Abstract
Limb-kinetic apraxia, the loss of the ability to make precise, independent but coordinated finger and hand movements affects quality of life in patients with Parkinson’s disease. We aimed to examine the effects of anodal transcranial direct current stimulation of the left posterior parietal cortex and upper extremity motor practice on limb-kinetic apraxia in Parkinson’s disease. This study was conducted in a randomized, double-blind, sham-controlled fashion. Patients confirmed to have Parkinson’s disease were recruited. Twenty-eight participants completed the study and were randomized to two groups: anodal or sham stimulation. For participants assigned to active stimulation, anodal stimulation of the left posterior parietal cortex was performed using 2 mA current for 20 min. Patients received anodal or sham stimulation, followed by motor practice in both groups. The primary outcome measure was time-performing sequential buttoning and unbuttoning, and several secondary outcome measures were obtained. A statistically significant interaction between stimulation type and timepoint on time taken to perform buttoning and unbuttoning was found. Patients who received anodal stimulation were found to have a significant decrease in sequential buttoning and unbuttoning time immediately following stimulation and at 24 h in the medication-ON state, compared to the medication-OFF state (31% and 29% decrease, respectively). Anodal stimulation of the left posterior parietal cortex prior to motor practice appears to be effective for limb-kinetic apraxia in Parkinson’s disease. Future long-term, multi-session studies looking at the long-term effects of anodal stimulation and motor practice on limb-kinetic apraxia in Parkinson's disease may be worthwhile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder (the most common being Alzheimer’s disease) (Ascherio and Schwarzschild 2016). While symptoms of PD such as tremor, bradykinesia, and postural instability are easily noted, other symptoms that affect patients’ quality of life are often present, but may not undergo clinical evaluation (Foki et al. 2016). An example of this is apraxia, a phenomenon characterized as difficulty performing skilled or learned movements, which is also seen in other neurodegenerative disorders, including Alzheimer’s disease (Park 2017). Limb-kinetic apraxia is a subtype of apraxia that is characterized by the loss of the ability to make precise, independent but coordinated finger and hand movements (Park 2017). This type of apraxia is additive to the difficulties patients have due to bradykinesia. Therefore, patients with limb-kinetic apraxia experience difficulty performing their activities of daily living that require dexterity, such as buttoning and unbuttoning, using mobile phones and keyboards (Foki et al. 2016). Limb-kinetic apraxia not only affects quality of life, but can also be an independent predictor for quality of life in PD patients (Foki et al. 2016; Vanbellingen et al. 2018).
To date, anatomical and functional neuroimaging study results have indicated that the left posterior parietal, temporal, supplementary motor, premotor, and motor cortices are relevant in many aspects of praxis (Ogawa and Imai 2016; Kubel et al. 2017, 2018). The posterior parietal cortex (PPC) in particular has received much attention, as many studies suggest that it plays a major role in the voluntary control of upper limb movement (Filimon 2010). The PPC appears to be involved in shaping of the hand (regardless of whether or not there is a graspable object) (Klaes et al. 2015; Rathelot et al. 2017). Studies using noninvasive brain stimulation methods have attempted to stimulate brain regions considered to be relevant in various types of praxis (Bolognini et al. 2015; Bianchi et al. 2015). Noninvasive brain stimulation has also been used to prime potential effects of exercise in patient populations such as PD and stroke (Allman et al. 2016). Anodal stimulation of the primary motor cortex has been reported to improve manual dexterity in healthy human subjects (Pavlova et al. 2014). This beneficial effect appears to be present in both young and elderly adult groups when combined with motor training (Parikh and Cole 2014). In patients with stroke, anodal stimulation with or without upper extremity exercise has been found to improve manual dexterity (Fusco et al. 2014). Studies using anodal tDCS have found that stimulation of other movement-related cortical areas combined with physical training results in synergistic effects (Wang et al. 2021). In this study, we attempted to ameliorate symptoms of limb-kinetic apraxia in patients with PD by delivering anodal transcranial direct current stimulation (tDCS), a method of excitatory noninvasive brain stimulation to the left posterior parietal cortex followed by motor practice of the contralateral upper extremity.
Materials and methods
Subjects
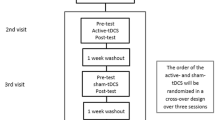
This study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital, and all study participants gave written informed consent. Patients confirmed to have PD according to the UK Parkinson’s Disease Brain Bank Diagnostic Criteria were enrolled. Inclusion criteria included the following: right-handedness, age 30–90 years, and Mini-Mental Status Examination (MMSE) score of at least 18. As the study involved the use of transcranial magnetic stimulation (TMS) for obtaining neurophysiological parameters, the following exclusion criteria were applied: history of seizures or epilepsy, metal in the eye or skull, presence of levodopa-induced dyskinesias, or history of having received deep brain stimulation. Thirty patients were recruited, and 28 patients completed the study. Two patients were withdrawn for reasons due to insufficient data collection and poor compliance. No major side effects occurred during the entire length of the study. See Fig. 1 for the study design.
Randomization and blinding
This study was conducted in a randomized, double-blind, sham-controlled fashion. Patients were randomized to either of the two groups: anodal or sham stimulation. An investigator (H.R.J.) who was not actively involved in the care of patients was responsible for the randomization. Once group allocation was determined via randomization, a code that determined the type of stimulation (anodal or sham) was programmed into the stimulator. The stimulator used in this study is capable of both active and sham stimulation (neuroConn DC-stimulator Plus, neuroConn, Ilmenau, Germany). All other investigators (including the rater) and patients were blinded to group allocation.
Transcranial direct current stimulation and motor practice
Transcranial direct current stimulation was delivered by a battery-driven current stimulator (neuroConn DC-stimulator Plus, neuroConn, Ilmenau, Germany). Two 5 × 7 cm2 tDCS electrodes were attached; the anodal electrode to the area corresponding to P3 according to the 10:20 electroencephalographic (EEG) system and the reference electrode to the right deltoid muscle. Participants received anodal or sham stimulation depending on their assigned group allocation, as previously noted. Of note, the stimulator operates such that sham stimulation also creates an initial brief ramping-up of electrical current, which is helpful so that the recipient is blinded to the type of stimulation. 1.5 mA current intensity was used for anodal stimulation, with fade-in/fade-out phases of 8 s each. All participants received 20 min of stimulation in the medication-ON state, with an investigator monitoring the stimulation session to ensure alertness.
Participants were assessed using a variety of measures in the following order: prior to stimulation, in the medication-OFF state (> 12 h since medication cessation), medication-ON state (30 min to 1 h following levodopa intake), 45 min following tDCS (“tDCS-immediate”), and 24 h post-tDCS. Participants were in the medication-ON state for both tDCS-immediate and 24 h post-tDCS evaluation. They were instructed to sequentially button and unbutton their hospital gown, which had five buttons aligned vertically (button diameter: 1.9 mm, 9.5 cm inter-button distance). All participants underwent evaluation using the Unified Parkinson's Disease Rating Scale (UPDRS) and the apraxia screen of test of upper limb apraxia (AST) (Vanbellingen et al. 2011; Movement Disorder Society Task Force on Rating Scales for Parkinson’s 2003). The AST was performed to confirm the absence of concurrent ideomotor apraxia. Participants also performed other manual activities including writing their name ten times using their dominant hand and flipping a coin ten times (100 Korean won coin: diameter of 2.5 cm) with their dominant and non-dominant hand, respectively. The time for completion of ten rotations represented the outcome measure, and participants picked up the coin in the case of drops. When the participant dropped the coin, the coin flip associated with the drop was excluded, and the participant resumed the coin flipping. The time measurement was discontinued and resumed when the coin was back in the starting position. 12-m gait examination was also conducted and videotaped for offline review.
All participants, regardless of the type of stimulation, underwent motor practice following the 20-min stimulation session, approximately 10 min after the end of stimulation. This motor practice was comprised of the activities described above (buttoning and unbuttoning, writing, and flipping a coin). These manual activities were repeated three times each. The time taken to perform these activities was not analyzed as the purpose was for motor practice. Following completion of this, participants were instructed to perform these activities in an order that minimized a practice effect and were videotaped for offline assessment.
All procedures took place during the participants’ in-hospital stay, to maintain a constant environment within and across all participants. At each time point (medication-OFF, ON, tDCS-immediate, and tDCS at 24 h), evaluations included UPDRS, buttoning/unbuttoning, writing, flipping a coin, 12-m gait, and resting motor threshold (RMT) obtained by TMS. The final evaluation was performed 24 h following the tDCS session on the second day of the hospital stay.
Transcranial magnetic stimulation
RMT measurements were obtained pre-, post, immediately, and 24 h following tDCS, using TMS (Magstim BiStim2, Magstim Company Ltd., Whitland, Dyfed, UK) and electromyography (EMG: Medelec Synergy, Oxford Instruments Medical, Inc. Surrey, UK) surface electrodes. The active surface electrode was placed over the muscle belly of the right first dorsal interosseous muscle, and the inactive surface electrode was placed over the metacarpophalangeal joint of the right thumb. The ground electrode was placed on the dorsum of the right hand. The motor hotspot was then found using a figure-of-eight TMS coil (external diameter of 70 mm) placed on the surface of the head corresponding to the left primary motor cortex, with the coil handle pointing backward and laterally at a 45° angle away from midline. This hotspot was found functionally by stimulating the area corresponding to the left primary motor cortex that evoked the largest MEP in the contralateral FDI; this spot was marked on a cap to ensure proper coil placement throughout the TMS procedure. RMT is defined as the minimum stimulation intensity producing motor-evoked potential (MEP) amplitude of 50 µV, in 5 out of 10 trials. Once the RMT was determined, MEPs were obtained with a figure-of-eight TMS coil (external diameter of 70 mm) placed on the surface of the head corresponding to the left primary motor cortex, using a stimulation intensity of 120% RMT.
Outcomes
The primary outcome measure was time taken to perform sequential buttoning and unbuttoning, as this is often a task performed daily in individuals, reflecting an activity representative of praxis. Secondary outcome measures included the UPDRS scores, RMT, time writing one’s name ten times, time flipping a coin using each hand, and time taken to walk 12 m, etc.
Statistical analysis
A power analysis was conducted prior to the study to determine sample size. Based on data obtained from a previous study, we postulated that buttoning and unbuttoning time might be 63 s in the sham tDCS group and 48 s in the active stimulation, with a standard deviation of 19 and 11 s, respectively (Park 2018). A sample size analysis was performed using a two-sample t test to achieve a significance level of 0.05% and 80% power, and a total of 12 participants were required for each group. To account for potential dropouts, a total requested accrual number of 30 was determined, with an aim to complete at least 24 participants.
Results
Twenty-eight participants completed the study, and the mean age in the active and sham group was 73 and 72 years (standard error [SE]: 5 and 9 years), respectively. Details of participants in both groups are shown in Table 1. Mean disease durations were 37 months and 41 months and mean UPDRS part I/II scores in the active and sham group were 4/11 and 3/9, in the active and sham group, respectively. Mean MMSE scores in the two groups were 25 and 26, respectively.
Mixed analysis of variance (ANOVA) was used to analyze the time taken to perform buttoning and unbuttoning data. There was a statistically significant interaction between the stimulation type and timepoint on time taken to perform buttoning and unbuttoning (F[3,78] = 3.520, p = 0.019, partial η2 = 0.119). Post hoc pairwise comparisons revealed that in the active stimulation group, there was a significant decrease in sequential buttoning and unbuttoning time immediately following tDCS and at 24 h, compared to the medication-OFF state (31% and 29% decrease, respectively, p = 0.001: see Fig. 2). Mixed ANOVA was also performed for the UPDRS part III and upper extremity scores, and no statistically significant group differences were found: F(1.650, 42.904) = 17.071, p = 0.277, partial η2 = 0.048 for UPDRS part III scores and F(3, 78) = 5.390, p = 0.075, partial η2 = 0.084 for upper extremity scores.
Sequential buttoning and unbuttoning time following active and sham stimulation. A Time taken to perform sequential buttoning and unbuttoning are shown for the two groups. The anodal stimulation group was found to have significant differences in OFF vs. tDCS-immediate, and OFF vs. tDCS-24 h. B The change in buttoning and unbuttoning time are shown in ratios
Writing time (time taken to write one’s name 10 times sequentially) data were also analyzed using a mixed two-way-ANOVA, which did not show a statistically significant group difference (F[2.195, 57.061] = 0.441, p = 0.664, partial η2 = 0.017). No group differences were found (F[3,78] = 8.167, p = 0.167, partial η2 = 0.062) for time flipping a coin ten times with the right hand. No statistically significant results were found for other secondary outcome measures including RMT, 12 m-gait time, and time flipping the coin with the left hand.
Discussion
Limb-kinetic apraxia is characterized by difficulty making independent but coordinated finger and hand movements (Quencer et al. 2007). As this phenomenon is often overlooked in the clinic as a matter of clumsiness, it is seldom addressed and no specific treatment is available (Vanbellingen et al. 2017). However, impaired dexterity can impact the quality of patients’ lives, as activities of daily living often require the use of the upper limb (Vanbellingen et al. 2018). This phenomenon has been observed in a variety of neurodegenerative disorders including PD and has been found to be independent of bradykinesia (Foki et al. 2015). While anodal tDCS of the primary motor cortex has been applied to patients with PD and improvement of bradykinesia has been observed, to date, few studies have addressed the effects of tDCS on limb-kinetic apraxia (Fregni et al. 2006).
The main purpose of our study was to assess the effects of combining anodal tDCS of the left posterior parietal cortex with motor practice on limb-kinetic apraxia by observing changes in the time taken to perform manual tasks, such as sequential buttoning and unbuttoning, flipping a coin using both the dominant and non-dominant hand, and writing one’s name. While there are several available measures that are used to assess dexterity, we chose these tasks among those available as they reflect some of the daily activities that require fine motor skills of the upper extremity (Foki et al. 2016). Subjects underwent motor practice shortly following tDCS (approximately 10 min after the end of stimulation), taking into account the duration of increased motor cortical excitability following tDCS in humans (Nitsche and Paulus 2001). Only the group of patients who received active (i.e., anodal) stimulation were found to have significant improvement in sequential buttoning and unbuttoning, but not other manual tasks (flipping a coin or writing). Therefore, we conclude that the significant improvement in sequential buttoning and unbuttoning is a change independent of bradykinesia that was found to be unchanged in both groups, as shown by the lack of significant differences in UPDRS part III and upper extremity scores. As previous study results suggest that the parietal cortex is involved in the control of upper limb movements, it is possible that in our subjects who received active stimulation of this brain region prior to motor practice, stimulation resulted in sustained effects of improvement in limb-kinetic apraxia seen at up to 24 h. The lack of improvement for other manual tasks might indicate that the ability to flip a coin or write (activities generally regarded to require fine motor skill), may remain intact in limb-kinetic apraxia.
Noninvasive brain stimulation methods such as tDCS and TMS are often used to study or treat disorders in the field of neurology, psychiatry, and rehabilitation (Lefaucheur 2016). While effects of tDCS have been noted to be limited in duration, this method of noninvasive brain stimulation is often more easily tolerated than TMS, and has a more favorable side effect profile (Woods et al. 2016). Therefore, among the various noninvasive brain stimulation methods, tDCS has much potential in terms of its feasibility. The current understanding of the mechanism of tDCS is expanding and it is now thought that effects are widespread; both neuronal and non-neuronal cells including endothelial cells, lymphocytes, or glial cells appear to be affected (Jackson et al. 2016). Furthermore, the effects of tDCS are thought to be both immediate and remote; observations on effects persisting beyond the time frame of intervention have been reported (Nitsche and Paulus 2001; Nitsche et al. 2003). Even weak anodal tDCS has been shown to have relatively sustained effects in increasing motor cortical excitability, at up to 90 min (Nitsche and Paulus 2001). TDCS is also thought to involve change in a variety of neurotransmitters, resulting in glutamatergic, GABAergic, serotonergic, dopaminergic, and cholinergic activity modulation (Medeiros et al. 2012; Nitsche et al. 2006).
Studies using noninvasive brain stimulation methods such as tDCS or TMS commonly combine stimulation with motor practice of the corresponding body region. Examples include tDCS or TMS followed by treadmill training in patients with PD (Chung et al. 2020). While results are mixed among studies, there are reports of increased gait velocity and improved balance with a combination of tDCS and physical training (Kaski et al. 2014a, b). TDCS has also been combined with motor training of the upper extremity in healthy humans, and has been found to enhance motor learning (Galea and Celnik 2009). In patients with stroke, combining anodal or cathodal tDCS with motor learning resulted in improved manual performance, as shown by change in Jebsen Taylor hand function test scores (Fleming et al. 2017). Improvement in fine motor control following anodal tDCS of the primary motor cortex was also observed in patients with subacute/chronic stroke (Pavlova et al. 2020; Ilic et al. 2016). A study also found that tDCS appeared to boost handwriting performance when combined with upper extremity training in patients with PD (Broeder et al. 2019). Studies have also targeted movement-related cortical areas beyond the primary motor cortex, including the cerebellum, prefrontal cortex, and somatosensory cortices (Wang et al. 2021). Based on the results of these previous studies, we aimed to study the effects of anodal tDCS targeting the posterior parietal cortex combined with motor practice in patients with PD.
Our study is not without limitations. We conducted a single-session tDCS session, and while a significant change in our primary outcome measure was found, studies conducting multiple stimulation sessions are necessary to further evaluate the effects. Furthermore, while participants were studied at four different time points, they were not followed up at a term interval longer than 24 h. Therefore, future studies employing multiple stimulation sessions with assessments at both short and long term are necessary to fully evaluate the effects of anodal tDCS and motor practice. While we found that only the group that received anodal stimulation had improvement in sequential buttoning and unbuttoning, we found that both groups had improvement in writing and flipping a coin (the latter, using the dominant hand). As these manual activities are also reflective of praxis, it is possible that the lack of a group difference may be a result of an insufficient sample size or number of stimulation sessions. Finally, while we aimed to study the effects of combining anodal tDCS with motor practice in this study, it would also be useful to study the effects of anodal tDCS alone on limb-kinetic apraxia.
In summary, anodal tDCS of the left posterior parietal cortex prior to motor practice appears to be effective for limb-kinetic apraxia in PD. Limb-kinetic apraxia is a phenomenon that deserves attention as it affects patients’ quality of life and is found not only in PD but also in other neurodegenerative diseases such as Alzheimer’s disease. Based on the findings of our study, future studies looking at the long-term effects of anodal tDCS and motor practice on limb-kinetic apraxia in PD and other neurodegenerative disorders may be worthwhile.
Availability of data and materials
Data will be made available on reasonable request.
Code availability
Not applicable.
References
Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, Stagg CJ, Johansen-Berg H (2016) Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med 8:330re1
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15:1257–1272
Bianchi M, Cosseddu M, Cotelli M, Manenti R, Brambilla M, Rizzetti MC, Padovani A, Borroni B (2015) Left parietal cortex transcranial direct current stimulation enhances gesture processing in corticobasal syndrome. Eur J Neurol 22:1317–1322
Bolognini N, Convento S, Banco E, Mattioli F, Tesio L, Vallar G (2015) Improving ideomotor limb apraxia by electrical stimulation of the left posterior parietal cortex. Brain 138:428–439
Broeder S, Nackaerts E, Cuypers K, Meesen R, Verheyden G, Nieuwboer A (2019) tDCS-enhanced consolidation of writing skills and its associations with cortical excitability in Parkinson disease: a pilot study. Neurorehabil Neural Repair 33:1050–1060
Chung CL, Mak MK, Hallett M (2020) Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann Neurol 88:933–945
Filimon F (2010) Human cortical control of hand movements: parietofrontal networks for reaching, grasping, and pointing. Neuroscientist 16:388–407
Fleming MK, Rothwell JC, Sztriha L, Teo JT, Newham DJ (2017) The effect of transcranial direct current stimulation on motor sequence learning and upper limb function after stroke. Clin Neurophysiol 128:1389–1398
Foki T, Pirker W, Geissler A, Haubenberger D, Hilbert M, Hoellinger I, Wurnig M, Rath J, Lehrner J, Matt E, Fischmeister F, Trattnig S, Auff E, Beisteiner R (2015) Finger dexterity deficits in Parkinson’s disease and somatosensory cortical dysfunction. Parkinsonism Relat Disord 21:259–265
Foki T, Vanbellingen T, Lungu C, Pirker W, Bohlhalter S, Nyffeler T, Kraemmer J, Haubenberger D, Fischmeister FP, Auff E, Hallett M, Beisteiner R (2016) Limb-kinetic apraxia affects activities of daily living in Parkinson’s disease: a multi-center study. Eur J Neurol 23:1301–1307
Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MT, Barbosa ER, Nitsche MA, Pascual-Leone A (2006) Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord 21:1693–1702
Fusco A, Iosa M, Venturiero V, De Angelis D, Morone G, Maglione L, Bragoni M, Coiro P, Pratesi L, Paolucci S (2014) After vs. priming effects of anodal transcranial direct current stimulation on upper extremity motor recovery in patients with subacute stroke. Restor Neurol Neurosci 32:301–312
Galea JM, Celnik P (2009) Brain polarization enhances the formation and retention of motor memories. J Neurophysiol 102:294–301
Ilic NV, Dubljanin-Raspopovic E, Nedeljkovic U, Tomanovic-Vujadinovic S, Milanovic SD, Petronic-Markovic I, Ilic TV (2016) Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restor Neurol Neurosci 34:935–945
Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, Bikson M (2016) Animal models of transcranial direct current stimulation: Methods and mechanisms. Clin Neurophysiol 127:3425–3454
Kaski D, Allum JH, Bronstein AM, Dominguez RO (2014a) Applying anodal tDCS during tango dancing in a patient with Parkinson’s disease. Neurosci Lett 568:39–43
Kaski D, Dominguez RO, Allum JH, Islam AF, Bronstein AM (2014b) Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clin Rehabil 28:1115–1124
Klaes C, Kellis S, Aflalo T, Lee B, Pejsa K, Shanfield K, Hayes-Jackson S, Aisen M, Heck C, Liu C, Andersen RA (2015) Hand shape representations in the human posterior parietal cortex. J Neurosci 35:15466–15476
Kubel S, Stegmayer K, Vanbellingen T, Pastore-Wapp M, Bertschi M, Burgunder JM, Abela E, Weder B, Walther S, Bohlhalter S (2017) Altered praxis network underlying limb kinetic apraxia in Parkinson’s disease—an fMRI study. Neuroimage Clin 16:88–97
Kubel S, Stegmayer K, Vanbellingen T, Walther S, Bohlhalter S (2018) Deficient supplementary motor area at rest: neural basis of limb kinetic deficits in Parkinson’s disease. Hum Brain Mapp 39:3691–3700
Lefaucheur JP (2016) A comprehensive database of published tDCS clinical trials (2005–2016). Neurophysiol Clin 46:319–398
Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS, Fregni F, Caumo W, Torres IL (2012) Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry 3:110
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2003) The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 18:738–750
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901
Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W (2003) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301
Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W (2006) Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci 23:1651–1657
Ogawa K, Imai F (2016) Hand-independent representation of tool-use pantomimes in the left anterior intraparietal cortex. Exp Brain Res 234:3677–3687
Parikh PJ, Cole KJ (2014) Effects of transcranial direct current stimulation in combination with motor practice on dexterous grasping and manipulation in healthy older adults. Physiol Rep 2:e00255
Park JE (2017) Apraxia: review and update. J Clin Neurol 13:317–324
Park JE (2018) Repetitive transcranial magnetic stimulation for limb-kinetic apraxia in Parkinson’s disease. J Clin Neurol 14:110–111
Pavlova E, Kuo MF, Nitsche MA, Borg J (2014) Transcranial direct current stimulation of the premotor cortex: effects on hand dexterity. Brain Res 1576:52–62
Pavlova EL, Semenov RV, Guekht AB (2020) Effect of tDCS on fine motor control of patients in subacute and chronic post-stroke stages. J Mot Behav 52:383–395
Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F, Heilman KM (2007) Limb-kinetic apraxia in Parkinson disease. Neurology 68:150–151
Rathelot JA, Dum RP, Strick PL (2017) Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci USA 114:4255–4260
Vanbellingen T, Kersten B, Van de Winckel A, Bellion M, Baronti F, Muri R, Bohlhalter S (2011) A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). J Neurol Neurosurg Psychiatry 82:389–392
Vanbellingen T, Nyffeler T, Nigg J, Janssens J, Hoppe J, Nef T, Muri RM, van Wegen EEH, Kwakkel G, Bohlhalter S (2017) Home based training for dexterity in Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord 41:92–98
Vanbellingen T, Hofmanner D, Kubel S, Bohlhalter S (2018) Limb kinetic apraxia is an independent predictor for quality of life in Parkinson’s disease. Mov Disord Clin Pract 5:156–159
Wang B, Xiao S, Yu C, Zhou J, Fu W (2021) Effects of transcranial direct current stimulation combined with physical training on the excitability of the motor cortex, physical performance, and motor learning: a systematic review. Front Neurosci 15:648354
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127:1031–1048
Funding
This work was supported by the Korean National Research Foundation (Grant # 2017R1C1B5018) Dr. Hallett is supported by the NINDS Intramural Program. Dr. Jeong-ho Park and other authors have nothing to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No relevant conflicts of interest reported.
Ethics approval
This study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital. All the clinical work was done at Dongguk University.
Consent to participate
All study participants gave written informed consent.
Consent for publication
Not applicable.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, J.E., Hallett, M., Jang, HR. et al. Effects of anodal stimulation and motor practice on limb-kinetic apraxia in Parkinson’s disease. Exp Brain Res 240, 1249–1256 (2022). https://doi.org/10.1007/s00221-021-06293-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06293-4