studies have focused on quantifying the impact of stimulus frequency (i.e., duration of motion stimuli (Valko et al. 2012)) or comprehensively assessing rotation and tilt motion perception (Kobel et al. 2023) and potential factors influencing perception of linear motion remain incompletely characterized.
For linear motions sensed by the otoliths (i.e., the utricle and saccule), perceptual sensitivity has been reported to be influenced by multiple experimental characteristics including (1) motion relative to the head/otoliths, (2) motion relative to gravity (i.e., earth-vertical vs. earth-horizontal), and (3) body orientation (e.g., upright, supine, side-lying) (Agrawal et al. 2013; Benson et al. 1986; Kobel et al. 2021a; MacNeilage et al. 2010). Yet, the only prior quantification of vestibular contributions to translation perception in the absence of vestibular function focused only on the assessment of the impact of stimulus frequency for inter-aural y-axis translations (“y-translations”) and superior–inferior z-axis translations (“z-translations”) while in an upright body orientation (Valko et al. 2012). Thus, insight into the maximal extent to which non-vestibular influences may play a role in these factors proposed to influence translation perception are limited.
Our past study (Kobel et al. 2021a) in young (< 40 years of age), healthy adults quantified the impact of these three factors proposed to influence perceptual sensitivity of translations. We assessed y-translations, naso-occipital x-axis translations (“x-translations”), and z-translations in three body orientations (upright, supine, and side-lying). Overall, when assessing the impact of motion relative to the head/otoliths, we identified that thresholds for x-translations and y-translations, with predominant utricular contributions, were lower than z-translation thresholds, with predominant saccular contributions (Kobel et al. 2021a) in line with past results (Agrawal et al. 2013; Benson et al. 1986; Bremova et al. 2016; Kingma 2005). These results have been interpreted to suggest that perceptual assays may capture aspects of peripheral sensitivity (Agrawal et al. 2013; Karmali et al. 2017; Kobel et al. 2021a) as afferent recordings show enhanced sensitivity of the utricle relative to the saccule (Fernandez and Goldberg 1976). However, if differences in motion perception sensitivity on the basis of a motion relative to head (i.e., motions with predominant sensory contributions from the utricle or saccule) are impacted by peripheral afferent input have yet to be assessed. By measuring thresholds for multiple motions relative to the head/otoliths, this allows quantification of the maximal extent that non-vestibular (e.g., tactile, somatic graviception) cues can contribute to differences in perceptual sensitivity on the basis of changes in axis of motion.
In addition, in healthy young adults, we identified that thresholds for earth-vertical (i.e., parallel to gravity) motions were approximately 2× higher than thresholds for earth-horizontal (i.e., perpendicular to gravity) motions (Kobel et al. 2021a) in seeming contrast to past results (MacNeilage et al. 2010). This impact of motion relative to gravity when assessed while upright, supine, and side-lying was only seen for motions with predominant utricular contributions (i.e., y-translations, x-translations) and not those with predominant saccular contributions (i.e., z-translations) yielding two earth-horizontal and two earth-vertical motions for each axis of motion relative to the head (Fig. 1; Kobel et al. 2021a). MacNeilage et al., (2010) did not identify a main impact of gravity for an experimental paradigm which only included y-translation and z-translation stimuli while upright and side-lying, yielding one earth-vertical and one earth-horizontal motion per axis of translation. As such, inclusion of multiple earth-horizontal conditions allowed identification of a main effect of motion relative to gravity. In addition, a previous study of thresholds in patients with complete bilateral vestibular loss revealed earth-vertical z-translation thresholds were ~ 8–50 times elevated relative to healthy control data while earth-horizontal y-translations thresholds were only ~ 1.5–4 times higher (Valko et al. 2012). Together, these studies suggest that the previously observed effect of motion relative to gravity on vestibular thresholds may be mediated by the otoliths given their role in the encoding of gravity and linear acceleration (i.e., translation) (Fernandez and Goldberg 1976). However, this prior study of patients with vestibular loss only included upright thresholds and two different axes of translation relative to the head. Thus, whether this impact of motion relative to gravity is truly vestibular in origin and if a differential effect of gravity is seen on the basis of end-organ stimulation has yet to be determined.
A Graphical depiction of our experimental paradigm. Axes (x-axis, y-axis, z-axis) refer to direction of translation in head coordinates, which is depicted by arrows. Conditions that are earth-vertical (i.e., motions are parallel to gravity) are highlighted by blue boxes. All other translations are earth-horizontal (i.e., translations are perpendicular to gravity). B Example threshold tests for a healthy control and Patient B for an upright z-translation motion for 2 Hz stimuli. Red dots represent the subject’s binary response with 0 indicating perceived downward and 1 perceived upward motion. The solid blue line corresponds to the estimated psychometric curve using a maximum likelihood estimate via a bias-reduced generalized linear model and a probit link function. The standard deviation (σ) of this cumulative Gaussian distribution corresponds to the reported threshold, which is the stimulus level which corresponds to 84.1% of responses correct. The mean of the fitted curve (µ) corresponds to the bias
Past research has suggested that assessing perceptual measures in non-upright body orientations (e.g., supine) leads to global decreases in perceptual sensitivity (Hummel et al. 2016; MacNeilage et al. 2010) due to measuring perception in a position where daily motion is not routinely experienced. Our previous results from healthy young asymptomatic participants, however, suggest that for linear motion, these body orientation effects at least were partially driven by orientation relative to gravity. We identified that z-translations across all body orientations (e.g., upright, supine, side-lying) were equivalent and earth-horizontal y-translation and x-translation thresholds were equivalent, regardless of body orientation (Kobel et al. 2021a). These results suggest that changes in body orientation from upright (e.g., supine, side-lying) do not necessarily lead to systematic increases in threshold (i.e., decreased perceptual sensitivity), and rather that previous identified changes may reflect changes in orientation to gravity and/or changes in availability of useful non-vestibular cues for motion perception (e.g., tactile). Assessment of perceptual thresholds in the absence of otolith function decouples the effects of orientation relative to gravity from the effect of motion relative to the otoliths. Thus, this allows insight into the impact of body orientation independent of changes modulated on the basis of motion relative to the head and motion relative to gravity.
Thus, to unravel the relative contributions of the non-vestibular factors impacting translation motion perception, this project compared a comprehensive set of vestibular perceptual thresholds between patients with a complete absence of vestibular function and a cohort of healthy controls. Translation thresholds were measured at multiple frequencies, and with the head and body positioned to allow motion trajectories that altered the orientation of the motion relative to the head and relative to gravity. As orientation relative to gravity is defined on the basis of both motion relative to the head and body orientation, these influences cannot be definitively disambiguated within the confines of gravity on earth. However, through assessing in the absence of peripheral vestibular function, we aimed to determine the maximal extent that sensory cues that were not peripheral vestibular afferent cues can influence factors that contribute to linear motion sensitivity.
Methods
Participants
Two participants with neuro-fibromatosis type II (NF-2) and complete bilateral surgical ablation of the labyrinths were enrolled to complete vestibular perceptual threshold assessments. In order to ensure complete bilateral vestibular ablation, we recruited patients who had both undergone bilateral trans-cochlear nerve sections with labyrinthectomies to remove vestibular schwannomas bilaterally. Our past research suggests that patients identified with severe bilateral vestibular hypofunction of the horizontal semicircular canals on the basis of traditional clinical testing (i.e., calorics, rotational testing) did not exhibit consistent loss of function across all end-organs. While these patient exhibited elevated yaw rotation thresholds, consistent with horizontal canal dysfunction, z-translation and roll tilt thresholds were equivalent to healthy control data, suggesting otolith and vertical canal peripheral function was at least partially intact (Priesol et al. 2014). As well, others have reported that in patients identified with bilateral loss, clinical measures of otolith function (i.e., vestibular evoked myogenic potentials) do not reveal consistent abnormalities which are associated to vestibular perceptual thresholds (Agrawal et al. 2013), suggesting that clinical testing is insufficient to confirm absence of vestibular function. By only enrolling patients who underwent bilateral trans-cochlear nerve sections with labyrinthectomies, we could ensure surgical confirmation of complete bilateral vestibular deafferentation to determine the maximal extent to which non-vestibular cues contributed to motion perception.
Both participants enrolled were included in an earlier study (Valko et al. 2012) that focused on determining the vestibular contributions to whole-body direction-recognition tasks when measured across a broad range of frequencies. However, we were unable to identify additional patients meeting this strict criteria (i.e., bilateral trans-cochlear nerve sections) who were willing to participate in our research efforts despite substantial efforts to enroll additional participants through local and nationwide searches over a span of several years. Patient A is a 34-year-old female who underwent right- and left-sided labyrinthectomies at age 9 and 18, respectively. She currently competes in marathons and triathlons. Patient B is a 37-year-old male who had a right-sided labyrinthectomy at age 5 and a left-sided labyrinthectomy at age 20. He reports difficulty perceiving motion when flying. However, he is a frequent traveler, and denies difficulty with his balance on a daily basis and is able to independently ride a bicycle. Both Patient A and Patient B have profound hearing loss when unaided. Both are current auditory brainstem implant (ABI) users and retain open set verbal communication with the use of the ABI. For the earlier initial study, both patients underwent neurological exams and no other substantial sensory abnormalities outside of hearing and vestibular function were reported. As well, assessment of pressure sensation on the trunk and buttocks using Semmes–Weinstein monofilaments was completed at the time of the initial evaluation. Despite the presence of spinal tumors in both patients, pressure thresholds were comparable between patients and normal control subjects (Valko et al. 2012).
Translation thresholds were also measured in 12 young healthy adults (6F/6M; 26.57 ± 4.01 years); these data were previously published (Kobel et al. 2021a). All control participants denied a history of vestibular disorders, neurological disorders, major health conditions (e.g., cancer), or recent orthopedic injury.
Motion stimuli and psychophysical threshold tests
In head coordinates, translation perception was assessed using naso-occipital x-axis translations (“x-translations”), inter-aural y-axis translations (“y-translations”), and superior-inferior z-axis translations (“z-translations”). Each translation was provided while the padded chair affixed to the motion device was positioned upright, supine, and on-side (Figs. 1 and 2) using both 1 Hz and 2 Hz stimuli (i.e., motions lasting 1 s and 0.5 s, respectively). Past evidence suggests that lower frequency stimuli maximize vestibular contributions (Valko et al. 2012). However, 1 and 2 Hz stimuli were included to assess potential frequency effects and to ensure that the bilateral loss patients could complete testing at the displacement limits of our equipment for at least one frequency for each motion condition. Order of testing was randomized to the extent possible across body orientations for x-translations, y-translations, and z-translations. Thresholds for 1 Hz were always collected prior to 2 Hz motion stimuli.
Motion paradigms and psychophysical procedures employed have been previously published in detail (e.g., Chaudhuri et al. 2013; Grabherr et al. 2008; Karmali et al. 2016). Vestibular thresholds were assessed using a standard one-interval forced-choice direction recognition task (e.g., “did I move right or left?”). Subjects were instructed to attend to motion in a single dimension for each set of trials (e.g., did they move left/right, front/back, head/toes) and to respond after motion offset which direction of motion they perceived. When subjects were unsure, they were encouraged to make their best guess. Responses were reported using buttons held in each hand. Patient B exhibited weakness in his right-hand, thus, for some test sessions performed in the right side-lying orientation (i.e., while lying on his right arm), he provided verbal answers that were entered by the experimenters.
Participants were seated in a padded chair on top of a MOOG six degree of freedom platform (6DOF2003E; East Aurora, New York) with the head secured in a helmet which was mounted to the platform. Custom written software was used to control the motion platform and implement the staircase procedure. To minimize non-vestibular contributions to motion perception, the test room was light-tight to remove visual cues and directional auditory cues were masked with ~ 60 dB sound pressure level (SPL) of white noise during motion trajectories. Both bilateral loss patients use an ABI for communication, thus this white noise stimulus was provided via Bluetooth connectivity to provide a cue for motion stimulus. Motion stimuli were single cycles of sinusoidal acceleration [a(t) = A sin(2πft)] in which A is the acceleration amplitude and f is the motion frequency. This yields a bell-shaped velocity trajectory (Benson et al. 1989; Grabherr et al. 2008) in which peak acceleration (A), peak velocity (vp), and total displacement are proportional (vp = A/πf = 2f∆p or ∆p = 2fvp = A/2πf2). The platform did not return to its starting position after each test motion; after each motion trajectory, at least a 3 s pause was provided to reduce potential motion after effects (Crane 2012a, b).
All 1 Hz and 2 Hz threshold tests had an initial velocity of 0.16 m/s for the first trial. These starting stimuli were chosen to be supra-threshold for most motion conditions, including for the bilateral loss patients. Practice trials in the light and dark were provided until the participants reported comfort with the task. Practice was performed in the light to ensure that the motion was supra-threshold for our bilateral loss participants and to help diminish the potential impact of fatigue by providing room light every ~ 10–12 min.
To optimize efficiency, an initial 2-down/1-up (2D/1U) staircase was used in which stimulus magnitude was halved after two consecutive correct responses until the first incorrect answer. After this initial staircase, a 4-down/1-up (4D/1U) adaptive staircase procedure was implemented for the vast majority of the data collection in which stimulus size decreased after four consecutive correct responses and increased after each incorrect response. Step sizes after the initial staircase were selected using parameter estimation by sequential testing (PEST) rules (Taylor and Creelman 1967). For each threshold test of 100 total trials, only one type of motion (e.g., upright 1 Hz z-translation) was assessed. Some thresholds for the bilateral loss patients had to be terminated early as the stimulus magnitude exceeded the displacement limits. Prematurely terminated thresholds were repeated and data from two or more sessions were combined to achieve 100 total trials for data analysis. Thresholds for 1 Hz stimuli required ~ 12 min and thresholds for 2 Hz stimuli required ~ 8 min. Testing for each subject was completed in 2-to-3-h sessions with breaks every ~ 30–40 min of testing. Bilateral loss participants completed ~ 15 h of testing during their visit to our lab over 2–3 days, thus, shorter test sessions were often completed due to scheduling/time constraints and to lessen the potential impact of fatigue.
Data analysis
Psychometric curves were fit to the binary (e.g., left/right) experimental data to obtain estimates of thresholds as previously described in detail (Chaudhuri and Merfeld 2013; Lim and Merfeld 2012; Merfeld 2011). A Gaussian cumulative distribution psychometric function defined by the standard deviation (σ; “threshold”) and mean (µ; “bias”) was fit using a maximum likelihood estimate via a bias-reduced generalized linear model and a probit link function. The threshold parameter represents the “one-sigma” vestibular threshold, as has been commonly reported (Bermúdez Rey et al. 2016; Karmali et al. 2021; Suri and Clark 2020; Valko et al. 2012), and represents: (1) the standard deviation of the underlying distribution function and (2) the stimulus level that would be expected to yield 84.1% accuracy in the absence of bias (Merfeld 2011). The bias-reduced method used for fitting our psychometric functions accounts for the known serial dependency associated with staircase methods that have previously been shown to underestimate thresholds (Chaudhuri and Merfeld 2013; Kaernbach 2001; Klein 2001). Inclusion of lapses (i.e., errors made by an observer that are independent of the test stimulus) is likely to impact the precision of estimates (Clark and Merfeld 2021; Wichmann and Hill 2001). Thus, a lapse-identification algorithm (Clark and Merfeld 2021) was used that implements a standard delete-one jackknife procedure when fitting the psychometric function to identify probable outliers. Standard errors of the parameter estimates were also quantified using this jackknife approach (Quenouille 1956; Tukey 1958). For all thresholds, results are presented in terms of peak velocity (i.e., cm/s, or deg/s). All fits were completed in MATLAB using the Statistics and Machine Learning Toolbox version 11.4.
Similar to earlier studies (Benson et al. 1989; Bermúdez Rey et al. 2016), thresholds for the healthy control subjects displayed a lognormal distribution; therefore, we report geometric means and the corresponding 95% confidence intervals (CIs), and statistical analyses were performed using log-transformed threshold data. As these translation threshold data from healthy controls were previously published, prior analyses were not repeated herein (Kobel et al. 2021a). But these results from the healthy control participants, based on statistical analyses, are summarized to facilitate comparison with our patients with absent vestibular function. In brief, separate linear mixed effect models for each frequency (i.e., 1 Hz and 2 Hz) were completed including fixed effects of motion relative to gravity, motion relative to the head/otoliths, body orientation and subject as a random effect to account for repeated measures.
For the two patients with vestibular loss, 95% CIs of the threshold (σ) parameter estimate were calculated for each motion condition and differences between vestibular thresholds were considered meaningful if 95% CIs did not include any overlap. This approach is conservative, which is appropriate given the small patient sample size. This approach enabled quantitative assessments of whether threshold measures differed between motion conditions and relative to healthy controls to determine the degree to which thresholds were elevated in the absence of vestibular function.
To assess our three main hypotheses on vestibular contributions to linear motion perception, three separate comparisons were used: (1) impact of motion relative to the head/otoliths was assessed comparing thresholds and associated 95% CIs between y-translation, x-translation, and z-translation thresholds for earth-horizontal translations and separately for earth-vertical translations, (2) impact of orientation relative to gravity was determined by comparing CIS for earth-vertical thresholds to earth-horizontal thresholds for each head axis of translation (i.e., separately for x-translations, y-translations, and z-translations), and (3) finally, to determine potential impact of body orientation, earth-horizontal thresholds obtained for each head axis of motion (e.g., upright y-translation, supine y-translation) were compared. In addition, impact of frequency of translation stimuli was evaluated through comparing thresholds and 95% CIs between 1 and 2 Hz thresholds.
Results
Table 1 provides translation thresholds and accompanying 95% CIs for each axis of translation relative to the head in each body orientation. Patient B was able to complete 1 Hz upright z-translation thresholds, however, a substantial bias (> 100% of estimated thresholds) was observed. Staircase stimulus selection only provides accurate threshold estimates when biases are small relative to the threshold parameter (i.e., less than 50%) (Merfeld 2011). Thus, this threshold was not included in final data analyses but is presented in Table 1 for completeness. No other threshold for Patient A or Patient B displayed bias parameter estimates that were greater than 30% of the estimated threshold. Bias values and accompanying 95% CIs for each axis of translation relative to the head in each body orientation are provided in Table 2.
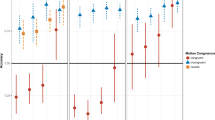
Overall, both patients displayed translation thresholds that were approximately 2–45 times greater than healthy controls. The 95% CIs of the individual patient threshold estimates did not overlap with those healthy controls, suggesting that these were meaningful elevations (Figs. 3, 4, 5 and 6). Patient thresholds normalized by the respective control threshold (i.e., ratio of the patient threshold to the geometric mean of the control threshold) for all motion conditions are provided in Table 3 for both Patient A and Patient B.
Average thresholds for control participants and patient A and patient B with complete bilateral vestibular loss for upright, supine, and side-lying thresholds across each axis of motion in head coordinates. Error bars for controls represent 95% CIs of the mean. For the patients, error bars represent the 95% CI of the threshold parameter estimate. x = naso-occipital x-translations; y = inter-aural y-translation; z = superior–inferior z-translation
Average thresholds for control participants and for patient A and patient B with complete bilateral vestibular loss for earth-horizontal and earth-vertical motions. Error bars for controls represent 95% CIs of the mean. For the patients, error bars represent the 95% CI of the threshold parameter estimate. Patient B thresholds for 1 Hz earth-vertical z-translation threshold (upper right panel) exhibited a significant bias (> 100%), thus is excluded as this threshold estimate may be inaccurate
Average thresholds for control participants and patient A and patient B with complete bilateral vestibular loss for x-translations, y-translation, and z-translations for each body orientation. Error bars for controls represent 95% CIs of the mean. For the patients, error bars represent the 95% CI of the threshold parameter estimate
Average thresholds for control participants and patient A and patient B with complete bilateral vestibular loss for x-translations, y-translations, and z-translations across stimulus frequency. Error bars for controls represent 95% CIs of the mean. For the patients, error bars represent the 95% CI of the threshold parameter estimate. Patient B thresholds for 1 Hz upright z-translation threshold (lower left panel) exhibited a significant bias (> 100%), thus is excluded as this threshold estimate may be inaccurate
Effect of motion relative to head
Thresholds for each body orientation and each axis of head motion are shown in Fig. 3 for both 1 Hz and 2 Hz stimuli. To assess impact of motion relative to the head, all earth-horizontal thresholds for each axis of translation (i.e., x-translation, y-translation, and z-translation) were compared to each other. Similarly, all earth-vertical thresholds were compared to assess if systematic differences between thresholds modulated on the basis of motion relative to head were identified.
In healthy controls—i.e., individuals without surgical removal of otoliths—1 Hz and 2 Hz x- and y-translation thresholds, motions with predominant utricular contributions, were on average lower than z-translation thresholds, a motion with predominant saccular contributions (Kobel et al. 2021a). For Patients A and B—individuals without otolith organs—no systematic differences between x-translation, y-translation, and z-translation thresholds were seen when comparing earth-horizontal motions or earth-vertical motions for either 1 Hz or 2 Hz stimuli.
Effect of orientation relative to gravity
Vestibular thresholds for earth-horizontal and earth-vertical motions are displayed in Fig. 4 for healthy controls and both bilateral loss patients. In healthy controls, thresholds for earth-vertical motions (i.e., linear motion parallel to gravity) were approximately a factor of 1.5 to 2 higher than earth-horizontal motions (i.e., linear motion perpendicular to gravity) for both x-translations and y-translations at 1 Hz and 2 Hz. For z-translation thresholds, earth-vertical and earth-horizontal thresholds were equivalent. These data suggest that motions with predominant utricular contributions are preferentially impacted by gravitational cues in healthy adults (Kobel et al. 2021a).
Overall, Patient A displayed qualitatively similar elevations in earth-vertical thresholds relative to earth-horizontal thresholds for 1 Hz stimuli but a consistent effect of orientation relative to gravity was not seen for 2 Hz stimuli. Thresholds for Patient A were 14–28 times higher than healthy controls for earth-vertical x-, y- and z-translations, while earth-horizonal x-, y-, and z-translation thresholds were 2.5–22 times higher. For 1 Hz stimuli, the 95% CIs surrounding Patient A’s earth-vertical translation thresholds did not overlap with earth-horizontal thresholds, suggesting that the increase in thresholds was meaningful. For 2 Hz stimuli, earth-vertical y- and x-translation thresholds were similar to the corresponding earth-horizontal thresholds; however, 2 Hz earth-vertical z-translations were still elevated relative to earth-horizontal z-translation thresholds. The diminished effect of orientation relative to gravity at 2 Hz may reflect the increase in non-vestibular contributions previously identified at higher frequencies of motion (Valko et al. 2012).
However, the effect of orientation relative to gravity for Patient B was less consistent relative to Patient A. For Patient B, earth-vertical thresholds were 6–30 times higher in comparison to controls and earth-horizontal thresholds were approximately 5–25 times higher. While thresholds for Patient B tended to be higher for earth-vertical motions in comparison to earth-horizontal thresholds, the 95% CIs for earth-vertical and earth-horizontal thresholds overlapped for most conditions except 2 Hz y-translation and 2 Hz x-translation thresholds. Thresholds for 2 Hz y-translations showed increases in earth-vertical thresholds, but 2 Hz x-translations showed an opposite pattern, with earth-horizontal thresholds being higher than earth-vertical thresholds. The contradictory finding for 2 Hz x-translation may have resulted from the large increase in earth-horizontal side-lying x-translations thresholds for this subject (see “Impact of body orientation”).
Overall, in healthy controls, 1 Hz earth-vertical thresholds were higher than the average of both earth-horizontal thresholds by 2.60×, 2.402×, and 1.19× for x-translations, y-translations, and z-translations, respectively. For Patient A, 1 Hz earth-vertical thresholds were higher by 9.98×, 16.34×, and 5.02× for x-translations, y-translations, and z-translations. For Patient B, earth-vertical x-translations were 1.5× higher while earth-vertical y-translations were 2.2× higher than earth-horizontal.
For 2 Hz thresholds, overall, a smaller impact of orientation relative to gravity was noted. Healthy control thresholds were 2.45×, 1.54×, and 1.08× higher for earth-vertical relative to earth-horizontal thresholds for x-translations, y-translations, and z-translations. For Patient A, earth-vertical thresholds were 5.69×, 3.39×, and 13.57× higher for x-translations, y-translations, and z-translations, while Patient B displayed elevations of 0.59×, 7.97×, and 2.21× for earth-vertical thresholds. Thus, as a whole, larger elevations in earth-vertical thresholds, as quantified by ratio of earth-vertical relative to earth-horizontal thresholds, were seen for both patients relative to healthy controls. For Patient B 1 Hz and 2 Hz ratios for x-translations were not significantly elevated, reflecting the large impact of body orientation on-side-lying earth-horizontal thresholds, as noted above.
Overall, Patient A displayed a larger impact of orientation relative to gravity as quantified by increase of earth-vertical thresholds relative to the earth-horizontal thresholds. When comparing Patient A and Patient B, thresholds for all conditions were largely equivalent, as indicated by overlapping 95% CIs. Thus, differences in the effect of motion relative to gravity (i.e., earth-vertical vs. earth-horizontal) on translation thresholds between patients may be indicative of the known variability in threshold measures and the large standard errors associated with increased thresholds.
Effect of body orientation
Figure 5 displays thresholds for motions performed in each of the three axes of head translation across each body orientation. In the control participants, differences in thresholds across body orientation were consistent with the expected changes in thresholds resulting from the aforementioned impact of orientation relative to gravity on both x-translations and y-translation thresholds. All earth-horizontal thresholds for x-translation and y-translations were equivalent regardless of “upright” vs. “non-upright” (i.e., supine or side-lying) body orientations. As well, no differences in z-translation thresholds were noted between any of the assessed body orientations (Kobel et al. 2021a). However, in patients with complete bilateral vestibular loss, there was evidence to suggest that body orientation impacted vestibular perceptual thresholds for some motion conditions, particularly x-translations.
For earth-horizontal thresholds, in our bilateral loss patients, a consistent change in threshold based on body orientation suggesting elevations of thresholds in non-upright orientations was not identified for either z-translation or y-translations. For both patients, 1 Hz and 2 Hz earth-horizontal y-translation thresholds were equivalent when measured in upright and supine orientations. A similar pattern was seen for 1 Hz and 2 Hz earth-horizontal z-translation thresholds as thresholds measured in supine were equivalent to side-lying thresholds. The exception being 1 Hz thresholds for Patient A as 95% CIs did not overlap, and thresholds displayed a small elevation of the supine z-translation threshold relative to side-lying z-translation (difference of 0.163 cm/s), potentially reflect inherent variability in the data. However, for both patients, 1 Hz and 2 Hz side-lying (i.e., earth-horizontal) x-translation thresholds were approximately 2–3 × higher than upright (i.e., also earth-horizontal) thresholds. The elevations in side-lying x-translation thresholds were meaningful (i.e., 95% CIs did not overlap) except for Patient A’s 1 Hz thresholds. This also partially reflects the higher uncertainty (i.e., larger standard error) associated with higher threshold values.
A significant impact of body orientation was not identified for earth-vertical translation thresholds in our bilateral loss patients. Specifically, for Patient A, all earth-vertical translation thresholds (i.e., upright, supine, and on-side) had overlapping 95% CIs for both 1 Hz and 2 Hz stimuli. For Patient B, 1 Hz earth-vertical thresholds were also largely similar across the different axes of head translation (i.e., motion relative to head/otoliths). The one exception to this was that Patient B displayed 2 Hz side-lying y-translation thresholds (i.e., earth-vertical) that were higher than 2 Hz x- and z-translation earth-vertical thresholds, suggesting a possible effect of absolute body orientation.
Effect of frequency
Figure 6 shows thresholds for each body orientation and axis of head translations across 1 Hz and 2 Hz stimulus frequencies. For healthy controls, thresholds were lower for 2 Hz translations in comparison to 1 Hz translations, consistent with past data (Roditi and Crane 2012; Valko et al. 2012). A similar pattern was seen for both of our patients with vestibular loss, with 2 Hz thresholds being generally lower than 1 Hz thresholds, suggesting that lower frequency translations (i.e., 1 vs. 2 Hz) may better reflect an isolated measure of the vestibular contributions to linear motion perception. For supine and side-lying y-translations, a seemingly opposite pattern was seen for Patient B in which 2 Hz thresholds were higher than 1 Hz thresholds. However, since the 95% CIs overlapped between the two patients at each frequency and between the two frequencies for each subject, this does not appear to represent a meaningful difference and likely reflects inherent variability in the threshold parameter.
Discussion
Our primary finding was that whole-body direction recognition translation thresholds were between 2 and 45 times larger for individuals with a complete absence of vestibular function in comparison to young, healthy adults. These thresholds represent the stimulus magnitudes at which we expect non-vestibular cues to contribute to linear motion perception. As thresholds following bilateral vestibular ablation were meaningfully elevated related to healthy controls, these data confirm that vestibular translation thresholds are dominated by vestibular cues.
Impact of motion direction relative to the head and otoliths
We aimed to assess three factors previously proposed to influence translation perception: (1) motion orientation relative to the head/otoliths, (2) motion orientation relative to gravity (i.e., earth-vertical, or earth-horizontal), and (3) body orientation.
As previously reported in depth (Kobel et al. 2021a), in this study of healthy adults, we identified differences in perceptual sensitivity on the basis of motion relative to the head (i.e., the otoliths) as y-translation and x-translation threshold thresholds were significantly lower than z-translation thresholds. These results are in agreement with multiple reports of other perceptual threshold data (Benson et al. 1986; Bermúdez Rey et al. 2016; Bremova et al. 2016; MacNeilage et al. 2010; Roditi and Crane 2012; Valko et al. 2012). Past interpretation of this differential sensitivity has been proposed to potentially reflect differences in peripheral sensitivity between the utricles and saccules (Karmali et al. 2017; Kobel et al. 2021a). Peripheral afferent recordings in the squirrel monkey have identified a lower sensitivity of the saccule in comparison to the utricle (Fernandez and Goldberg 1976), putatively due to the smaller surface area of the saccule and the decreased number of hair cells (Merchant et al. 2000; Naganuma et al. 2001, 2003; Takagi et al. 1988). However, the extent to which peripheral anatomy can explain the behavioral differences seen has been questioned, as otolith afferent recordings in the rhesus monkey have revealed similar gains for horizontal and vertical motions (Jamali et al. 2009; Yu et al. 2012).
In our bilateral loss patients, we failed to identify systematic changes in perceptual thresholds on the basis of motion relative to the head. Earth-vertical 1 Hz and 2 Hz thresholds were largely equivalent for all motions relative to the head (i.e., x-translations, y-translations, and z-translations) regardless of concomitant changes in body orientation. Similarly, all earth-horizontal 1 Hz and 2 Hz thresholds were essentially equivalent regardless of the orientation of the motion relative to the head, with the exception of x-translations (discussed more below in “Impact of body orientation”). However, due to the small sample size, we may have failed to identify an effect that would be uncovered when assessed in a larger population.
As the effect of motion relative to the head (i.e., relative to the otoliths) was seen only for those with intact vestibular inputs, and not in our bilateral loss patients, this effect of motion relative to the head may be at least partially driven by the heightened sensitivity of the utricular, relative to the saccular, end-organs in the control subjects. However, this pattern may be driven by changes in central processing and weighting of afferent vestibular input. Further studies should examine potential differences in motion perception sensitivity in patients with localized damage to one otolith organ (i.e., isolated utricular pathology, or isolated saccular pathology).
Impact of gravity
The otoliths encode both linear acceleration and gravity, as the afferent signal is proportional to the net gravitoinertial force (GIF) (Fernandez & Goldberg 1976), rather than either input in isolation. Einstein’s equivalence principle states that all linear accelerometers must encode both linear acceleration and gravity equivalently; However, past literature suggests neural processing can decompose this ambiguous afferent signal into independent estimates of gravity and linear acceleration through the use of internal models of gravity (Angelaki et al. 1999; Merfeld et al. 1993, 1999). This assertion is supported by data showing that reflexive responses (Angelaki et al. 1999; Merfeld et al. 1999), perceptual responses (Angelaki et al. 1999; Angelaki and Hess 1994; Merfeld et al. 1999), and neural encoding at multiple levels including the vestibular nuclei, brainstem, and thalamus (Angelaki et al. 2004; Angelaki and Hess 1994; Laurens et al. 2013a, b)are made in response to central estimates of linear acceleration, rather than the net GIF encoded by the otolith afferent neurons.
However, the majority of these past efforts have focused on assessing the ability to resolve ambiguous otolith signals to parse tilts from translations, and not the ability to disambiguate linear acceleration from co-linear gravitational forces. Our past data in healthy young adults found that earth-vertical translation thresholds were significantly higher than earth-horizontal translation thresholds for y-translations and x-translations (Kobel et al. 2021a, b). This suggests that internal models of gravity influence translation perception, as earth-vertical thresholds, where the translation stimulus must be disambiguated from a co-linear gravitational force, were significantly higher than earth-horizontal thresholds, where the linear motion is independent of (i.e., perpendicular to) gravitational acceleration.
In the present study, patients with complete bilateral loss, overall, tended to display earth-vertical thresholds that were increased for all axes of head translations (x-translation, y-translation, z-translation) when compared to earth-horizontal thresholds, with larger increases relative to healthy controls. These results suggest that vestibular loss led to impaired internal models of gravity and decreased perceptual precision when patients were required to differentiate co-linear translation from gravity leading to higher elevations of earth-vertical thresholds relative to healthy controls. While speculative, this suggests that vestibular contributions are more evident for the perception of earth-vertical motions in comparison to earth-horizontal motions However, at this time, we cannot be certain that this effect is not driven by extra-vestibular cues (e.g., tactile, somatic graviception) without manipulation of gravity. These results may also reflect Weber’s law, a fundamental tenet of psychophysics stating that the ratio between the difference threshold magnitude and stimulus intensity for any given stimulus is constant. Gravity stimulates the otoliths when positioned in the plane of gravity (i.e., during an earth-vertical motion) resulting in an increased firing rate, thus, subjects must perceive a change of this elevated firing rate resulting from a linear acceleration. However, past vestibular studies have noted behavioral violations of Weber’s law, in part dependent on stimulus frequency (Mallery et al. 2010; Naseri and Grant 2012), therefore, further focused studies are needed to explicitly explore this hypothesis.
However, the effect motion relative to gravity was more prominent and consistent in Patient A vs. Patient B as earth-vertical thresholds were elevated by 9.98×–16.34× relative to earth-horizontal thresholds for Patient A while they were only elevated by 0.5×–7.97× for Patient B The 95% CIs of the threshold parameter estimate for earth-vertical and earth-horizontal translations consistently failed to overlap for Patient A, while a meaningful elevation in earth-vertical thresholds was less consistently seen for Patient B. However, as thresholds between our two patients were largely equivalent, this may reflect the variability inherent to our small sample size.
The effect of orientation relative to gravity was also larger at 1 Hz than at 2 Hz for both healthy controls and patients with vestibular ablation. This effect of stimulus frequency is consistent with past data that have posited increased non-vestibular contributions to linear motion perception at frequencies above 1 Hz—where non-vestibular inertial cues (tactile, kinesthetic, somatic, etc.) are greatest—as translation thresholds were more elevated at 1 Hz than at 2 and 5 Hz in patients with bilateral vestibular loss (Valko et al. 2012).
In our healthy control group, an impact of orientation relative to gravity was only seen for motions with predominant utricular contributions (i.e., y-translations and x-translations) and not those with predominant saccular contributions (i.e., z-translations). As humans are bipeds, and thus motion typically occurs while upright, this was taken to suggest that an absence of a gravity effect on superior-inferior translations may reflect an ecological adaptation to human motion whereby the brain has learned to adapt to compensate for co-linear gravity and linear acceleration during gait (Kobel et al. 2021a, b). However alternatively, this finding may reflect a lower sensitivity of the saccule relative to the utricle (Fernandez and Goldberg 1976). In our patients with complete bilateral vestibular loss, a cohort without afferent inputs from the utricles or saccules, earth-vertical thresholds were elevated a similar amount across the different axes of head translation (x-, y-, and z-translation). As these patients were each ambulatory, and thus also experience motion each day that is similar to that of healthy controls, this suggests that the lack of an impact of orientation relative to gravity on superior-inferior translations in healthy controls may not reflect changes in ecological coding strategies and may instead be a result of differences in peripheral encoding of linear motion and/or the sensitivity of peripheral afferent neurons.
Impact of body orientation
Past data have suggested that body orientation may modulate visual and vestibular perception, with non-upright orientations having been proposed to yield a decreased reliability of sensory estimates; however, inconsistent results have been previously noted in part dependent on availability of sensory cues (Graybiel and Patterson 1955; Hummel et al. 2016; MacNeilage et al. 2010; Mikellidou et al. 2015). The proposed effect of body orientation may reflect an increased sensitivity to motions that occur in orientations that mirror those typically experienced in daily life (i.e., while upright). However, our data show that in general, earth-horizontal, as well as earth-vertical, thresholds measured with the body tilted away from upright (e.g., side-lying or supine) were equivalent to thresholds measured when upright for healthy controls as well as our bilateral loss patients without vestibular function. This suggests that overall changes in perception on the basis of body orientation may be reflective of the relatively large effect of motion direction relative to gravity (i.e., ~ two time increase in thresholds for earth-vertical relative to earth-horizontal motions). As on earth, changes in body orientation and changes in orientation relative to gravity are inherently coupled, relative effects of change for each factor on perceptual sensitivity cannot be definitively parsed without artificial manipulations of gravity.
However, we did identify an impact of body orientation for x-translations in our bilateral vestibular loss patients. For both patients, side-lying earth-horizontal x-translation thresholds were significantly higher than upright earth-horizontal x-translation translation thresholds. Side-lying earth-horizontal z-translations thresholds were equivalent to upright earth-horizontal z-translation thresholds, suggesting that this side-lying orientation did not lead to an overall increase in vestibular thresholds and non-vestibular contributions to side-lying x-translations may be unique to this specific motion paradigm. In side-lying, a smaller surface area of the body is supported by the chair relative to the upright and supine orientations. In our set-up, only the right side of the torso and legs rest on the lower edge of the chair when in side-lying, while in upright and supine, the majority of the body (i.e., buttocks and back) rests securely on the chair. Useful tactile cues for motion perception may therefore be reduced for x-translations, as sensing fore-aft motion on this limited surface area is difficult, while z-translations can be perceived on the basis of head and/or buttocks pressure.
Beyond vestibular graviception, somatic graviception, from graviceptors in the trunk, has been shown to contribute to gravitational vertical (Mittelstaedt 1983, 1995, 1996) and may have provided additional extra-vestibular cues for motion perception. Particularly, gravity’s pull on the abdominal viscersa as may weight of blood pooling in the trunk may influence perceived gravitational vertical (Mittelstaedt 1995, 1996; Mittelstaedt and Fricke 1988; Vaitl et al. 1997, 2002). Experiments with paraplegic and nephrectomized subjects suggest afferent input from the kidneys (Mittelstaedt 1995, 1996; Mittelstaedt and Mittelstaedt 1996; Vaitl et al. 2002) and contributions from visceral mechanoreceptors in the pericardium and inferior vena cava (Goodman-Keiser et al. 2010; Kostreva and Pontus 1993a, b). Past research suggests that somatic graviception and other visceral signals for motion perception can influence vestibular processing (Catanzaro et al. 2014; Suzuki et al. 2012). As such, somatic graviception cues, particularly those resulting from re-orientation of the body in supine and side-lying, may have influenced perception. As both patients reported spinal tumors, these may have potentially influenced availability and integrity of somatic graviceptive and other visceral cues influencing motion perception. However, the impact of interoceptive cues on motion perception has not been rigorously assessed and future studies should further examine potential influences.
Limitations
The primary limitation of this study was the number of bilateral loss patients (n = 2) that we were able to recruit. However, to state with certainty that the thresholds measured in our patients only reflected contributions of other sensory systems, and thereby define stimulus levels at which non-vestibular contributions (e.g., tactile, somatic graviception) can become relevant for motion perception, we required individuals with a complete loss of vestibular function. To ensure complete bilateral ablation, patients were selected on the basis of having bilateral labyrinthectomies, and not bilateral nerve sections. Both patients tested in this study were included in a previous study (Valko et al. 2012). Reflecting the rarity of this patient population, we were unable to identify additional patients with a total bilateral loss of vestibular function despite searches both locally and nationally over several years. However, due to the large differences in vestibular thresholds seen, this limitation did not preclude our ability to draw inferences into vestibular contributions to linear motion perception.
Of note, both of our bilateral loss patients are exceptionally high performing and had previous vestibular experience prior to surgical ablation. Patient A is actively participating in competitive athletic events, including running, swimming, and riding a bike. Similarly, Patient B is an avid international and domestic traveler and still rides a bike for recreation. Both patients have had several years (i.e., 15+ years) to adapt to the loss of vestibular function and presumably have maximized use of non-vestibular cues for motion perception. While we attempt to minimize non-vestibular contributions to motion perception through testing in complete darkness and masking directional auditory cues, the interface between participants and the chair and helmet is unavoidable, thus, all participants have access to tactile cues. As such, thresholds obtained in our bilateral loss patients likely reflect the smallest stimulus magnitudes at which non-vestibular cues can contribute to linear motion perception. We posit that adults with less experience in the substitution of vestibular cues for motion perception would require larger stimuli to use non-vestibular information for motion direction recognition. Similarly, as both patients experienced vestibular afferent input for several years prior to surgical intervention, their ability to use non-vestibular information for motion perception may differ from those with congenital vestibular loss.
In addition, a neurological exam and test of pressure sensation was last assessed ~ 10 years prior when both patients were included in a previous study (Valko et al. 2012); thus, changes in sensory function from these initial assessments could have influenced our results. However, at both time points, upright translation thresholds were assessed and were similar for y-translations (1 Hz: 2.112 cm/s vs 2.4855 cm/s; 2 Hz: 1.227 cm/s vs. 1.6775 cm/s) and z-translations (1 Hz: 31.890 cm/s vs. 25.736 cm/s; 2 Hz: 11.105 cm/s vs. 17.627 cm/s) suggesting that potential changes in sensory function over time did not impact perceptual precision. Our patients (age 34 and 37) were approximately 10 years older than the mean of the HC participants included (26.57 years). However, our previous data suggest that thresholds are on average relatively constant up to age 40 (Bermúdez Rey et al. 2016), thus a substantive effect of age is not expected to influence the vestibular perceptual thresholds measured in this study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrawal Y, Bremova T, Kremmyda O, Strupp M, MacNeilage PR (2013) Clinical testing of otolith function: perceptual thresholds and myogenic potentials. JARO J Assoc Res Otolaryngol 14:905–915
Angelaki DE, Hess BJ (1994) Inertial representation of angular motion in the vestibular system of rhesus monkeys. I. vestibuloocular reflex. J Neurophysiol 71:1222–1249
Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJM (1999) Computation of Inertial Motion: Neural Strategies to Resolve Ambiguous Otolith Information. J Neurosci 19:316–327
Angelaki DE, Shaikh AG, Green AM, Dickman JD (2004) Neurons compute internal models of the physical laws of motion. Nature 430:560–564
Benson AJ, Spencer MB, Stott JRR (1986) Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med 57:1088–1096
Benson AJ, Hutt ECB, Brown SF, Hutt C, Brown SF, Hutt ECB, Brown SF (1989) Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60:205–213
Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM (2016) Vestibular perceptual thresholds increase above the age of 40. Front Neurol. https://doi.org/10.3389/fneur.2016.00162
Bremova T, Caushaj A, Ertl M, Strobl R, Böttcher N, Strupp M, MacNeilage PR (2016) Comparison of linear motion perception thresholds in vestibular migraine and Menière’s disease. Eur Arch Otorhinolaryngol 273:2931–2939
Catanzaro MF, Miller DJ, Cotter LA, McCall AA, Yates BJ (2014) Integration of vestibular and gastrointestinal inputs by cerebellar fastigial nucleus neurons: multisensory influences on motion sickness. Exp Brain Res 232:2581–2589
Chaudhuri SE, Merfeld DM (2013) Signal detection theory and vestibular perception: III. Estimating unbiased fit parameters for psychometric functions. Exp Brain Res 225:133–146
Chaudhuri SE, Karmali F, Merfeld DM (2013) Whole body motion-detection tasks can yield much lower thresholds than direction-recognition tasks: implications for the role of vibration. J Neurophysiol 110:2764–2772
Clark TK, Merfeld DM (2021) Statistical approaches to identifying lapses in psychometric response data. Psychon Bull Rev 28:1433–1457
Crane BT (2012a) Fore-aft translation aftereffects. Exp Brain Res 219:477–487
Crane BT (2012b) Roll aftereffects: influence of tilt and inter-stimulus interval. Exp Brain Res 223:89–98
Fernandez C, Goldberg JM (1976) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force response relations. J Neurophysiol 39:985–995
Goodman-Keiser MD, Qin C, Thompson AM, Foreman RD (2010) Upper thoracic postsynaptic dorsal column neurons conduct cardiac mechanoreceptive information, but not cardiac chemical nociception in rats. Brain Res 1366:71–84
Grabherr L, Nicoucar K, Mast FW, Merfeld DM (2008) Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186:677–681
Graybiel A, Patterson JRJL (1955) Thresholds of stimulation of the otolith organs as indicated by the oculogravic illusion. J Appl Physiol 7:666–670
Hummel N, Cuturi LF, MacNeilage PR, Flanagin VL (2016) The effect of supine body position on human heading perception. J vis 16:19–19
Kaernbach C (2001) Slope bias of psychometric functions derived from adaptive data. Percept Psychophys 63:1389–1398
Jamali M, Sadeghi SG, Cullen KE (2009) Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J neurophysiol 101:141–149
Karmali F, Chaudhuri SE, Yi Y, Merfeld DM (2016) Determining thresholds using adaptive procedures and psychometric fits: evaluating efficiency using theory, simulations, and human experiments. Exp Brain Res 234:773–789
Karmali F, Rey MCB, Clark TK, Wang W, Merfeld DM (2017) Multivariate analyses of balance test performance, vestibular thresholds, and age. Front Neurol 8:1–16
Karmali F, Goodworth AD, Valko Y, Leeder T, Peterka RJ, Merfeld DM (2021) The role of vestibular cues in postural sway. J Neurophysiol 125:672–686
King S, Priesol AJ, Davidi SE, Merfeld DM, Ehtemam F, Lewis RF (2019) Self-motion perception is sensitized in vestibular migraine: pathophysiologic and clinical implications. Sci Rep 9:1–12
Kingma H (2005) Thresholds for perception of direction of linear acceleration as a possible evaluation of the otolith function. BMC Ear Nose Throat Disord 6:1–6
Klein SA (2001) Measuring, estimating, and understanding the psychometric function: a commentary. Percept Psychophys 63:1421–1455
Kobel MJ, Wagner AR, Merfeld DM (2021a) Impact of gravity on the perception of linear motion. J Neurophysiol 126:875–887
Kobel MJ, Wagner AR, Merfeld DM, Mattingly JK (2021b) Vestibular thresholds: a review of advances and challenges in clinical applications. Front Neurol 12:203
Kobel MJ, Wagner AR, Merfeld DM (2023) Evaluating vestibular contributions to rotation and tilt perception. Exp Brain Res 241:1873–1885
Kostreva DR, Pontus SP (1993a) Hepatic vein, hepatic parenchymal, and inferior vena caval mechanoreceptors with phrenic afferents. Am J Physio Gastrointest Liver Physiol 265:G15–G20
Kostreva DR, Pontus SP (1993b) Pericardial mechanoreceptors with phrenic afferents. Am J Physiol Heart Circulatory Physiol 264:H1836–H1846
Laurens J, Meng H, Angelaki DE (2013a) Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci 16:1701–1708
Laurens J, Meng H, Angelaki DE (2013b) Neural representation of orientation relative to gravity in the macaque cerebellum. Neuron 80:1508–1518
Lewis RF, Priesol AJ, Nicoucar K, Lim K, Merfeld DM (2011a) Dynamic tilt thresholds are reduced in vestibular migraine. J Vestib Res Equilib Orient 21:323–330
Lewis RF, Priesol AJ, Nicoucar K, Lim K, Merfeld DM (2011b) Abnormal motion perception in vestibular migraine. Laryngoscope 121:1124–1125
Lim K, Merfeld DM (2012) Signal detection theory and vestibular perception: II. Fitting perceptual thresholds as a function of frequency. Exp Brain Res 222:303–320
MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE (2010) Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci 30:9084–9094
Mallery RM, Olomu OU, Uchanski RM, Militchin VA, Hullar TE (2010) Human discrimination of rotational velocities. Exp Brain Res 204:11–20
Merchant SN, Tsuji K, Wall C, Velázquez-Villaseñor L, Glynn RJ, Rauch SD (2000) Temporal bone studies of the human peripheral vestibular system. Ann Otol Rhinol Laryngol 109:3–13
Merfeld DM, Young LR, Oman CM, Shelhamer MJ (1993) A multidimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestib Res 3:141–161
Merfeld DM, Zupan L, Peterka RJ (1999) Humans use internal models to estimate gravity and linear acceleration. Nat 398:615–618
Merfeld DM (2011) Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp Brain Res 210:389–405
Mikellidou K, Cicchini GM, Thompson PG, Burr DC (2015) The oblique effect is both allocentric and egocentric. J vis 15:24–24
Mittelstaedt H (1983) A new solution to the problem of the subjective vertical. Naturwissenschaften 70:272–281
Mittelstaedt H (1995) Evidence of somatic graviception from new and classical investigations. Acta Otolaryngol 115:186–187
Mittelstaedt H (1996) Somatic graviception. Biol Psychol 42:53–74
Mittelstaedt H, Fricke E (1988) The relative effect of saccular and somatosensory information on spatial perception and control. Clinical testing of the vestibular system. Karger Publishers, Basel, pp 24–30
Mittelstaedt M-L, Mittelstaedt H (1996) The influence of otoliths and somatic graviceptors on angular velocity estimation. J Vestib Res 6:355–366
Naganuma H, Tokumasu K, Hashimoto S, Okamoto M, Yamashina S (2001) Three-dimensional analysis of morphological aspects of the human saccular macula. Ann Otol Rhinol Laryngol 110:1017–1024
Naganuma H, Tokumasu K, Hashimoto S, Okamoto M, Yamashina S (2003) Three-dimensional analysis of morphological aspects of the human utricular macula. Ann Otol Rhinol Laryngol 112:419–424
Naseri AR, Grant PR (2012) Human discrimination of translational accelerations. Exp Brain Res 218:455–464
Priesol AJ, Valko Y, Merfeld DM, Lewis RF (2014) Motion perception in patients with idiopathic bilateral vestibular hypofunction. Otolaryngol Head Neck Surg (united States) 150:1040–1042
Quenouille MH (1956) Notes on bias in estimation. Biometrika 43:353–360
Roditi RE, Crane BT (2012) Directional asymmetries and age effects in human self-motion perception. JARO J Assoc Res Otolaryngol 13:381–401
Suri K, Clark TK (2020) Human vestibular perceptual thresholds for pitch tilt are slightly worse than for roll tilt across a range of frequencies. Exp Brain Res 238:1499–1509
Suzuki T, Sugiyama Y, Yates BJ (2012) Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 302:R965–R975
Takagi A, Sando I, Takagi A, Sando I (1988) Computer-aided three-dimensional reconstruction and measurement of the vestibular end-organs. Otolaryngol Head Neck Surg 98:195–202
Taylor M, Creelman CD (1967) PEST: Efficient estimates on probability functions. J Acoust Soc Am 41:782–787
Tukey J (1958) Bias and confidence in not quite large samples. Ann Math Stat 29:614
Vaitl D, Mittelstaedt H, Baisch F (1997) Shifts in blood volume alter the perception of posture. Int J Psychophysiol 27:99–105
Vaitl D, Mittelstaedt H, Saborowski R, Stark R, Baisch F (2002) Shifts in blood volume alter the perception of posture: further evidence for somatic graviception. Int J Psychophysiol 44:1–11
Valko Y, Lewis RF, Priesol AJ, Merfeld DM (2012) Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci 32:13537–13542
Van Stiphout L, Lucieer F, Pleshkov M, Van Rompaey V, Widdershoven J, Guinand N, Pérez Fornos A, Kingma H, van de Berg R (2021) Bilateral vestibulopathy decreases self-motion perception. J Neurol. https://doi.org/10.3389/fneur.2022.856472
Wichmann FA, Hill NJ (2001) The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63:1293–1313
Yu X, Dickman JD, Angelaki DE (2012) Detection thresholds of macaque otolith afferents. J Neurosci 32:8306–8316
Acknowledgements
We would like to thank our bilateral loss participants for their time, effort, and willingness to travel to participate in our research. We also thank Bob Grimes and Michael Hall for their technical assistance and Philippe deNeere for artistic assistance.
Funding
This research was supported by the National Institute on Deafness and Other Communication Disorders Grants (R01DC014924) and the National Institute on Aging (R01AG073113). Department of Defense Congressionally Directed Medical Research Programs (CDMRP) Award Number W81XWH192000. MK was supported in part by a The Ohio State University Graduate School’s Alumni Grants for Graduate Research and Scholarship (AGGRS) Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by MJK and ARW. Data analysis and the first draft of the manuscript was written by MJK. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Ethical approval
All study procedures were approved by the Ohio State University Institutional Review Board.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobel, M.J., Wagner, A.R. & Merfeld, D.M. Vestibular contributions to linear motion perception. Exp Brain Res 242, 385–402 (2024). https://doi.org/10.1007/s00221-023-06754-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06754-y