Abstract
During precise gaze shifts, eye, head, and body movements exhibit synergic relations. In the present study, we tested the existence of behavioural synergic relations between eye and postural movements in a goal-directed, precise, visual search task (locate target objects in large images). More precisely, we tested if postural control could be adjusted specifically to facilitate precise gaze shifts. Participants also performed a free-viewing task (gaze images with no goal) and a fixation task. In both search and free-viewing tasks, young participants (n = 20; mean age = 22 years) were free to move their eyes, head, and body segments as they pleased to self-explore the images with no external perturbation. We measured eye and postural kinematic movements. The results showed significant negative correlations between eye and postural (head and upper back) movements in the precise task, but not in the free-viewing task. The negative correlations were considered to be stabilizing and synergic. Indeed, the further the eyes moved, the more postural variables were adjusted to reduce postural sway. These results suggest that postural control was adjusted to succeed in subtle and active self-induced precise gaze shifts. Furthermore, partial correlations showed significant relations between (1) task performance to find target objects and (2) synergic relations between eye and postural movements. These later results tend to show that synergic eye-postural relations were performed to improve the task performance in the precise visual task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When people stand upright, they sway and need to control their equilibrium (Ivanenko and Gurfinkel 2018). The literature reports also show that young healthy individuals sway less when they need to perform precise short and successive gaze shifts (< 15°) on visual targets than unprecise control visual tasks (Stoffregen et al. 2007; Rougier and Garin 2007; Rodrigues et al. 2013). Here, precise tasks refer to tasks in which participants have to find and gaze subtle details in images, while unprecise tasks refer to tasks in which they randomly look at images with no goal. We found that young adults couple their postural movements to their eye movements to perform precise gaze shifts on small (Bonnet et al. 2017, 2019a) and large images (Bonnet et al. 2019b). In these studies, we showed that the further the eyes moved to search for subtle details in images, the more the participants stabilized their posture, and especially their head movements, to succeed. Furthermore, young adults exhibited stabilizing relations between eye and head movements in precise search tasks, while they failed to demonstrate such a pattern of results in unprecise free-viewing tasks.Footnote 1 These relations were considered “synergic”, because they showed that the postural control system was adjusted in interaction with characteristics of precise gaze shifts to be performed (Bonnet and Baudry 2016a).
In the present study, synergies concerned relations between kinematic variables in two systems (postural and visual). We define synergies as task-dependent negative co-variation between elemental visual and postural variables to stabilize performance (Latash 2008). Synergic relations between eye and postural movements refer to how postural sway (analyzed at the head, upper back and lower back levels) and eye movements actively work together with one another during the entire task. In our previous studies, we used artificial search tasks that did not represent meaningful ecological tasks encountered during activities of daily living (Bonnet et al. 2017, 2019a; b). Furthermore, visual search in these tasks was unstructured and random, because targets could be located anywhere in the images with no clue where they could be located. However, in daily activities, people are often searching for things placed at expected locations. For example, when they are looking for a loaf of bread in the kitchen, they direct our gaze to flat horizontal surfaces instead of vertical walls. In the current study, we tested the existence of synergic relations between eye and postural movements, while people searched for objects located at conventional places in scenes showing rooms in residential homes.
The present study’s primary objective was to test the existence of synergic relations between eye and postural (head, upper back, and lower back) movements in young adults searching for objects in rooms in homes. Our main hypothesis was to find significant stabilizing negative correlations between eye and postural movements, especially significant correlations between eye and head movements, even stronger than in our previous studies (Bonnet et al. 2017, 2019a; b). We expected so because the target objects were located at conventional locations and gaze shifts could be directed toward specific and logical zones of interest, which was not the case in our previous studies. As postural control should be adjusted adaptively to succeed in precise tasks (Bonnet and Baudry 2016a), our secondary hypothesis was to find better task performance (finding targets) related to stronger relations between eye and head movements.
Methods
Participants
Twenty healthy, young adults (12 males, 8 females) from the University of Lille participated in the study. Their mean age, body mass, and height were: 22 ± 2 years, 70 ± 15 kg, and 1.70 ± 0.08 m, respectively. All the participants had a good or adequately corrected visual acuity. If glasses or contact lenses were required in everyday life, they were worn during the tasks. The study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by our local ethical committee. The participants gave their written, informed consent to participation.
For the calculation of the sample size, we used our previous data with young adults (Bonnet et al. 2019b) and the bivariate normal model in G*power (Faul et al. 2009). We estimated the effect size f = 0.79 based on this previous study. For a test using two tails, α = 0.05, power = 0.8, and correlation phi (H0) = 0, the required sample size = 9. We recruited 20 young adults to increase the statistical power in our analyses.
Apparatus
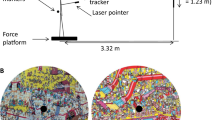
Three video-projectors (Optoma HD83, London, England) were used to project large experimental images onto a full panoramic display (radius: 2.04 m; height: 2 m; circumference: 12.8 m; Fig. 1A). The images used were all images of rooms inside houses (e.g., living-room; bedroom; Fig. 1B). One image was used in each trial.
A Figure showing the position of the participants with respect to the semi-circular panoramic display. The 12 images were projected onto that panoramic display (2.04 m radius, 2 m high). The participants stood 3.75 m behind the semi-circular panoramic display and therefore could see the images subtending a visual angle of maximum 120°; B two images shown during the study. Any of the 12 images could be projected in the free-viewing and search tasks. Here, for our example, the top image would be shown in the free-viewing task as there is not list of objects at the top of the image. In the free-viewing task, the participants were given instruction to look at the image as they pleased. The bottom image would be shown in the precise search task as the participants could see a list of five objects (at the top of the image) to search. C Schematic representation of the environmental set-up
Three markers (Polhemus Liberty 240/8-8 System, Colchester, VT) were used to record head, upper back, and lower back movements at 240 Hz. The markers were placed on a helmet worn by the participants, at the seventh cervical vertebra, and on a chest belt approximately at the fifth lumbar vertebra, respectively (Fig. 1C). An eye tracker (SensoMotoric Instruments, Teltow, Germany) was used to measure monocular eye movements at 50 Hz. The eye tracking goggle was set on the helmet worn by the participants (Fig. 1C). The iViewX system recorded the pupil position and a video recorded what the right eye was looking at in the visual environment. A MATLAB (MathWorks, Natick, MA, USA) script was used to synchronize all apparatus (magnetic tracking system and eye tracker); at the same time, the images were projected onto the wall.
Participants stood in a standardized position of the feet (14 cm, 17°; McIlroy and Maki 1997). The participant was located 3.75 m from the panoramic display, so that the participants could see all images projected onto the panoramic display subtending a visual angle of maximum 120° left–right and 23.17° up–down (Fig. 1C). The experimenter was located behind and on the left of the participants to check compliance with instruction.
Tasks and instruction
The participants performed three tasks (fixation, free-viewing and search) and six trials per task (50 s per trial). All tasks were performed one after another, but the order of tasks was randomized. One of the 12 images projected in each trial was chosen randomly and could never appear twice in the search and free-viewing tasks. The six images projected in the fixation task were the same as in the free-viewing task for two reasons. First, the participants did not look at the image in the fixation task. Second, the stationary task was a control task of the free-viewing task, without any eye movement in the first task and with eye movement in the second task.
In the fixation task, the participants had to stare at a black cross (2°) projected in front of them for the duration of the trial. The black cross was located at the center of the images (Fig. 1B) projected in front of the participants. In the free-viewing and search task, the participants also stared at the same black cross for the first 5 s, then the cross disappeared, and the participants were free to look at the image as they chose. In the free-viewing task, the participants were given instruction to be interested in the content of the image and to look at it as they pleased (Fig. 1B, top one). In the search task, they were given instruction to detect the location of five objects in each image. The name of these objects was displayed at the top of images (Fig. 1B, bottom image). Before the study began, a document with all the names and figures of the objects to be searched was shown to the participants. When the participants performed the search task, they had to stare at each target for approximately 5 s to show that they had found them. We indeed judged that if the participants had only looked at some objects 2–3 s, it would not necessarily mean that they wanted to show us that they had found a target object. However, for our analyses, we did not need the participants to gaze each target object for 5 s; we only considered the x longest target objects fixated. Here, “x” corresponded to the number of target objects found by the participants. In fact, after each trial, the participants had to report how many objects they had found during the trial. Also after each trial, they had to rate their level of confidence concerning their task performance [from 1 (low), to 5 (high)]. The participants were invited to restart the search task if they could find the five objects by the end of the trial. We used this methodology to avoid some trials in the search task to be finished before the end of the trial duration. We agree that the search task became easier when the participants restarted searching to locate the objects, but this situation rarely happened (see “Results”).
For general information, before the study, we had prepared a list of five target objects in each of the 12 images. The five objects were randomly located in the image. The Matlab script selected which images would be projected in each task and therefore selected in which images the list of five objects should be projected (for the search task). The characteristics of the objects to be found can be summarized as follows:
-
Small objects (< 3°): 1.85° ± 0.85°; mid-size objects ([3°–7°]): 4.63° ± 0.90°; bigger objects (> 7°): 12.11° ± 5.97.
-
Centered objects (< 17° of the center): 10.32° ± 4.69°; half centered objects ([18°–34°]: 25.08° ± 5.44°; decentered objects (> 34°): 40.98° ± 5.61°
In all trials, the participants were told to relax and hold their hands by the side of the body (Fig. 1C). They had to avoid any voluntary movement (e.g., hand movements and speak to the instructor) unrelated to the task performed. However, they were allowed to move their head and other body parts to be able to look at the images in the most comfortable way. The present study used an original methodology in the free-viewing and search tasks. In fact, the participants were free to move their eyes whenever and wherever they liked; they could also rotate their body whenever they liked to perform the task. Such freedom in eye and body movement is rare in the literature on postural control.
Procedure
Once the participants arrived in the experimental room, they signed the informed consent forms. Next, they were given instruction for the various tasks. To explain the task, we showed them images of rooms inside houses with the five names of object listed at the top of the image. Once the instruction was all understood, the participants were invited to take their shoes off. The Polhemus markers were affixed on the head, the upper back and lower back levels (Fig. 1C). The light was turned off so that the participants could clearly see the images. To aid relaxation, participants were given instruction to sit down and rest after each task.
Dependent variables
To analyze synergic relations between postural and eye movements, we used classical variables of postural sway, i.e., the range (R), standard deviation (SD), and mean velocity (V) on the medio-lateral (ML) and antero-posterior (AP) axes. To analyze the eye movement time-series, we used closely related variables (R, SD, V) in both left/right and up/down directions. These variables were already used in our three previous studies analyzing synergic relations between postural and eye movements (Bonnet et al. 2017, 2019a, b). We used many less variables as we did in these past studies as (1) we did not use path length and ellipse area variables of eye and postural movements, (2) we did not measure and report data of the center of pressure, and (3) we only studied the kinematics of eye movements and did not use variables of eye movement related to characteristics of fixation and saccade.
In the search task, a performance was considered ‘correct’ if the participant fixated on the appropriate object. For task performance, we evaluated several variables: the number of correct objects found, the percentage of incorrect objects found, the time spent to gaze the correct objects found, the eccentricity and size of correct objects found, as well as the confidence score to have found the correct objects.
Preparation of the data
The head, upper back and lower back time-series were all resampled at 50 Hz, as the eye movement time-series. Three reasons explained why the SMI eye tracker did not record some data: (1) when the participants’ eyes were closed (e.g., during blinks), (2) in case of increased extra pupil dilatation caused by the lighting of the room turned offFootnote 2, and (3) when the participants did not look through the small window of the eye tracker (diameter of that window: 40°). The eye movement files in which there were more than 20% of missing values were excluded to analyze only very good recordings. For eye and postural movement data, there were on average 0.21% and 0.61% of outliers per spreadsheet, respectively. The remaining visual files contained, on average 89.48 ± 4.90% data.
In the search task, the successive imposed 5 s fixations when finding a target may be a confounding factor. To control this issue of fixation in the search task, we deleted the longest fixations corresponding to the number of objects found—in the eye time-series and data at the corresponding moments in all other files, i.e., head, neck, and lower back markers. By doing this, we avoided to induce significant positive correlations between eye and postural movements simply due to fixation, as shown in Bonnet (2019).
Statistical analyses
The synergic model focuses on the regulation of postural control to actively facilitate successful gaze shifts toward precise locations. To analyze relations between eye and postural movements, we performed multiple Pearson correlations in the free-viewing and search tasks separately, as in our previous studies (Bonnet et al. 2017, 2019a; b). Pearson correlations are indeed classically used in analyses of synergic relation (Bruton and O’Dwyer 2018). In these analyses, only the data of the free-viewing and search tasks were included for analyses.
Partial correlations were also performed to investigate the influence of these relations between eye and postural movements on task performance. The dependent variable “number of correct objects found” was controlled—partialed out—in the previous significant Pearson correlations between eye and postural movements. We were interested in non-significant partial correlations, showing that controlling for the task performance canceled the significant correlations between eye and postural movements. In our analyses, if the original Pearson correlation between eye and postural movements was significant and if the partial correlation (additionally controlling for the number of correct objects found) was also significant, the number of correct objects found was supposed not to be a biased variable. However, if the original Pearson correlation between eye and postural movements was significant and the partial correlation was not significant anymore, number of correct objects found was supposed to have played a role in the previously significant Pearson correlation. In this second scenario, the significant correlation between eye and postural movements may be only indirect and caused by a common causal factor (here the change in the number of correct objects found).
For complementary purposes, we used repeated-measure analyses of variance (ANOVAs) to contrast postural sway and body rotations between the fixation and the two other tasks (free-viewing and search). These additional analyses served to show that the participants swayed more and rotated their body more in both free-viewing and search tasks than in the fixation task. For this analysis, we only used the variables range AP, range ML, range yaw, and range pitch of head movements. They were used to discuss that functional relations between eye and postural movements—presumably found in the search task—can exist even if the participants sway and rotate their body significantly more than in quiet stance. All analyses were performed with Statistica 10 software (Statsoft Inc., Tulsa, OK, USA).
Before performing analyses, data were tested for normal distribution and homoscedasticity (homogeneity of variance) using the Shapiro–Wilk and Levene tests, respectively. In case outliers could be detected by box plots, they were deleted in the spreadsheets for analyses as recommended by Tabachnik and Fidell (2006).
Results
Relations between eye and postural movements in Pearson’s correlations
In the search task, Pearson correlations between eye and postural movements showed that the young group only exhibited significantly negative correlations both in the ML/left–right direction (Fig. 2A–G) and in the AP/up–down direction (Fig. 2H–M). Overall, three Pearson correlations were found significant between eye and head movements, six ones were found significant between eye and upper back movements, and four ones were found significant between eye and upper lower movements (Fig. 2A–M). In considering only eye movements, these analyses showed that only the SD of eye movement, not R or V of eye movement, was related to various characteristics of postural (head, upper back and/or lower back) movements in both the ML/left–right and AP/up–down directions. In the free-viewing control task, no significant correlations between eye and postural movements were found, ns (Fig. 2A–M).
Pearson correlations between eye movements and postural movements (lower back, upper back and head). The correlations were significant in the search task (p < 0.01) but not in the free-viewing task (p > 0.01). A–G Correlations between the standard deviation of eye movement in the left–right direction (SDleft/right) and body movement in the medio-lateral (ML) directions. These significant relations were found between eye SDleft/right and head ML range (RML; A), head ML standard deviation (SDML; B), head ML mean velocity (VML; C), upper back RML (D), upper back SDML (E), upper back VML (F), and lower back movement VML (G). H–M Correlations between the standard deviation of eye movement in the up–down direction (SDup/down) and body movement in the antero-posterior (AP) directions. These significant relations were found between eye SDup/down and upper back RAP (H), upper back SDAP (I), upper back VAP (J), lower back RAP (K), lower back SDAP (L), and lower back VAP (M). The units are pixels (px), centimeters (cm), and seconds (s)
Partial correlations showed that some relations between eye and postural movements were lost when controlling for the number of objects found by the participants. It was the case specifically in the ML/left–right direction and for the amplitude of movement (SD and R), not for the velocity of movement (Table 1). In other words, these negative correlations between eye and postural movements showed in the ML/left–right direction were significantly related to task performance.
Task performance in the search task
For the task performance, young adults succeeded well in the task to find many objects (Table 2). On average, the participants indeed found 5.16 objects per trial, which means that they found an object each 8.72 s. As they spent, on average 4.23 s to look at these objects, they needed on average 4.5 s to find an object (Table 2). They were quite accurate as we could only notice in total five inaccurate objects found, which represented 1.12% (Table 2). The objects found were not necessarily the biggest and/or more centrally located objects. In fact, young adults found objects located everywhere and of all sizes in approximately the same proportion (Table 2).
Range of head sway and head rotation in the three tasks
For complementary purposes, we analyzed the head sway and head rotation in the three tasks. The results showed that participants clearly exhibited larger range of head sway in both free-viewing and search tasks than in the fixation task on both AP axis (fixation: 1.37 ± 0.86 cm; free-viewing: 10.74 ± 3.40 cm; search: 10.08 ± 3.16 cm) and ML axis (fixation: 2.89 ± 1.05 cm; free-viewing: 9.07 ± 2.66 cm; search: 8.18 ± 2.39 cm). Participants also clearly exhibited larger range of head rotation in both free-viewing and search tasks than in the fixation task in both yaw (left–right) direction (fixation: 5.57° ± 3.01°; free-viewing: 77.22° ± 14.66°; search: 75.70° ± 14.91°) and pitch (up–down) direction (fixation: 1.73° ± 0.59°; free-viewing: 8.63° ± 2.81°; search: 8.61° ± 2.56°). All these analyses were significant (Fs > 106.10, p < 0.01).
Discussion
In the present study, we investigated how postural control is actively adapted to perform self-induced gaze shifts to search for target objects in large images. The results showed that the young participants exhibited stabilizing relations between eye and (1) head and (2) upper back movements when searching for target objects in large familiar scenes (rooms in houses). These relations were absent when participants looked at these images with no specific goal. Furthermore, synergic relations between eye, head and back movements were correlated with task performance, suggesting that they may serve to facilitate task performance.
Significant relations between eye and head, upper back and lower back movements in the precise search task
Previously, it has been suggested that postural control may not be controlled for its own sake, but for the achievement of other goals (Stoffregen et al. 1999, 2000). Latash and colleagues have invoked the idea of synergies, specifically muscle synergies serving for action stability or stability of performance variable (Park et al. 2012; Latash and Huang 2015; Yamagata et al. 2018). Our proposal of synergy between eye and postural variables is the same as forwarded by these investigators as our variables exhibit task-dependent negative co-variation to stabilize overall performance (stable posture).
Consistent with this definition of synergies serving for action stability, we proposed a synergic model of postural control (Bonnet and Baudry 2016a, b). We suggested that postural sway should be adjusted actively to successfully perform precise gaze shifts in self-induced eye and body movements with no external perturbations. Eye and postural movements are expected to be coordinated in synergies, that is, they are expected to work together to succeed in precise gaze shifts. Based on our model, we also predicted that eye and postural movement would not be coupled in unprecise random exploration of images (Bonnet and Baudry 2016a).
The present study supported our initial hypothesis, because the young participants exhibited negative (stabilizing) correlations between eye and postural movements in the precise search task (Fig. 2A–M). On one hand, these results were strong, because (1) we found more significant relations (13 ones) than in all three previous studies (four significant relations were found in each of the three previous studies, cf. Bonnet et al. 2017, 2019a; b) and (2) with a fewer analyses performed (see “Methods”). These results confirmed our main prediction that relations between eye and postural movements would be negatively correlated during precise visual and ecological search tasks. In our images, target objects were located at usual locations (e.g., a fork on a table and an oven close to the lavatory in a kitchen furniture) and were clearly visible.
Unexpectedly, the significant relations between eye and postural movements were not only found at the head level but at all levels of the body segments involved in this study (head, upper back and lower back; Fig. 2A–M). In Bonnet et al. (2019a, b), we already showed that the addition of a counting (subtracting) task led to significant relations between eye and postural movements at all levels of the body and not only at the head level. We should still mention that the significant relations between eye and head movements found in the ML/left–right direction (Fig. 2A–C) were strong and expected. Indeed, the panoramic display was much more extended in the left–right direction than up–down direction (Fig. 1C), thus requiring more stabilizing relation in left–right than in up–down direction. The more participants needed to extend their visual search in the ML/left–right direction, the more they had to stabilize their posture to avoid postural instability and failure to gaze specific locations (Fig. 2). Consistently, the task performance to find target objects in the images was significantly related to these synergic relations between eye and head movements in the ML/left–right direction (Table 1; see also “Main differences between young adults, older adults and PD patients” below).
The results supported our initial hypothesis also because the young participants did not exhibit negative (stabilizing) correlations between eye and postural movements in the unprecise control free-viewing task (Fig. 2A-M). These results are consistent with our three previous studies (Bonnet et al. 2017, 2019a; b) and also with Haworth et al. (2014). In this later study, the young participants looked at various visual stimuli (a sine, chaos, surrogate, or random noise trajectory). The results showed that postural sway was not affected by visual stimulation, while gaze shifts were affected by the complexity of the stimuli (Haworth et al. 2014). In this later study, there was no relation between eye and postural movement, probably because the participants did not need to couple them to succeed in the task. Figure 2A–M also shows that relations between eye and postural movements are different in search and free-viewing tasks. In fact, Fig. 2 shows that (1) behavioral relations always involved longer eye movements in the search task than in the free-viewing task (relations more on the right on these figures) and (2) behavioral relations were more extended in the search task than in the free-viewing task. Taken together, these new characteristics—never discussed in past studies—showed that eye and postural movements had to be coordinated when more variability of movements was engaged in the search task. They provide a new way to characterize synergic control between eye and postural movements.
Main differences between young adults, older adults and PD patients
We recall that young adults—in the present study—exhibited significant stabilizing relations, and only stabilizing ones, between eye and postural movements to perform the precise search task. In another study recently published, 20 PD patients and 20 age-matched controls performed the same experimental set-up (Bonnet et al. 2021). The contrast in the results is clear. Indeed, healthy older adults did not exhibit any significant relations between eye and postural (head and/or upper back and lower back) movements. In other words, older adults were not able to use stabilizing relations between eye and postural movements to perform the precise search task. The PD patients presented even worse patterns of results than their age-matched controls. Indeed, the PD patients only exhibited destabilizing relations between eye and postural movements to perform the precise search task. Therefore overall, all these results together showed a continuum with stabilizing relations for young adults, ns for older healthy adults and destabilizing relations for PD patients. We already showed significant stabilizing relations between eye and postural movements—mostly between eye and head movements—in young adults who performed precise search tasks (Bonnet et al. 2017, 2019a; b). In these studies, the experimental set-up was different than in the present one because the search task was artificial. Indeed, young participants searched to locate Waldo located anywhere in densely furnished cartoon images or searched to locate unrealistic depixelized animals randomly located anywhere in images of landscape. In the present study, the search task was ecological as participants searched for conventional objects displayed at conventional locations.Therefore, the results in the present study are novel and complementary to previous published ones (Bonnet et al. 2017, 2019a; b). They show for the first time that young adults use significant stabilizing relations between eye and head or more generally between eye and postural movements to perform precise search tasks in exploring ecological environment in the standing position.
Relation between synergic movements and task performance
Young participants exhibited a good visual task performance in finding objects in the search task (cf. Table 2). As expected, this task performance was significantly related to synergic eye and postural movements. Indeed, some significant correlations between eye and postural movements were not significant anymore after controlling for the effects of task performance in partial correlations (cf. Table 1). These findings should be considered carefully for two reasons. First, the control of task performance only minimally changed the p value (these values were still below p < 0.05, Table 1). Also, these results do not prove any causal relation as they are just correlations. However, the significant relations between task performance and synergic eye and postural movements seemed functional as they were only found at the head and upper back levels, not at the lower back level. Also, they were only found in the wider ML/left–right direction and not in the relatively smaller AP/up–down direction. Overall, these significant relations may be useful for successful task performance. This general finding goes in line with the general conceptualisation of ‘synergies for action stability’ already mentioned previously (Latash 2008; Park et al. 2012; Yamagata et al. 2018) and in populations affected by some diseases such as Parkinson’s disease (Latash and Huang 2015). Indeed, synergies are useful for goal-directed reasons; they are useful to stabilize the body for successful task performance. We also reiterate that in our study, the task performance was related to the supra-postural task performed and not to the control of the upright stance, as in other studies (Ting and McKay 2007; Degani et al. 2010; Park et al. 2012). To our knowledge, this is the first study showing significant relations between synergic control and task performance.
Limitations and conclusions
One main limitation of the present study is the use of many Pearson and partial correlations, thus limiting the impact of the findings. We needed to do so to analyze subtle interactions between eye and postural movements in each task separately. Another limitation was to look at target words (e.g., “red pelow”) and thus to conceptualize the visual objects to be found in the image. This cognitive bias could have minimized, at least not facilitated, significant findings. For this reason, the significant relations between eye and postural movements that we found were strong in our study. A third limitation is that we did not study characteristics of gaze (e.g., number of fixations and duration of fixation) as well as visual strategies used to search for target objects as already performed in other studies (Henderson 2003; Oliva et al. 2004; Torralba et al. 2006; Boot et al. 2009). We specifically focused on relations between kinematic eye and postural movements and not on any strategies. Our data complement these other findings. A fourth limitation is that we did not measure the dominant eye of each participant but only the right eye. If we had recorded the dominant eye, significant relations between eye and postural movements may have been stronger. We will be careful about this aspect in the future.
The present study clearly showed that young adults actively adapted their postural control to perform and succeed in precise gaze shifts. It showed that young adults establish synergic relations between eye and postural movements to better find target objects in large images. The present results are important, because they were found in activities of daily living. They are also useful to better understand published results with PD patients and age-matched controls in the same experimental context (Bonnet et al. 2021).
Availability of data and materials
None.
Notes
In the present manuscript, search tasks are considered as precise tasks, and therefore, the terms ‘search tasks’, ‘precise tasks’, and ‘precise search tasks’ are all equivalent (the term ‘precise’ is only an adjective to quality the task). In the same way, ‘unprecise tasks’, ‘free-viewing tasks’, and ‘unprecise free-viewing tasks’ are equivalent. The precise task is the experimental task and the unprecise task is the control task.
When the pupil diameter is too large, the eye tracking could not detect accurately enough the position of gaze anymore and reported no data.
References
Bonnet CT (2019) Positive relations between vision and posture in the fixation task performed upright. Mot Control. https://doi.org/10.1123/mc.2018-0094
Bonnet CT, Baudry S (2016a) A functional synergistic model to explain postural control during precise visual tasks. Gait Posture 50:120–125. https://doi.org/10.1016/j.gaitpost.2016.08.030
Bonnet CT, Baudry S (2016b) Active vision task and postural control in healthy, young adults: synergy and probably not duality. Gait Posture 48:57–63. https://doi.org/10.1016/j.gaitpost.2016.04.016
Bonnet CT, Szaffarczyk S, Baudry S (2017) Functional synergy between postural and visual behaviors when performing a difficult precise visual task in upright stance. Cogn Sci 41:1675–1693. https://doi.org/10.1111/cogs.12420
Bonnet CT, Davin T, Baudry S (2019a) Interaction between eye and body movements to perform visual tasks in upright stance. Hum Mov Sci 68:102541. https://doi.org/10.1016/j.humov.2019.102541
Bonnet CT, Davin T, Hoang J-Y, Baudry S (2019b) Relations between eye movement, postural sway and cognitive involvement in unprecise and precise visual tasks. Neuroscience 416:177–189. https://doi.org/10.1016/j.neuroscience.2019.07.031
Bonnet CT, Delval A, Singh T, Defebvre L (2021) Parkinson’s disease-related changes in the behavioural synergy between eye movements and postural movements. Eur J Neurosci 54:5161–5172. https://doi.org/10.1111/ejn.15351
Boot WR, Becic E, Kramer AF (2009) Stable individual differences in search strategy? The effect of task demands and motivational factors on scanning strategy in visual search. J vis 9:7–7. https://doi.org/10.1167/9.3.7
Bruton M, O’Dwyer N (2018) Synergies in coordination: a comprehensive overview of neural, computational, and behavioral approaches. J Neurophysiol 120:2761–2774. https://doi.org/10.1152/jn.00052.2018
Degani AM, Danna-Dos-Santos A, Robert T, Latash ML (2010) Kinematic synergies during saccades involving whole-body rotation: a study based on the uncontrolled manifold hypothesis. Hum Mov Sci 29:243–258. https://doi.org/10.1016/j.humov.2010.02.003
Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160. https://doi.org/10.3758/BRM.41.4.1149
Haworth JL, Vallabhajosula S, Stergiou N (2014) Gaze and posture coordinate differently with the complexity of visual stimulus motion. Exp Brain Res 232:2797–2806. https://doi.org/10.1007/s00221-014-3962-5
Henderson J (2003) Human gaze control during real-world scene perception. Trends Cogn Sci 7:498–504. https://doi.org/10.1016/j.tics.2003.09.006
Ivanenko Y, Gurfinkel VS (2018) Human postural control. Front Neurosci. https://doi.org/10.3389/fnins.2018.00171
Latash ML (2008) Synergy. Oxford University Press
Latash ML, Huang X (2015) Neural control of movement stability: lessons from studies of neurological patients. Neuroscience 301:39–48. https://doi.org/10.1016/j.neuroscience.2015.05.075
McIlroy WE, Maki BE (1997) Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech Bristol Avon 12:66–70. https://doi.org/10.1016/s0268-0033(96)00040-x
Oliva A, Wolfe JM, Arsenio HC (2004) Panoramic search: the interaction of memory and vision in search through a familiar scene. J Exp Psychol Hum Percept Perform 30:1132–1146. https://doi.org/10.1037/0096-1523.30.6.1132
Park E, Schöner G, Scholz JP (2012) Functional synergies underlying control of upright posture during changes in head orientation. PLoS ONE 7:e41583. https://doi.org/10.1371/journal.pone.0041583
Rodrigues ST, Aguiar SA, Polastri PF et al (2013) Effects of saccadic eye movements on postural control stabilization. Mot Rev Educ Física 19:614–619. https://doi.org/10.1590/S1980-65742013000300012
Rougier P, Garin M (2007) Performing saccadic eye movements or blinking improves postural control. Mot Control 11:213–223. https://doi.org/10.1123/mcj.11.3.213
Stoffregen TA, Smart LJ, Bardy BG, Pagulayan RJ (1999) Postural stabilization of looking. J Exp Psychol Hum Percept Perform 25:1641–1658. https://doi.org/10.1037/0096-1523.25.6.1641
Stoffregen TA, Pagulayan RJ, Bardy BG, Hettinger LJ (2000) Modulating postural control to facilitate visual performance. Hum Mov Sci 19:203–220. https://doi.org/10.1016/S0167-9457(00)00009-9
Stoffregen TA, Hove P, Bardy BG et al (2007) Postural stabilization of perceptual but not cognitive performance. J Mot Behav 39:126–138. https://doi.org/10.3200/JMBR.39.2.126-138
Tabachnik BB, Fidell LS (2006) Using multivariate statistics, 6th edn. Pearson
Ting LH, McKay JL (2007) Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 17:622–628. https://doi.org/10.1016/j.conb.2008.01.002
Torralba A, Oliva A, Castelhano MS, Henderson JM (2006) Contextual guidance of eye movements and attention in real-world scenes: the role of global features in object search. Psychol Rev 113:766–786. https://doi.org/10.1037/0033-295X.113.4.766
Yamagata M, Falaki A, Latash ML (2018) Stability of vertical posture explored with unexpected mechanical perturbations: synergy indices and motor equivalence. Exp Brain Res 236:1501–1517. https://doi.org/10.1007/s00221-018-5239-x
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
We received approval for this study from our local ethical committee (CER VIPOSAD).
Consent to participate
All participants signed a consent to participation.
Consent for publication
All investigators consented to send this manuscript to publication.
Additional information
Communicated by Melvyn A. Goodale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonnet, C.T., Barela, J. & Singh, T. Behavioral synergic relations between eye and postural movements in young adults searching to locate objects in room inside houses. Exp Brain Res 240, 549–559 (2022). https://doi.org/10.1007/s00221-021-06276-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06276-5