Abstract
The attenuation of sensory inputs via various methods has been demonstrated to impair balance control and alter locomotor behavior during human walking; however, the effects of attenuating foot sole sensation under distinct areas of the foot sole on lower extremity motor output remains poorly understood. Thus, the purpose of this study was to attenuate cutaneous feedback via regional hypothermia under five different areas of the foot sole and investigate the resultant modulation of kinematic and muscle activity during level walking. Electromyography from eight lower leg muscles, kinematics, and location of center of pressure was recorded from 48 healthy young adults completing walking trials with normal and reduced cutaneous sensation from bilateral foot soles. The results of this study highlight the modulatory response of the tibialis anterior in terminal stance (propulsion and toe-off) and medial gastrocnemius muscle throughout the entire stance phase of gait. The topographical organization of foot sole skin in response to the attenuation of cutaneous feedback from different areas of the foot sole significantly modified locomotor activity. Furthermore, the locomotor response to cutaneous attenuation under the same regions that we previously facilitated with tactile feedback do not oppose each other, suggesting different physiological changes to foot sole skin generate unique gait behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cutaneous mechanoreceptors in the foot sole skin play an important role in dynamic balance control and human locomotion. As the foot contacts the external environment, these receptors convey loading, pressure distribution and touch feedback to the central nervous system (CNS) to modulate smooth movement patterns. Lending support to this hypothesis, the experimental attenuation of sensory inputs has been demonstrated to impair balance control and alter locomotor behavior as the system searches for functioning sensory cues to generate motor output. Despite the evidence supporting the importance of cutaneous feedback, the effects of attenuating foot sole sensation to distinct areas of the foot sole on lower extremity motor output remains poorly understood. Thus, the purpose of this study was to attenuate cutaneous feedback (via hypothermia) under five different areas of the foot sole and investigate the resultant modulation of kinematic and muscle activity during level walking.
Static and dynamic balance control is achieved through the interplay of various somatosensory systems working together. This is emphasized in studies that alter the environmental conditions and investigate the relative contribution of each sensory system (“sensory re-weighting”) according to the demands of the task (Assländer and Peterka 2014). For example, the importance of vision has been well-established in postural control literature, whereby the removal of visual input can drastically alter one’s ability to discriminate between a successful vs. unsuccessful recovery of a balance control perturbation (Ray et al. 2008; Sozzi et al. 2011; Sarabon et al. 2013). In static stance balance tasks, the magnitude of center of pressure (CoP) sway appears proportionate to the magnitude of sensory impairment from foot sole skin (Wang and Lin 2008). In static tasks requiring increased balance control (for example, double limb support vs. single limb support), our system relies more heavily on cutaneous feedback, especially when visual input is removed (Mckeon and Hertel 2007).
When skin makes physical contact with the external environment, cutaneous mechanoreceptors are the physiological structures responsible to transmit touch, pressure, vibration, and skin stretch information to the CNS (Macefield 1998; McGlone and Reilly 2010; Geffeney and Goodman 2012; Abraira and Ginty 2013; Zimmerman et al. 2014). Four types of receptors, slow-adapting type I and II and fast-adapting type I and II, have specialized mechanoreceptor endings within the dermis and epidermis of human glabrous skin (Macefield 1998; Zimmerman et al. 2014). As force changes across the foot sole during gait, these receptor endings are impinged, stretched and vibrated, transducing mechanical forces into nerve impulses, and generating an influx of afferent feedback into the spinal cord (Hao et al. 2015). The distribution of these receptors are widespread across the foot sole (Strzalkowski et al. 2018), thus emphasizing their vital role in the maintenance of dynamic stability and smooth transitions across each phase of gait. Although several studies have explored the effects of facilitating cutaneous mechanoreceptor output (Zehr and Stein 1999; Nakajima et al. 2006; Perry et al. 2008; Zehr et al. 2014; Robb and Perry 2022), the experimental attenuation of sensory inputs appears to impair locomotor strategies and highlights the importance of this cutaneous feedback.
A common method of attenuating cutaneous feedback is by placing ice directly onto foot sole skin (experimental hypothermia). The underlying assumption, as supported in several studies that have previously iced cutaneous tissue (Perry et al. 2000; Nurse and Nigg 2001; Eils et al. 2004), is that ice applied to skin reduces feedback from cutaneous mechanoreceptors (Lowrey et al. 2013) while leaving other sensory inputs, such as feedback from muscle spindles and Golgi tendon organs intact. During gait, the removal of cutaneous feedback along the entire foot sole has been demonstrated to reduce walking velocity (McDonnell and Warden-Flood 2000; Taylor et al. 2004) and significantly reduce the ground reaction forces at initial contact and toe-off phases of gait. These mechanical changes have been described as a more ‘cautious gait pattern’ (Eils et al. 2004). The ankle remains more dorsiflexed, the knee increases in flexion, tibialis anterior (TA) increases in activation, and medial gastrocnemius (MG) muscle activity is reduced at initial contact (Eils et al. 2004; Hohne et al. 2012). Peak pressures under the forefoot are reduced at toe-off (Eils et al. 2002; Taylor et al. 2004) with marked increases in soleus and TA activation (Hohne et al. 2012). More interestingly, the removal of cutaneous feedback under specific areas of the foot sole results in plantar pressure shifts to areas of normal cutaneous sensation. For example, the experimental attenuation of cutaneous feedback under the forefoot increases pressures under the rearfoot, and vice versa, with attenuation under the rearfoot increasing pressure under the forefoot (Nurse and Nigg 2001). Consequently, cutaneous feedback is evidently important and our CNS seeks and relies on cutaneous feedback in the regulation of normal human walking.
Despite understanding how global attenuation of cutaneous feedback modifies locomotion (attenuation to the entire foot sole, entire forefoot or rearfoot), it remains unknown if the attenuation to specific areas under the foot sole modifies locomotor patterns differently across site. Therefore, the purpose of this study was to attenuate cutaneous feedback from different areas of the foot sole and investigate the kinematic and muscle activity changes during steady-state locomotion. Microneurographic recordings have recently mapped the regional variability of cutaneous afferent firing thresholds, receptive field sizes, afferent distribution and density across nine foot sole regions (Strzalkowski et al. 2018). When considering the unique skin characteristics along the foot sole, it is hypothesized that kinematic and muscle activity modulation will be unique to the area of cutaneous feedback attenuation.
Methods
Participants
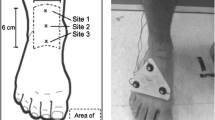
Convenience sampling around the local university permitted for the recruitment of 48 participants (23.3 ± 4.1 years; 15 males, 33 females; 172.7 ± 8.2 cm; 70.8 ± 15.0 kg) for this experimental protocol. All participants completed a screening questionnaire and were excluded if they self-reported a neurological and/or musculoskeletal disorder, vestibular and/or balance impairments or a history of frostbite to either foot. Normal tactile sensory thresholds (below 1.65 filament corresponding to 0.008 g force, Semmes–Weinstein monofilaments, North Coast Medical, Inc., Morgan Hill, CA) on the foot sole was also required and confirmed prior to participation. Five foot sole sites were evaluated: medial and lateral forefoot, medial and lateral midfoot and central calcaneus. Informed consent was provided by all participants and the university’s ethics review board (REB#5583) approved the protocol prior to data collection.
Instrumentation
Kinetic and electromyography (EMG) data (1000 Hz) were collected using AMTI force plates (OR6-5-2000; AMTI, Watertown, Massachusetts, USA) and a wireless EMG collection system (Ultium, Noraxon, Scottsdale, AZ, USA). This EMG protocol has been previously described elsewhere (Robb and Perry 2022). Briefly, muscle activity was collected in eight muscles: four lower leg muscles with surface EMG (TA, MG, peroneus longus (PL), and extensor digitorum longus (EDL)) and four muscles with fine-wire EMG (tibialis posterior (TP), extensor hallucis longus (EHL), flexor digitorum longus (FDL) and flexor hallucis longus (FHL)). Two surface and two fine-wire insertions were performed per leg and the selection of muscles per left and right leg rotated across participants.
Surface landmarking initially identified the sites of electrode placement. Hair was removed from the lower leg and skin was cleaned with Nuprep abrasive gel. Following SENIAM guidelines (Hermens et al. 1999), disposable bipolar surface electrodes (HEX 272S, Ag/AgCL, Noraxon, USA, Inc.) were placed on each muscle belly at an inter-electrode distance of 2 cm. While monitoring live EMG signals, the integrity of each signal was confirmed with active resisted muscle testing of the TA, PL, MG and EDL. All fine-wire insertions were performed under ultrasound guidance (Eco 6, CHISON Medical Technologies Co., Ltd) (Perotto 2011). Paired fine-wire electrodes (Chalgren Enterprises, Inc., [30 mm (1.25″) × 27 g] (000-318-130); 50 mm (2.00″) × 25 g] (000-318-150)) punctured the skin surface and were carefully guided into the TP, EHL, FDL and FHL muscles.
Following the EMG setup and participants self-reported comfort to stand upright, infrared emitting diode markers were placed on 12 anatomical landmarks: the xyphoid, center of the frontal bone, bilateral acromions, anterior superior iliac spines, ankle joints and 3rd metatarsal heads. Kinematic data was sampled at 100 Hz and collected with the Optotrak Certus motion capture system (Northern Digital Inc., Waterloo, Ontario, CAN).
Experimental protocol
Each participant was appropriately sized to a prefabricated insole (D609561, Sole Thin Sport, Edge Marketing Corp; Alberta, AB, Calgary) and bare feet were placed inside Rockport WT casual dress shoes. Next, ten self-selected velocity walking trials were performed (normal sensory condition) prior to icing one of five locations under the foot sole. Along the walking trajectory, participants contacted two force plates on steps 3 and 4 respectfully. Randomly across participants, ice was placed under bilateral foot soles to either the medial forefoot, lateral forefoot, medial midfoot, lateral midfoot, or calcaneus (Fig. 1). Participants were asked to sit in a chair with both knees at a 90° angle. With both feet touching the floor, a hard, square ice pack was carefully placed to the specific region under each foot. The ice packs were held in place by the participant between the floor and the bottom of their feet. During the icing protocol, the experimenter monitored the participant to ensure the ice pack remained cool and that participants were not moving around on the ice and/or offloading the foot from the ice. The ice pack remained in contact with the specific area of each foot for 15 min and tactile thresholds (cutaneous reduction site only) were re-evaluated with Semmes Weinstein (North Coast Medical, Inc., Morgan Hill, CA) monofilaments. It should be noted that this icing protocol was intended to attenuate the cutaneous sensation from distinct regions under the foot sole and assumed to isolate the effects of reduced skin input while not altering foot and ankle proprioception. Ice was returned under the foot for an additional minute prior to placing feet inside the footwear (to minimize any temperature increases to foot sole skin during the monofilament testing). Five walking trials were performed immediately following the icing protocol (< 30 s following footwear application). If participants experienced delays (example: shoelaces untied) during these five walking trials, subjectively reported their sensation returning, or verbally commented that their feet were ‘feeling warmer’, footwear was removed and ice was returned to their foot soles prior to resuming collection. Tactile thresholds were re-evaluated immediately following the final walking trial. Given our sample size of 48 participants, each region of sensory reduction had 9–10 participants per icing group.
The topographical division of each region of foot sole cutaneous attenuation. The forefoot (A—lateral forefoot, B—medial forefoot) was divided from the midfoot (C—lateral midfoot, D—medial midfoot) proximal to the metatarsal head. The medial and lateral forefoot was divided at the second metatarsal webspace to the distal aspect of the natural curvature of the medial longitudinal arch. The calcaneus (E) was divided from the midfoot at the distal aspect of the calcaneus bone on the plantar surface of the foot
Data acquisition and analysis
To interpolate small gaps in marker trajectories, a cubic spline function processed kinematic data in a custom-made Optofix software. Data was then filtered with 6 Hz low-pass dual-pass Butterworth filter. The sagittal plane kinematics of the hip, knee and ankle were calculated from the anterior–posterior and vertical coordinates of the markers. A static stance trial at the beginning of each testing session standardized the zero-reference position for each joint. Kinematic markers on the metatarsals and ankle joints, relative to the force plates, allowed us to identify five zones of the foot where the CoP could reside. These corresponded to the cutaneous reduction areas under the foot sole. This CoP analysis was selected to represent the percentage of time (% CoP) in single stance when the CoP trajectory deviated from the area of cutaneous reduction under the foot sole. EMG signals were amplified at a gain 500 × and band pass filtered between 10 and 500 Hz. Then post-collection EMG signals were unbiased, full-wave rectified, and linear enveloped with a 40 Hz dual-pass Butterworth filter. Fine-wire EMG signals that were saturated with noise or demonstrated poor signal quality were removed from the dataset. This was quite rare (< 5% of data) at this stage of data processing, as most signal errors were corrected during live collection. The gait cycle (100%) was divided into ten equal sized epochs, where each epoch represented 10% of the gait cycle. Data were analyzed for epochs 1–6 representing the stance phase of gait. Within each epoch, for each trial per participant, the average EMG (aEMG) data were normalized to the peak EMG (%Pk) of each muscle within 100% of that same walking trial.
Statistical analysis
Repeated measures analysis of variance (SAS University Edition, 2.8.1, version 9.4) were run for each variable (normalized EMG of each muscle per epoch of the gait cycle; % CoP; gait parameters; and hip, knee and ankle kinematics) by factor of the location of cutaneous attenuation (medial forefoot, lateral forefoot, calcaneus, medial midfoot, lateral midfoot). The 10% epochs were labeled as distinct phases of stance: epoch 1: initial contact, epoch 2: loading response, epoch 3: midstance, epoch 4: heel rise, epoch 5: propulsion, epoch 6: toe-off. All data met normality and Tukey’s honest significant difference (HSD) post hoc comparisons were run when main effects were significantly different. Statistical significance was determined at p < 0.05 a priori.
Results
To investigate the effects of attenuating cutaneous feedback under distinct regions of the foot sole, changes in muscle activity, % CoP, sagittal plane kinematics, and gait parameter results (walking velocity, step length and step width) for each region of cutaneous attenuation were compared to the normal walking condition. Complete results are provided in Tables 1, 2, 3, and 4. The monofilament tactile thresholds increased from baseline (prior to ice) to immediately post-icing confirming that the protocol successfully reduced cutaneous feedback from the foot sole. Furthermore, monofilament testing immediately following the walking trials had minimally changed from the start of the sessions (post-icing) indicating sustained reduction across the duration of all trials (Fig. 2). With the exception of ice applied to the lateral midfoot (which also significantly reduced step length), walking velocity was slightly but significantly increased (Table 4) when cutaneous feedback was reduced under all foot sole regions (F5,96 = 23.83, p < 0.0001).
Attenuation of cutaneous feedback under the forefoot
When cutaneous feedback was attenuated under the medial forefoot, participants walked with significantly reduced hip (F5,96 = 17.40, p < 0.0001) and knee extension (F5,95 = 17.84, p < 0.0001), increased ankle dorsiflexion (F5,92 = 7.16, p < 0.0001), and reduced ankle plantarflexion (F5,92 = 6.55, p < 0.0001), compared to walking with normal cutaneous feedback. Surprisingly, the percentage of time that pressure remained under the medial forefoot significantly increased (F5,96 = 2.41, p = 0.0351) with the attenuation of cutaneous feedback to this region. The magnitude of MG muscle activity was significantly greater at initial contact (F5,96 = 5.57, p < 0.0001), loading response (F5,96 = 5.26, p = 0.0001), and heel rise (F5,96 = 8.16, p < 0.0001) phases of gait (Fig. 3). TP muscle activity was significantly reduced at initial contact (F5,76 = 11.13, p < 0.0001), whereas TA activity was significantly greater at propulsion (F5,96 = 8.21, p < 0.0001) and toe-off (F5,96 = 8.00, p < 0.0001) (Fig. 4a, b) compared to walking with normal cutaneous feedback.
The attenuation of cutaneous feedback under the lateral forefoot resulted in participants walking with increased hip range of motion (F5,96 = 10.87, p < 0.0001), increased knee extension (F5,95 = 17.84, p < 0.0001), and reduced ankle plantarflexion (F5,92 = 7.54, p < 0.0001). No significant changes in % of COP were observed. EHL muscle activity was significantly greater at initial contact (F5,85 = 5.12, p = 0.0001) compared to walking with normal sensation. During midstance (F5,96 = 4.41, p = 0.0006) and heel rise (F5,96 = 3.83, p = 0.002), PL activation was significantly greater, whereas MG activation was significantly reduced during the loading response (F5,95 = 5.26, p = 0.0001) and heel rise (F5,95 = 8.16, p < 0.0001) phases of gait (Fig. 3). In later stance, TA muscle activity was significantly reduced at propulsion (F5,96 = 8.21, p < 0.0001) (Fig. 4a) and TA (F5,96 = 8.00, p < 0.0001) and EHL (F5,86 = 8.81, p < 0.0001) were reduced at toe-off (Fig. 4b).
Attenuation of cutaneous feedback under the midfoot
When walking with reduced cutaneous feedback under the medial midfoot, participants demonstrated significantly less knee extension (F5,95 = 17.84, p < 0.0001) and reduced knee range of motion (F5,96 = 16.65, p < 0.0001) compared to walking with normal sensation. The percentage of CoP (F5,96 = 6.45, p < 0.0001) time under the lateral forefoot significantly increased and, comparatively, was significantly reduced under the calcaneus. At initial contact, TA (F5,96 = 1.65, p = 0.0048) muscle activity was significantly reduced and MG (F5,95 = 5.57, p < 0.0001) activation was significantly greater. During the loading response, EDL (F5,95 = 5.13, p = 0.0001) and MG (F5,95 = 5.26, p < 0.0001) were both significantly greater compared to walking with normal sensation. Nearing terminal stance, MG activity remained greater at heel rise (F5,96 = 8.16, p < 0.0001) (Fig. 3), along with TA activation at propulsion (F5,96 = 8.21, p < 0.0001) and toe-off (F5,96 = 8.00, p < 0.0001,) (Fig. 4a, b).
Under the lateral midfoot, when cutaneous feedback was attenuated participants walked with significantly less hip extension (F5,96 = 17.40, p < 0.0001), increased hip range (F5,96 = 10.87, p < 0.0001) of motion, and increased ankle dorsiflexion (F5,92 = 7.16, p < 0.0001) with reduced plantarflexion (F5,92 = 6.55, p < 0.0001). Pressure significantly increased (F5,96 = 6.21, p < 0.0001) under the medial forefoot, lateral forefoot, and calcaneus. At initial contact, PL (F5,96 = 8.65, p < 0.0001), TP (F5,76 = 11.13, p < 0.0001,), and MG (F5,95 = 5.57, p < 0.0001) muscle activity significantly increased. MG activation continued into the loading response (F5,95 = 5.26, p = 0.0001) of gait (Fig. 3). EDL significantly increased from midstance (F5,95 = 6.79, p < 0.0001), heel rise (F5,95 = 3.77, p = 0.0023) and into propulsion (F5,95 = 5.05, p = 0.0002). MG activation was also significantly greater at heel rise (F5,95 = 8.16, p < 0.0001) and propulsion (F5,95 = 7.03, p < 0.0001). TA and FHL were significantly reduced at propulsion (TA: F5,96 = 8.21, p < 0.0001; FHL: F5,74 = 3.51, p = 0.0042) into toe-off (TA: F5,96 = 8.00, p < 0.0001; FHL: F5,70 = 6.25, p < 0.0001) and significantly greater in FDL (propulsion: F5,81 = 2.24, p = 0.048; toe-off: F5,80 = 5.53, p < 0.0001).
Attenuation of cutaneous feedback under the calcaneus
Attenuating cutaneous feedback under the calcaneus resulted in increased hip range of motion (F5,96 = 10.87), increased ankle dorsiflexion (F5,92 = 7.16, p < 0.0001), and reduced ankle plantarflexion (F5,92 = 6.55, p < 0.0001) during walking. No significant changes in % of COP were observed. During loading response, PL (F5,96 = 3.94, p < 0.0016) and MG (F5,95 = 5.26, p = 0.0001) muscle activity significantly increased (Fig. 3), whereas TP (F5,76 = 5.61, p < 0.0001) activation significantly reduced. At heel rise and propulsion, TA activation (heel rise: F5,96 = 3.10, p = 0.009; propulsion: F5,96 = 8.21, p < 0.0001) increased, as TP activation (heel rise: F5,75 = 2.44, p = 0.0343; propulsion: F5,76 = 2.51, p = 0.0297) was significantly reduced. MG activation was also reduced at propulsion (F5,95 = 7.03, p < 0.0001). At toe-off, FHL activation (F5,70 = 6.25, p < 0.0001) was significantly reduced, whereas EHL was significantly increased (F5,86 = 8.81, p < 0.0001) compared to walking with normal sensation.
Discussion
The aim of this study was to independently attenuate cutaneous feedback from different areas of foot sole skin to investigate the consequential changes in walking behaviour when the foot sole had areas of reduced sensory feedback to the CNS. As hypothesized, the kinematic and muscle activity modulation was unique to the location of cutaneous attenuation, suggesting topographical differences in modulatory behavior based on the foot sole region of sensory impairment. With the exception of reduced feedback from the lateral midfoot, participants walked significantly faster when feedback was removed, contrary to a previously reported observation that reduced sensory feedback typically generates a more hesitant walking behavior (Eils et al. 2004). In our study, the removal of cutaneous feedback demonstrated the largest modulation to the TA at the terminal stance (propulsion and toe-off) and to the MG throughout the entire stance phase of gait.
Motor output modulation secondary to the attenuation of cutaneous input
Both the importance of sensory feedback and modulatory behavior of locomotor muscle activity has been well established (Winter 1983; Zehr and Stein 1999; Alfuth and Rosenbaum 2012). Cutaneous feedback provides an influx of sensory information to regulate spinal locomotor circuitry (Zehr and Stein 1999; Sinkjaer et al. 2000; Zehr et al. 2014) and motor output consists of muscular activation to contract concentrically, eccentrically, and to stabilize the foot as it progresses through different phases of gait. Thus, when cutaneous feedback is removed, one should expect motor output changes and/or velocity changes as a required adaptation to this reduction in cutaneous input. In static stance, reduced feedback from the entire foot sole has been demonstrated to increase triceps surae muscle activity as a mechanism of reducing CoP displacement (Billot et al. 2015). Despite growing our understanding of motor output changes when reducing cutaneous input during static balance control, the applicability of these results are minimal to our study’s protocol which tested participants during steady-state walking trials. During gait, the removal of cutaneous feedback from the rearfoot has been previously shown to increase load under the forefoot. This pressure redistribution is accompanied by increased MG activation and attributed to a reflexive response to load the forefoot at toe-off (Nurse and Nigg 2001). The findings of our study are not consistent with these results as the removal of calcaneus feedback reduced MG activation at propulsion and toe-off. In fact, after ice was applied under the calcaneus, the entire flexor group (MG, TP, FDL and FHL) reduced muscle activation at propulsion (Fig. 3). A possible explanation for this discrepancy is that the attenuation of calcaneus input in our study reduced the time that the CoP trajectory remained under the forefoot (Fig. 5), as opposed to increasing the time as observed in Nurse and Nigg (2001). Furthermore, the boundaries between the rearfoot and midfoot are not clearly demarked in Nurses’ protocol, whereas our protocol separated these regions of cutaneous attenuation; therefore, we may be comparing a larger region of sensory reduction in their study compared to ours. As cutaneous mechanoreceptor distribution and density varies across different regions of the foot sole (Strzalkowski et al. 2018), this is a likely explanation for the changes in pressure distribution. As minimal research has explored the role of topographically attenuating cutaneous input on muscle activity during gait, the following results remain novel to the scientific community, but challenges our ability to draw comparisons to previous literature.
A visual interpretation of the center of pressure (CoP) percentage (%) change (% CoP attenuated—%CoP normal) within each topographical region. Blue is representative of areas in which the % of CoP time was reduced compared to normal (no cutaneous attenuation), and red is representative of areas in which the % of CoP time was increased compared to normal. Each darker color is suggestive of larger reduction or increases within the region. For example, when cutaneous feedback was attenuated under the lateral forefoot, the % of CoP time under the lateral midfoot and calcaneus experienced a large pressure increase compared to normal sensory feedback. Conversely, when cutaneous feedback was attenuated under the lateral forefoot, the % of CoP time under the medial forefoot was largely reduced. (blue % change range, from darkest blue to lightest blue: > − 40, − 39 to − 20, − 19 to − 10, − 9 to − 1; red % change range, from lightest red to darkest red: + 1 to + 10, + 11 to + 20, > + 20)
The role of the ankle joint with the attenuation of cutaneous input
The topographic attenuation of cutaneous input from the foot sole appears to be largely modulated at the ankle joint. For example, in early stance (initial contact and loading response), MG muscle activity increased with the reduction of cutaneous feedback under the medial forefoot, and medial and lateral midfoot compared to walking with normal sensation. With the exception of the lateral midfoot, increases in walking velocity may partially be attributed to the accompanying EMG amplitude increases while also suggesting a more confident walking pattern (Table 4). This muscle activation remained consistently greater than walking with normal sensation in heel rise and the propulsive phases of stance. As the ankle experienced increased dorsiflexion (Table 3), the MG remained isometrically contracted to reduce load time under the areas of minimal cutaneous feedback (midfoot and medial forefoot). Interestingly at propulsion, MG activation was significantly reduced, accompanied by increases in TA, when cutaneous input was attenuated under the calcaneus and medial midfoot. These motor patterns are consistent with the principle that we search and rely on areas of normal sensory feedback to effectively navigate the external environment (Nurse et al. 2005). Another important observation is the variability in ankle kinematics compared to the hip and knee (Fig. 6). When changing the location of cutaneous attenuation under the foot sole, sagittal plane kinematics of the hip and knee joints demonstrated similar range of motion across gait. Conversely, the ankle joint experienced large fluctuations in response to different areas of cutaneous attenuation. Overall, the results of this study highlight the important role of the ankle joint, more specifically the TA and MG muscle activation, in response to changes in cutaneous input under the foot sole.
The attenuation of cutaneous feedback does not oppose cutaneous facilitation
Previous work in our laboratory has studied the effects of facilitating cutaneous input via the addition of texture under the foot sole to these same topographical regions (Robb and Perry 2022). It is worth noting that the reduction of cutaneous input to each region under the foot sole does not oppose the results of cutaneous afferent facilitation. The neurological mechanisms between electrical and mechanical stimulation are inherently different, and the reduction of cutaneous input is yet again a differing physiological phenomenon to foot sole skin regardless of the cutaneous input traveling through similar pathways. Although this study provides insight into understanding how reduced cutaneous sensation alters locomotion, we cannot assume that a region of skin with reduced sensory response that is secondary to neuropathy (or other medical conditions) would generate similar locomotor patterns as observed in this study. This may be a function of cutaneous receptor density across the foot sole skin (Strzalkowski et al. 2018, 2019) or the complexity of cutaneous afferent’s ability to modulate spinal (Fallon et al. 2005) and transcortical pathways (Gill et al. 2022) during locomotion. Despite this complexity, the results of this study generate a new avenue of research to compare clinical populations with impaired cutaneous feedback to distinct regions under the foot sole to kinematic and muscle activation changes during steady-state locomotion.
Limitations
It should be recognized that cutaneous hypothermia assumes that targeted anesthesia to foot sole skin removes feedback from skin receptors, although feedback from muscle spindles and Golgi tendon organs remains intact. This overall assumption may be flawed, as despite one study reporting a reduction in cutaneous afferent discharge rates following an icing protocol (Lowrey et al. 2013), the discharge properties of proprioceptors of the foot immediately following cutaneous hypothermia have yet to be investigated. Thus, it remains plausible that the kinematic and muscle activity changes observed in this study are also influenced by reduced proprioception and local hypothermia changes affecting intrinsic foot muscles during gait.
Conclusion
Despite this limitation, the results of this study highlight the topographical organization of foot sole skin in response to the attenuation of cutaneous feedback from different areas of the foot sole. Furthermore, this manuscript focuses on changes to the ankle joint following cutaneous hypothermia, although researchers and clinicians should appreciate the complexity of this experimental protocol and EMG fluctuations were also observed in the deep compartment posterior muscles (FDL, FHL and TP) throughout stance (Fig. 3). Overall, by understanding how lower limb muscle activity changes with topographically facilitated (Robb and Perry 2022) and reduced feedback from foot sole skin, these results can assist in the development of new clinical interventions within the footwear and orthotic industry. In conclusion, this study provides benchmark data to compare gait modulation in neurological and/or diseased populations experiencing sensory loss under distinct areas of the foot sole.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abraira VE, Ginty DD (2013) The sensory neurons of touch. Neuron 79:618–639. https://doi.org/10.1016/j.neuron.2013.07.051
Alfuth M, Rosenbaum D (2012) Effects of changes in plantar sensory feedback on human gait characteristics: a systematic review. Footwear Sci 4:1–22
Assländer L, Peterka RJ (2014) Sensory reweighting dynamics in human postural control. J Neurophysiol 111:1852–1864. https://doi.org/10.1152/jn.00669.2013
Billot M, Handrigan GA, Simoneau M, Teasdale N (2015) Reduced plantar sole sensitivity induces balance control modifications to compensate ankle tendon vibration and vision deprivation. J Electromyogr Kinesiol 25:155–160. https://doi.org/10.1016/j.jelekin.2014.06.003
Eils E, Nolte S, Tewes M et al (2002) Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech 35:1307–1313. https://doi.org/10.1016/S0021-9290(02)00168-9
Eils E, Behrens S, Mers O et al (2004) Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20:54–60. https://doi.org/10.1016/S0966-6362(03)00095-X
Fallon JB, Bent LR, McNulty PA, Macefield VG (2005) Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol 94:3795–3804. https://doi.org/10.1152/jn.00359.2005
Geffeney SL, Goodman MB (2012) How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 74:609–619. https://doi.org/10.1016/j.neuron.2012.04.023
Gill G, Forman DA, Bent LR, Taylor JL (2022) Location-specific cutaneous electrical stimulation of the footsole modulates corticospinal excitability to the plantarflexors and dorsiflexors during standing. Physiol Rep 10:1–15. https://doi.org/10.14814/phy2.15240
Hao J, Bonnet C, Amsalem M et al (2015) Transduction and encoding sensory information by skin mechanoreceptors. Eur J Physiol 467:109–119. https://doi.org/10.1007/s00424-014-1651-7
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G (1999) European recommendations for surface electromyography: results of the SENIAM project. Roessingh Research and Development, Enschede
Hohne A, Ali S, Stark C, Brüggemann G-P (2012) Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. Eur J Appl Physiol 112:3829–3838. https://doi.org/10.1007/s00421-012-2364-2
Lowrey CR, Strzalkowski NDJ, Bent LR (2013) Cooling reduces the cutaneous afferent firing response to vibratory stimuli in glabrous skin of the human foot sole. J Neurophysiol 109:839–850. https://doi.org/10.1152/jn.00381.2012
Macefield VG (1998) The signaling of touch, finger movements and manipulation forces by mechanoreceptors in human skin. In: Morley JW (ed) Neural aspects of tactile sensation. Elsevier Science Publishers, Amsterdam, pp 89–130
McDonnell M, Warden-Flood A (2000) Effect of partial foot anaesthesia on normal gait. Aust J Physiother 46:115–120. https://doi.org/10.1016/S0004-9514(14)60319-6
McGlone F, Reilly D (2010) The cutaneous sensory system. Neurosci Biobehav Rev 34:148–159. https://doi.org/10.1016/j.neubiorev.2009.08.004
Mckeon P, Hertel J (2007) Diminished plantar cutaneous sensation and postural control. Percept Mot Skills 104:56–66
Nakajima T, Sakamoto M, Tazoe T, Endoh T (2006) Location specificity of plantar cutaneous reflexes involving lower limb muscles in humans. Exp Brain Res 175:514–525. https://doi.org/10.1007/s00221-006-0568-6
Nurse MA, Nigg BM (2001) The effect of changes in foot sensation on plantar pressure and muscle activity. Clin Biomech 16:719–727. https://doi.org/10.1016/S0268-0033(01)00090-0
Nurse MA, Hulliger M, Wakeling JM et al (2005) Changing the texture of footwear can alter gait patterns. J Electromyogr Kinesiol 15:496–506. https://doi.org/10.1016/j.jelekin.2004.12.003
Perotto A (2011) Anatomical guide for the electromyographer - the limbs and trunk, 5th edn. Charles C Thomas Springfield, Illinois
Perry SD, McIlroy WE, Maki BE (2000) The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res 877:401–406. https://doi.org/10.1016/S0006-8993(00)02712-8
Perry SD, Radtke A, McIlroy WE et al (2008) Efficacy and effectiveness of a balance-enhancing insole. J Gerontol 63:595–602. https://doi.org/10.1093/gerona/63.6.595
Ray CT, Horvat M, Croce R et al (2008) The impact of vision loss on postural stability and balance strategies in individuals with profound vision loss. Gait Posture 28:58–61. https://doi.org/10.1016/j.gaitpost.2007.09.010
Robb KA, Perry SD (2022) The effect of texture under distinct regions of the foot sole on human locomotion. Exp Brain Res. https://doi.org/10.1007/s00221-022-06402-x
Sarabon N, Rosker J, Loefler S, Kern H (2013) The effect of vision elimination during quiet stance tasks with different feet positions. Gait Posture 38:708–711. https://doi.org/10.1016/j.gaitpost.2013.03.005
Sinkjaer T, Andersen JB, Ladouceur M et al (2000) Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523:817–827
Sozzi S, Monti A, Marco A et al (2011) Sensori-motor integration during stance : time adaptation of control mechanisms on adding or removing vision. Hum Mov Sci 30:172–189. https://doi.org/10.1016/j.humov.2010.06.002
Strzalkowski NDJ, Peters RM, Inglis JT, Bent LR (2018) Cutaneous afferent innervation of the human foot sole: what can we learn from single-unit recordings? J Neurophysiol 120:1233–1246. https://doi.org/10.1152/jn.00848.2017
Strzalkowski NDJ, Ali RA, Bent LR (2019) The firing characteristics of foot sole cutaneous mechanoreceptor afferents in response to vibration stimuli. J Neurophysiol. https://doi.org/10.1152/jn.00647.2016
Taylor AJ, Menz HB, Keenan AM (2004) Effects of experimentally induced plantar insensitivity on forces and pressures under the foot during normal walking. Gait Posture 20:232–237. https://doi.org/10.1016/j.gaitpost.2003.02.001
Wang TY, Lin SI (2008) Sensitivity of plantar cutaneous sensation and postural stability. Clin Biomech 23:493–499. https://doi.org/10.1016/j.clinbiomech.2007.11.014
Winter DA (1983) Biomechanical motor patterns in normal walking. J Mot Behav 15:302–330. https://doi.org/10.1080/00222895.1983.10735302
Zehr EP, Stein RB (1999) What functions do reflexes serve during human locomotion? Prog Neurobiol 58:185–205. https://doi.org/10.1016/S0301-0082(98)00081-1
Zehr P, Nakajima T, Barss T et al (2014) Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci Med Rehabil 6:33. https://doi.org/10.1186/2052-1847-6-33
Zimmerman A, Bai L, Ginty DD (2014) The gentle touch receptors of mammalian skin. Science 346:950–954. https://doi.org/10.1126/science.1254229
Acknowledgements
This work was supported by the Natural Science and Engineer Research Council of Canada (NSERC) Discovery Grant [RGPIN-2015-06481] and the Canadian Foundation for Innovation [5141].
Funding
This article is funded by Pedorthic Research Foundation of Canada, Natural Science and Engineer Research Council of Canada, RGPIN-2015-06481, Stephen D. Perry, Canadian Foundation for Innovation, 5141, Stephen D. Perry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robb, K.A., Perry, S.D. The topographical attenuation of cutaneous input is modulated at the ankle joint during gait. Exp Brain Res 242, 149–161 (2024). https://doi.org/10.1007/s00221-023-06737-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06737-z