Abstract
When lifting or moving a novel object, humans are routinely able to quickly characterize the nature of the unknown load and swiftly achieve the desired movement trajectory. It appears that both tactile and proprioceptive feedback systems help humans develop an accurate prediction of load properties and determine how associated limb segments behave during voluntary movements. While various types of limb movement information, such as position, velocity, acceleration, and manipulating forces, can be detected using human tactile and proprioceptive systems, we know little about how the central nervous system decodes these various types of movement data, and in which order or priority they are used when developing predictions of joint motion during novel object manipulation. In this study, we tested whether the ability to predict motion is different between position- (elastic), velocity- (viscous), and acceleration-dependent (inertial) loads imposed using a multiaxial haptic robot. Using this protocol, we can learn if the prediction of the motion model is optimized for one or more of these types of mechanical load. We examined ten neurologically intact subjects. Our key findings indicated that inertial and viscous loads showed the fastest adaptation speed, whereas elastic loads showed the slowest adaptation speed. Different speeds of adaptation were observed across different magnitudes of the load, suggesting that human capabilities for predicting joint motion and manipulating loads may vary systematically with different load types and load magnitudes. Our results imply that human capabilities for load manipulation seems to be most sensitive to and potentially optimized for inertial loads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans have an excellent capability for manipulating unknown objects in daily life. When reaching and grasping a cup of coffee, for example, humans can readily estimate its weight and can rapidly generate the required muscle forces to complete the desired motion (Bock 1990). Interestingly, it has been claimed that humans may exhibit different performances when manipulating various types of external loads. This could be because of humans’ different sensitivities to various forms of body movement signals, such as the position, velocity, and acceleration of body joints as measured by somatosensory systems, when recognizing/predicting body movements with loads imposed on the upper limb. In this study, we sought to determine if humans have varying capabilities for predicting different types of body movement, which potentially result in different adaptation speeds across load types.
Several previous studies (Johansson and Westling 1988; Bock 1990) have shown that intact humans exhibit a proficient capacity to adapt to different weights during voluntary limb movements. Even if mass estimates are not that accurate, humans can swiftly adjust muscle forces (Bock 1990; Crevecoeur et al. 2020) to complete the planned movements, and adaptation to different weights or motor correction can be successfully achieved within just one or two movement trials (Johansson and Westling 1984; Westling and Johansson 1984; Bock 1990). Other groups of studies have also reported quick and efficient motor adaptation during point-to-point target reaching tasks with a velocity-dependent load (Crevecoeur et al. 2020) or spring load (Flash and Gurevich 1997b).
While manipulating an external load, it appears that both tactile and proprioceptive feedback systems help humans develop an accurate prediction of load properties and how associated limb segments behave when novel loads are moved through space. For example, when lifting and moving an object with the hand, humans generate lifting forces and grip forces efficiently, so there is a relatively small margin between the lifting forces and exerted forces to prevent slipping. Even if the imposed load has a novel surface, friction force, and weight, skin tactile and arm proprioceptive systems send afferent signals to our Central Nervous System (CNS) to predict joint motion, and this serves to notify any discrepancies between the planned and current movement. This means that humans can correct the ongoing movement and exert forces readily to avoid slipping (Johansson and Westling 1984; Westling and Johansson 1984; Forssberg et al. 1991, 1992). Based on afferent signals from somatosensory systems, the CNS can update the internal model of the limb and of the estimated load, which associates body states with dynamic environments, to correct the ongoing movement or prepare the next movement.
Nonetheless, a previous study (Hwang et al. 2006) has shown that the speed of motor adaptation to a given load can vary with the type of load imposed on the human upper limb. To explain different performances quantifying interactions with various load types, it has been suggested that humans may have different sensitivities to various types of afferent body signals, including position, velocity, and acceleration of joint motion. For example, during a pointing target task, a previous study (Hwang et al. 2006) reported that the CNS is better optimized for interacting with a velocity-dependent force field than an acceleration-dependent force field. This study tested whether the previously learned curl field in acceleration space during a target reaching task could be generalized to another reaching movement trajectory, which had the same acceleration field but different velocity fields. The acceleration-dependent curl field exerted forces which were perpendicular to the movement direction but changed their direction during the first and second stages of a reaching movement. After adapting to this curl field, subjects performed the reaching movements in the opposite direction with the same acceleration-dependent force field, but with the opposite velocity field, to determine which signal-dependent force field seemed to dominate. The results showed that adaptation to the acceleration-dependent curl field was not generalized to the reaching movement in the opposite direction with the same acceleration field but with the opposite velocity field. The authors concluded that the human CNS and internal models were better optimized for a velocity-dependent force field than for either position- or acceleration-dependent force fields (Hwang et al. 2006). From this study, the authors speculated that the human CNS was optimized for the velocity of joint motion rather than for its position and acceleration. This was linked to the assertion that muscle spindles, the major proprioceptive system in the human body, have a maximum sensitivity to the stretch velocity of a muscle. It remains unclear, however, how the CNS encodes these various types of somatosensory data and in which order or priority they are used.
While various types of limb movement information, such as position, velocity, and acceleration, as well as manipulating forces can be detected using human tactile and proprioceptive systems, we know little about how the central nervous system decodes these various types of movement data and in which order or priority they are used when developing predictions of motion and of the load during novel object manipulation. Although controversial, Sherrington enumerated the sources of muscular sense and claimed a key role for human muscles in position and movement senses, together with efference copy (Sherrington 1906). After Sherrington’s study, a lot of discussion has occurred to examine how humans recognize and sense the passive and active movements of body parts (Matthews 1982). Some recent studies, however, claimed that efference copy of the active muscle might not be enough to explain the velocity-dependent errors when estimating joint positions (Gritsenko et al. 2007). In addition, it has been claimed that sensory afferent signals from cutaneous and proprioceptive sensory systems might be sufficient for estimating passive movements, and those signals were transmitted to human brain and processed in relation to the referent signals, which were previously specified by the human brain (Feldman 2016). More specifically, it remains unclear whether the ability to predict motion and to manipulate external load is different between position-, velocity-, and acceleration-dependent body signals imposed by external loads.
We hypothesize that the prediction of the motion model is optimized for one or more of these types of movement signals, and if so, this potentially results in different adaptation speeds across load types. To simulate these types of joint motion, a simple passive mass, viscosity, and joint angular spring were implemented using a haptic device during a planar reaching task in intact human subjects. However, when testing different capabilities for manipulation of different load types, which are dependent on joint movement signals, the complexity of the given force field might also affect the speed of motor learning (Gribble and Scott 2002; Huang and Patton 2011). To avoid the possible confounding effect of such complexity on motor learning performance, load types need to be as simple as possible. Thus, the inertial (point mass), viscous (damper), and elastic (rotational spring) loads used in this study were designed to be simply proportional to the acceleration of a hand, velocity of the hand, and elbow joint angular movement, respectively (Stoeckmann et al. 2009). We then estimated the number of reaching trials needed to show a consistent movement trajectory, as found in the fully adapted trials. Some load types may show a quick adjustment of limb movement because those loads are easily recognizable. Other loads, however, may require a longer time frame to be identified and may show gradually increasing proficiencies with repeated exposure. In addition, humans may have different sensitivities in detecting various magnitudes of the external load from muscle afferent and cutaneous tactile sensory systems, and this might also affect the ability to develop accurate predictions of motion and capability for load manipulation. Thus, we further determined whether the speed of motor adaptation to external loads varied with different magnitudes of the imposed loads. We used two different (small and large) magnitudes of each load type, and different load magnitudes were tested on different days.
Materials and methods
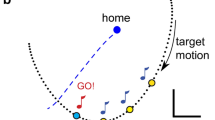
To examine how well speed of joint movement could be detected across different reaching tasks, a virtual load, which was dependent on a movement position, velocity, and acceleration, was implemented using a programmable haptic robot. To avoid potential confounding effects of load complexity on the difficulty of the task, our three load types were chosen to be as simple as possible. Thus, inertial and viscous loads were implemented as a simple passive point mass and velocity-dependent damper, which generated resistance to movement acceleration and velocity of the end effector (handle of the haptic robot), respectively (Fig. 1). The elastic load was implemented as a virtual rotational spring, which originated in the position of the elbow joint and exerted resisting forces in proportion to the joint angular deviation from the initial joint angle at the home position (Fig. 1). While a user was at the home position, there was no spring force, but as motion approached the target, the spring force increased in proportion to an increase in the angle of the elbow joint (\({\uptheta }_{{{\text{elbow}}}}\), Fig. 1). The direction of the possible movement (position, velocity, and acceleration of a subject’s hand) and direction of correspondingly generated virtual mechanical impedance are represented in Fig. 1.
(Top left) Different load types imposed on the handle of the HapticMaster for the planar reaching task and (Top right) the experimental setup. The inertial (blue solid arrow), elastic (red solid arrow), and viscous (yellow solid arrow) loads, which are dependent on the acceleration (blue dotted arrow), elbow joint angle (red dotted angle), and velocity (yellow dotted arrow) of the handle of the haptic robot, were virtually implemented as an acceleration-dependent simple point mass (blue solid circle), a viscosity resisting tangential movement velocity, and a joint angular spring located on the elbow joint (red empty circle), respectively. In the resting state before starting a target reaching, the elastic force was set to zero. (Bottom) Protocols of the experiment. Please note that this order of the load types is for an example, and the actual order of the load types was in a random order
Subjects
Ten neurologically intact human subjects (30.78 ± 4.85 yrs. old, five females and five males) participated in this study and conducted planar target reaching tasks using their dominant hand (as verbally reported by each participant). Subjects were seated on a height-adjustable chair, and their shoulder heights were matched to the height of a handle of a HapticMaster haptic device (Linde et al. 2002) (Moog FCS Robotics, East Aurora, NY) to ensure that all the reaching movements were performed on a horizontal plane. The upper arm was supported by and tied to a SaeboMAS mobile arm supporting device (Saebo, Inc., Charlotte, NC) to minimize fatigue and to free elbow movements during the reaching task. Although the subjects’ bodies were not tied to the chair during the task, they were asked to keep their initial positions to avoid undesired movements. Using the subject’s dominant side, each participant was asked to reach the designated targets shown in the display at a self-selected, consistent speed. The start position was 0.1 m anterior to the subject’s body. A target was located 0.12 m to the forward direction and 0.12 m to the direction of the dominant side of the hand, so the total travel distance was 0.170 m (Fig. 1). Visual feedback was provided on a monitor so participants could see a location of the start position (home), target, and current position in real time, which were all represented as circles with different colors. Once the color denoting the start position on the display changed from red to green, subjects were instructed to start voluntary movements and to reach the target. After staying at the target for one second, the haptic robot automatically returned to the home position, and the next trial began. All subjects signed an informed consent form, which was approved by the Northwestern University Institutional Review Board (IRB) before the experiments. The minimum number of subjects was determined using the power test. With a difference in the number of reaching trials required for the adaptation between the load types, which is one of the most important performance parameters in this study, a power of 0.90, and a significant level of 0.05 for ANOVA test, a calculated effect size was 0.77. The required number of samples is then nine; therefore, ten participants would be enough with this statistical power.
Protocols
Each subject participated in two-day experiments with a one-day break between each experiment day, and on each day, we tested either the small or large external loads with the same protocol. Each day included two sets of a planar reaching task, and each set tested the three types of mechanical impedance: inertial, elastic, and viscous loads.
Each experiment day began with 20 reaching trials without additional load as a baseline to allow the subject to become familiar with the haptic robot. The following two sets of reaching tasks were performed with unexpected loads on the subjects’ manipulator (Fig. 1). Each set consisted of three distinct blocks, and each block consisted of 15 reaching movements with one type of load (i.e., inertial, elastic, or viscous load). Load types were applied in a random order and were then changed to another load type after 15 reaching trials. On each day, in total, subjects were asked to perform 20 reaching trials as a baseline test without additional load, three blocks including a total of 45 reaching trials (15 trials for each block with a single load type, which was chosen randomly) in the first set, and 45 reaching trials with the same order of loads in the second set (Fig. 1).
All subjects were asked to reach the target as consistently as possible and to perform similar movement trajectories, independent of load type. During the baseline task without additional loads, subjects were verbally instructed to maintain a consistent time duration to acquire the target. Between each block of trials, subjects were given a 5-min break. Between two sets, a 15-min break was provided to minimize fatigue and to limit possible effects of the previous load type on the ensuing reaching trials. Based on a prior study by Scheidt and colleagues (Stoeckmann et al. 2009), the magnitude of each type of load was set to have the same effective net force, which is the cumulative impeding force over time, for all load types. To determine if the speed of motor adaptation varies with the magnitude of the given loads and if improved motor adaptation from the first day could affect the adaptation to loads with different magnitudes on another day, subjects also participated in a second-day experiment with the different magnitudes of loads. The small and large sets of impedances had inertial (5 or 7 kg), elastic (1.2 or 1.7 N-m/rad), and viscous (10 or 15 N-s/m) loads, respectively. The order of two-day experiments and the types of the given loads on each day, which were designed to simulate various loads in daily activities after the training, were selected randomly.
Data collection and analysis
During target reaching, movement trajectories were recorded at 2.048 kHz using HapticMaster. The kinematic data were filtered using a 5th-order Butterworth low-pass filter with a cutoff frequency of 5 Hz. Velocity profiles recorded from the last five reaching trials for each load type were averaged and then defined as a fully adapted (skilled) movement trajectory. When compared to this reference velocity profile, an estimated error (\(y_{i}\)) of the ith reaching trial performed with the randomly chosen impedances was calculated as follows:
where \(v_{i}\), \(v_{{{\text{ref}}}}\), and \(t_{{{\text{reach}}}}\) are the velocity profile of the ith reaching trial, fully adapted (reference) velocity profile, and reaching time duration, respectively. To have the same affected contact forces (or same magnitude of a time integral of the contact forces) across load types, the time integral of the absolute value of the contact forces exerted by a subject was calculated for each load type. A ratio of these cumulative forces to the maximum of the time integral of contact forces between load types was then used to normalize the estimated velocity error (Eq. 1). The estimated errors over the reaching trials were then plotted and fitted with a motor learning curve (Silva et al. 2013) as follows:
to compare motor adaptation speeds between different types of loads, where y, a, b, c, and x are the velocity errors from the fully adapted trial (Eq. 1), the initial performance of the target reaching, the speed of adaptation, the final error, and the number of reaching trials. From the fitted learning curve, the number of reaching trials (time) required to reduce the velocity deviation to 36.8%, which is a definition of the time constant (Lipták 2018), was calculated to quantitatively assess the speed of motor adaptation in response to the given load during the reaching task. This parameter, the time constant, has been generally used, especially for decaying exponential curves in control theories and was defined as an inverse of the exponent of the exponential curve, which represents how swiftly an exponential function decays. One of the important characteristics of the time constant is that with the reaching trial number of the time constant, the response (error) decreases to 36.8%. Since the authors tried to explain and fit the error curve with the decaying exponential motor learning curve (Eq. 2), it is possible to quantify how swiftly human subjects reduce the velocity error by estimating the number of reaching trials required to reduce the error below 36.8%.
For statistical analyses, 2-way ANOVAs were performed to determine if there was a significant difference in the performance parameters, including the number of reaching trials, to reduce the velocity error to 36.8% between the load types and load magnitudes and to see if there was a significant interaction between the load types and load magnitudes. For follow-up t-tests with multiple comparisons, the Tukey’s honest significant difference (HSD) criterion was used.
Results
All subjects completed the specified reaching task with virtually imposed inertial, elastic, and viscous loads. Following successful adaptation with the imposed loads, the velocity–time profiles during the loaded reaching tasks converged to a specific velocity–time profile for each load type. Figure 2 illustrates the response of the limb motion to the different load types. Especially with small inertial loads, most subjects overshot the target in the first few trials (blue up arrow in Fig. 2), but this overshoot and corresponding initial error diminished swiftly as adaptation progressed (Fig. 2). With the inertial load, the velocity–time profiles showed a single, symmetric, bell-shaped positive peak, as was initially recorded in the baseline task without additional loads. The elastic and viscous loads, however, showed a substantial undershooting in the first trial (red and yellow up arrows in Fig. 2), and their velocity–time profiles showed an asymmetric positive peak, which was skewed to the left (early phase of reaching).
Kinematic data during the target reaching task from a representative subject with a set of small (upper panels) and large loads (lower panels). From each load magnitude, each row indicates a position (top), velocity (middle), and acceleration (bottom). The first and last reaching trials are shown in blue and red solid lines, respectively. The blue arrow indicates that a subject overshoot the target with the inertial load. The red and yellow arrows indicate an undershoot
Figure 3 illustrates manipulating forces exerted by a user during the task. The inertial load showed both forward-directional (solid up arrows in Fig. 3) and backward-directional (dotted down arrows in Fig. 3) forces for the initial acceleration and deceleration phases, respectively. The viscous and elastic loads, however, showed only forward-directional (positive) manipulating forces with relatively small or almost zero negative manipulating forces (Fig. 3). While the viscous and inertial loads showed a single positive peak in the manipulating forces, the elastic load showed multiple, at least two, positive peaks in the force profiles (red arrows in Fig. 3).
The contact forces exerted by a representative subject with a set of small (top) and large (bottom) loads, which consisted of inertial (blue), elastic (red), and viscous (yellow) loads. Shaded areas indicate ± 1 SD. Solid and dotted arrows indicate positive and negative peak forces for acceleration and deceleration, respectively
Figure 4 shows the velocity error (Eq. 1) as a function of reaching trials from the 1st and 2nd set with each load magnitude and each load type. The velocity deviation between the load-imposed trials and the fully adapted trial for each load type was calculated (Eq. 1) and then fitted with the motor learning curve (Eq. 2) (Fig. 4). From all subjects, the initial velocity error (Eq. 1) for the small inertial, elastic, and viscous loads was 47.77 (mean) ± 28.02% (1SD), 65.54 ± 50.65, and 86.58 ± 31.84%, respectively (Fig. 5). The large inertial, elastic, and viscous loads showed initial errors of 55.23 ± 14.47, 80.95 ± 35.53, and 55.17 ± 20.76%, respectively (Fig. 5). A significant difference in the initial velocity error was found across the load types (p = 0.0066 with the small loads and p = 0.0022 with the large loads). The small inertial load showed a significantly smaller initial error than the small viscous load (p = 0.0046). The large inertial (p = 0.0063) and viscous loads (p = 0.0062) showed a significantly smaller initial error than the large elastic load (Fig. 5).
The estimated velocity error (Eq. 1) as a function of the number of reaching trials from all subjects. Different shapes of data points indicate different subjects. The error was specified as the velocity deviation between the fully adapted trials for the inertial (left), elastic (center), and viscous (right) load types during the first (gray markers) and second (black markers) sets and was then fitted with the motor learning curve (Eq. 2) for the first (thin solid lines) and second (thick solid lines) sets. A gray horizontal line indicates an error of 36.8%
Comparison of initial errors from the first reaching trial between the load types with the small (left) and large (right) magnitudes of the given loads. Different shapes of data markers indicate different subjects from the 1st (gray dashed lines) and 2nd (black solid lines) sets. Average values of the initial errors from all subjects with inertial, elastic, and viscous loads are represented in blue, red, and yellow, respectively. Error bars indicate ± 1 confidence intervals. (*p < .05, **p < .01, ***p < .001)
Figure 6 indicates how many reaching trials were required for all subjects to show a consistent movement trajectory and to reduce the velocity error (velocity deviation from the fully adapted trial) to 36.8%. The participants required 1.80 ± 1.23, 4.46 ± 3.27, and 5.26 ± 2.01 trials for the small inertial, elastic, and viscous loads to reduce the velocity error to 36.8%, respectively (Fig. 6). For the large inertial, elastic, and viscous loads, 3.56 ± 2.35, 7.95 ± 2.94, and 3.49 ± 2.10 trials were required, respectively (Fig. 6). Different load types showed a significantly different number of reaching trials required to reduce the velocity error to 36.8% (p < 0.0001) with small loads (p = 0.0012) and large loads (p < 0.0001). No difference between the load magnitudes (p = 0.0675) was found but a significant effect of interaction between the load type and load magnitudes was found (p = 0.0038). Adaptation in response to the small inertial load was significantly faster than that in response to the small elastic (p = 0.0157) and viscous loads (p = 0.0013) (Fig. 6). Both the large inertial and large viscous loads showed faster adaptation than the large elastic load (p < 0.0001 and p < 0.0001, respectively). The elastic load only showed different adaptation speed between its two different magnitudes (p = 0.0219), while inertial (p = 0.0500) and viscous (p = 0.0702) loads did not. Eight and five out of ten participants showed the fastest adaptation speed with the small inertial load and large inertial load compared to other load types, respectively. Five participants showed the fastest adaptation speed with the large inertial load. No one showed the fastest adaptation with the small and large elastic loads. These numbers of reaching trials were not significantly affected by the previous load type (p > 0.05) for all load types and all load magnitudes. Six out of ten participants verbally reported that they could not recognize the existence of the added small elastic or viscous loads during the test, and three of them could not reduce the error to 36.8% within ten reaching trials with these load types. None the less, they did not show a significant difference in the speed of motor adaptation compared to other participants, who recognized the existence of the small elastic (p = 0.6180) or viscous (p = 0.6285) loads.
Comparison of number of reaching trials to reduce the error to 36.8% between the load types with the small (left) and large (right) magnitudes of the given loads. Different shapes of data markers indicate different subjects from the 1st (gray dashed lines) and 2nd (black solid lines) sets. Average values of the initial errors from all subjects with inertial, elastic, and viscous loads were represented in blue, red, and yellow, respectively. Error bars indicate ± 1 confidence intervals. (*p < .05, **p < .01, ***p < .001)
Figures 7 and 8 illustrate how participants regulated their acceleration profiles and manipulating forces as a result of adaptation to each load type. Overall, no significant difference in the ratio of peak acceleration to peak deceleration was found across the load types (p = 0.0709 and p = 0.0594 for the small and large loads, respectively) (Fig. 7). The large inertial load showed a significantly larger maximum contact force during the 1st half of the reaching phase (the acceleration phase) than the large elastic load (p = 0.0023) (Fig. 8). The peak decelerating forces showed more substantial and statistically different changes across load types (p = 0.0257 and p < 0.0001 for the small and large loads, respectively) (Fig. 8). During the second half of the reaching phase, the inertial load showed a negative decelerating contact force. Interestingly, almost zero or positive contact force was found, which is significantly different from the decelerating contact force with the inertial load, with the small (p = 0.0258) and large (p < 0.0001) elastic load, and with the large viscous load (p < 0.0001) (Fig. 8). The peak decelerating force was significantly different between the large elastic and viscous loads (p = 0.0276) (Fig. 8).
Comparison of peak contact forces during the first (0–50%) and second half (51–100%) of the reaching phase between the inertial (blue), elastic (red), and viscous (yellow) loads and between the small (left panels) and large (right panels) loads from all subjects. Error bars indicate ± 1 confidence interval. (*p < .05, **p < .01, ***p < .001)
Discussion
As hypothesized, we found that for intact subjects manipulating upper extremity loads using a robot (the HapticMaster), different numbers of trials were needed to achieve a criterion level of adaptation between position-, velocity-, and acceleration-dependent loads, which were simple passive inertial, elastic, and viscous loads. There were also differences in adaptation for different load magnitudes. These results indicate that intact humans have different proficiencies in predicting joint motions between several types of mechanical loads, and as a result, they reveal different adaptation speeds for different loads.
We observed that the inertial loads showed an overshoot in the hand positional trajectory during the first reaching trial (blue arrow in Fig. 2). As subjects started their reaching movements and recognized the imposed inertial load, they increased manipulating forces to compensate for the decreased reaching speed (Fig. 2). Conversely, as they approached the target, they often did not appear ready to exert the proper decelerating forces near the target. Since the additional inertial load made it difficult to stop swiftly, this induced a large overshoot of the reaching trajectory. In contrast, the viscous and elastic loads always impeded the forward-directional movements, like mechanical stabilizers, and these impedances appeared to help the deceleration near the target and induced a relatively smaller overshooting or even undershooting (red and yellow up arrows in Fig. 2) of the positional trajectory.
Interestingly, although the inertial load showed a large overshoot in the positional trajectory (blue arrow in Fig. 2), it showed faster speed of adaptation compared to the other types of loads (Fig. 6). Within just 1.80 ± 1.23 trials or 3.56 ± 2.35 trials for the small and large inertial loads, respectively, the error in the velocity profile decreased sharply to 36.8% (Fig. 6). These results are in line with previous studies reporting that intact humans require only one or two trials for movement correction in grip force generation and motor learning with unexpected changes in the weight of the supported load (Johansson and Westling 1984; Westling and Johansson 1984; Bock 1990).
In contrast, small elastic load, small viscous load, and large elastic load showed relatively slower adaptation speeds than the other load types and needed a significantly larger number of reaching trials to reach criterion performance (Fig. 6). In line with this result, a previous study (Flash and Gurevich 1997a) also reported that intact human subjects required five to seven reaching trials to achieve straight line movement trajectories and bell-shaped velocity profiles with imaginary spring forces. These results imply that humans may have selective proficiencies in movement detection across joint position, velocity, and acceleration and in manipulating the different types of loads, regardless of whether the load helps users stabilize their movements. Possible explanations for different adaptation speeds varying with types of loads are discussed below.
Movement Kinematics: Each of the velocity profiles recorded after adaptation to a particular load type looked broadly like a standard bell-shaped velocity profile with a single positive peak (Fig. 2), which appears to optimize energy expenditures (Uno et al. 1989) and to minimize higher motion derivatives such as a jerk (Flash and Hogan 1985; Flash and Gurevich 1997b). As the inertial load increased, the shapes of the velocity profiles remained almost unchanged and seemed to simply scale up, appearing very similar to the symmetric velocity peaks (Fig. 2). Since manipulating forces with the simulated inertial load are proportional to the product of a magnitude of the imposed inertia and its acceleration, manipulating force profiles also look like the acceleration profiles (Fig. 2), which consist of significant positive and negative peaks for acceleration and deceleration, respectively (Fig. 3).
Since elastic and viscous loads always exert resisting forces against the movement direction, subjects in this study utilized these impeding forces in the deceleration phases of the limb trajectory (Stoeckmann et al. 2009). Interestingly, even with almost zero negative contact forces with the viscous load and with positive contact forces with the elastic load during the deceleration phase (Figs. 3 and 8), subjects could still successfully decelerate their hand speed and stop motion near the target. This result implies that the subjects utilized simulated impeding forces, which were imposed by the haptic device against users’ movements, to decelerate their movements effectively as they got closer to the target instead of exerting braking (decelerating) contact forces using muscular effort in elbow flexors. Since such a change in the decelerating contact forces was significant with the elastic and viscous loads compared to the inertial load, we can further speculate that the larger force deviations from the null task without additional loads may explain the relatively slower adaptation speeds with the elastic and viscous loads compared to the inertial load. Moreover, especially with the elastic load, the subjects needed to identify not only the magnitude of stiffness of the simulated elastic load but also where the spatial coordinates of the elastic load were located. A previous study also supports this analysis by comparing the speed of motor learning between force fields, which have a linear and nonlinear relationship between the position and perturbations (Hwang et al. 2003). These additional spatial characteristics of the elastic load could potentially make adaptation more complicated and difficult to acquire than for viscous and inertial loads.
Interestingly, six out of ten participants reported that after completing the given tasks with these load types, they could not distinguish the small elastic or viscous loads. When learning a novel movement, even though planning all the temporal relationship between the engaged muscles and body joints was mostly done unconsciously, one might begin coordinating the required muscles as a conscious act within the cerebrum (Brindley 1964). Thus, if some participants did not recognize the additional load, they might not be able to trigger a motor correction swiftly, to modulate the motor commands and to utilize the gained information about the loads even while performing the consistent movement trajectories.
A previous study (Johansson et al. 1992) also suggested that humans could correct the grip force during vertical movement once the magnitude of the applied load exceeded the load amplitude threshold. In addition, when comparing the virtually imposed elastic loads (rotational stiffness of 1.7 and 0.85 N-m/rad) to passive elbow joint stiffness in intact human subjects (0.83 N-m/rad during elbow extensions in a horizontal plane) (Zhang et al. 2017), humans may not be sensitive enough to sense a load that has a similar or smaller magnitude when compared to the intrinsic (passive) impedance of the human joint. The participants, who were not able to recognize the small elastic and viscous loads, however, did not show a significantly different speed of adaptation with these loads as compared to remaining subjects, who successfully recognized the existence of these loads. Thus, this study cannot conclude possible effects of the sensible load threshold on the speed of motor adaptation, and it needs to be further investigated.
It has been proposed that humans may possess predetermined “models” of specific load mechanics and of limb structures and mechanics, labeled an “internal model” (Wolpert et al. 1998; Kawato 1999; Tomi et al. 2008). The existence of such models and perhaps the different speeds needed for updating such models may explain the different adaptation speeds for various mechanical load types. Previous behavioral studies (Wolpert et al. 1998; Kawato 1999) have suggested that such types of models may help humans generate anticipatory manipulating forces for novel objects. For example, during cyclic (periodic) vertical movements with a hand-held object, intact humans can predict the exact timing of maximum vertical acceleration that utilizes a maximum grip force, known from previous motor commands. Intact human subjects can also successfully match the timing of the peak grip force with the peak acceleration timing (Kawato 1999). Given the significant time delay for error corrections (up to 150 ms) between sensory recognition and the ensuing voluntary motor commands (Cooke and Diggles 1984), such accurate coincidence between peak grip forces and peak vertical accelerations can best be explained by the predictive control of manipulating forces using somatosensory feedback systems. Interestingly, such a capacity for exerting anticipatory grip forces seems not to be preserved in stroke survivors after cerebellar stroke (Nowak et al. 2003).
Different speeds of adaptation to novel loads could potentially be explained considering such internal model theory with two possible scenarios. First, if humans do not possess accurate models of many other applied loads, then they may need to develop new internal models for novel load types, which will result in relatively slow motor adaptation. In this case, since humans did not have a predeveloped internal model of the imposed load, they may require a longer time to identify the loads and to develop correct movements. Furthermore, as adaptation progresses, they will likely show gradually increasing proficiencies rather than quick and/or sharp adjustments of movements.
Conversely, another possible scenario is the concept of “rescaling” motor outflow (Nowak et al. 2003) using the already existing internal model of the load. According to this hypothesis, humans routinely acquire and then store kinematic and kinetic data required to move the upper limb with additional loads. When trying to move novel objects, humans would only need to rescale parameters of an already existing internal model associating upper limb states with the novel imposed loads, so they can quickly generate the required muscular forces. In line with these findings, several previous studies (Flanagan and Wing 1997; Gribble and Scott 2002; Milner and Franklin 2005) have suggested that humans can formulate internal models of routinely experienced loads and of body segments in their cerebellum, especially when adapting to objects and learning goal-directed movements. Notably, there is a possibility that humans develop such internal models only for specific types of loads and that existing internal models may be related to how frequently humans manipulate each type of load in daily life.
McIntyre and colleagues reported that astronauts initiated catching movements earlier in a 0 g environment than in a 1 g environment in falling-ball catching tasks (McIntyre et al. 2001), which implies that they had already adapted to an altered gravitational field. In line with this study, another study (Häger-Ross et al. 1996) also reported that intact human subjects showed faster movement and grip force corrections when external loads were in the same direction as gravity. Although it is not yet clear whether such a model does indeed exist in the human CNS, since humans have many experiences in weight handling in a normal gravity field, they may already have an excellent model of how to manipulate various weights and inertial loads but may not for other more complicated load types. Even though data interpretation in this study was rooted in the different speeds of updating the internal model and the inertial load in general and the large viscous load showed more rapid adaptation than other load types as hypothesized, it is worth noting that this study cannot determine directly if humans do have indeed such a predeveloped internal model for the passive inertial load (mass) and its neural correlates but not for other loads examined in this study.
Another approach, the equilibrium point (EP) theory, can also provide some possible explanations for these results. While internal model theory suggests that humans utilize afferent signals from somatosensory systems to recognize deviations between the current and planned movement trajectories and to update the internal model for preparing anticipatory manipulating forces (Flanagan and Wing 1997; Wolpert et al. 1998; Kawato 1999), EP theory also suggests that such motor adaptation is possible within the EP theory itself. For example, when correcting lifting and grip forces during a vertical movement with an object (Johansson and Westling 1984; Westling and Johansson 1984; Forssberg et al. 1992), humans generally go to the first EP determined by the planned threshold position of the related muscles and the environment, and if the weight of the held object is heavier than expected, they can change the threshold position for the next movement until smooth object manipulation is achieved (Feldman and Levin 2009). Therefore, it seems that swift motor adaptation with an unexpected change in load during vertical cyclic movement can be explained by both the EP theory and internal model theory, even though these two different approaches are not equivalent. According to this EP theory, humans can achieve the new EP by changing the threshold position when and where the required muscles need to be engaged and recruited (Pilon et al. 2007). In line with this theory, our results showed that the subjects needed to change the timing (or threshold) of activation in elbow extensors with elastic load. They recruited elbow extensors not only in the first half of the reaching phase but also in the second half with the second positive peak force to reach the target against the increasing elastic resisting forces, so almost zero or no backward force for deceleration was recorded (Figs. 3 and 8). Even though the viscous load also showed almost zero decelerating forces compared to the inertial load, it seems that the participants just turned off or deactivated the elbow flexors during the second half of the reaching phase without changing the timing of the first peak force during the first half. The slower adaptation to the elastic load than the viscous load implies that it might be more difficult to change the activation threshold in elbow flexors with the elastic load than deactivating or turning off elbow flexors with the viscous load. Notably, as ongoing debates on motor learning and control theories evolve, further studies are needed to determine which theory can better explain how the CNS encodes somatosensory systems and generates anticipatory forces efficiently.
Complicated kinematic and kinetic characteristics of an additional load may be a source of inconsistencies between previous studies determining the different speeds of motor adaptation between load types. Our results imply that the inertial load (passive point mass) showed faster adaptation than the small elastic load, small viscous loads, and large elastic load. These results are in accordance with important previous results (Bock 1990), which reported that the movement strategy is swiftly altered within just one or two trials with inertial loading. Our results, however, differ from some other studies, which reported that the acceleration-dependent load is harder to learn than the velocity-dependent load (Hwang and Shadmehr 2005; Hwang et al. 2006) because proprioceptive cues are encoded in the human brain in the form of joint position and velocity (Cordo et al. 1994). The latter studies (Hwang and Shadmehr 2005; Hwang et al. 2006), however, used the curl force fields, which turn out to be more complicated than the simple load types used in this study. For example, the acceleration-dependent force field changes its direction multiple times while a subject is approaching a target, while the velocity-dependent force field does not. In line with this speculation, Huang and Patton (Huang and Patton 2011) compared within-day reductions in the error between a single negative viscous load and the same viscous load combined with the added inertia for a planar circle-drawing task. They reported faster error reduction in the single viscous load condition, which implies that more complicated loads may require a longer time for adaptation. Gribble and Scott (Gribble and Scott 2002) also suggested that the internal model for more complex loads might even be formed by combining internal models for simpler loads.
Although the upper arm of the subject was tied to a mobile arm supporting device and subjects were asked to maintain the body position by leaning back in the chair, there might be a small free movement of the trunk. If that happened and if subjects used their trunk to exert forces against the given mechanical loads, this could potentially be a source of the reduced elbow flexion/extension and could arouse the limited magnitude of external mechanical loads, especially the elastic force, which were designed to be proportional to the elbow joint angular movement.
Finally, this study can potentially be utilized when assessing the capability for motor adaptation in patients with neurological disorders, especially in patients with stroke or spinal cord injury. It can provide the user with opportunities for handling various types of mechanical loads (Stoeckmann et al. 2009) and for interacting with different virtual obstacles (Van Der Linde and Lammertse 2003; Vato et al. 2014). Some recent studies have reported that the human cerebellum plays a critical role in motor adaptation based on sensory data (Wolpert et al. 1998; Tseng et al. 2007; Sokolov et al. 2017). After a cerebellar stroke, stroke survivors could adapt to a first load while reaching a target but could not adapt to subsequent changes in applied limb load. In addition, cutaneous anesthesia induces relatively slower motor corrections during a vertical lifting movement (Johansson and Westling 1984). Interestingly, excluding visual feedback did not appear to limit the capability for motor adaptation in intact human subjects (Johansson and Westling 1984), implying the importance of sensory feedback systems on motor adaptation to external loads. Thus, the results from this study in intact human subjects with different load types can (in due course) be compared with the results in patients with neurological disorders to assess how the damaged cerebellum and correspondingly impaired capability for updating the internal model or impaired somatosensory systems after stroke (Vidoni and Boyd 2009; Cherpin et al. 2019) limit the capability for motor adaptation to novel loads.
Conclusions
In this study, we examined how quickly intact humans could adapt to various types of loads during a target reaching task implemented with the upper extremity. We also sought to determine whether different magnitudes of the given load affected the speed of motor adaptation. While the swift adaptation to a viscous load could be explained by mechanoreceptors in the human body, which mainly detect joint angular velocity, faster adaptation speeds with the inertial load suggest that humans might have optimized the capability for manipulation of the inertial load, thanks to the tactile and proprioceptive sensory systems. In addition, our results imply that the nature of motor adaptation may vary with both the types and magnitudes of imposed loads. This finding is potentially due to different adaptation strategies for each load type, such as altered braking strategies and newly generated timing of activation of elbow extensors with the elastic load compared to the inertial load. Our study findings can potentially be utilized to assess capacities for adapting to external loads in patients with neurological disorders.
Data availability
All data generated or analyzed during this study are included in this published article. The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Analysis of all data in this study was performed using the Matlab software and the code, which will be available on reasonable request.
References
Bock O (1990) Load compensation in human goal-directed arm movements. Behav Brain Res 41:167–177
Brindley G (1964) The use made by the cerebellum of the information that it receives from sense organs. Intern Brain Res Organ Bull 3:80
Cherpin A, Kager S, Budhota A et al (2019) A preliminary study on the relationship between proprioceptive deficits and motor functions in chronic stroke patients. 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR). IEEE, pp 465–470
Cooke J, Diggles VA (1984) Rapid error correction during human arm movements: evidence for central monitoring. J Mot Behav 16:348–363
Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK (1994) Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol 71:1848–1861. https://doi.org/10.1152/jn.1994.71.5.1848
Crevecoeur F, Thonnard J-L, Lefevre P (2020) A very fast time scale of human motor adaptation: within movement adjustments of internal representations during reaching. eNeuro. https://doi.org/10.1523/ENEURO.0149-19.2019
Feldman AG (2016) Active sensing without efference copy: referent control of perception. J Neurophysiol 116:960–976
Feldman AG, Levin MF (2009) The equilibrium-point hypothesis–past, present and future. Progress in motor control. Springer, pp 699–726
Flanagan JR, Wing AM (1997) The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17:1519–1528. https://doi.org/10.1007/s00221-008-1691-3
Flash T, Gurevich I (1997a) Arm trajectory generation and stiffness control during motor adaptation to external loads. Self-organization computational maps and motor control. Elsevier, pp 423–482
Flash T, Gurevich I (1997b) Models of motor adaptation and impedance control in human arm movements. Adv Psychol 119:423–481. https://doi.org/10.1016/S0166-4115(97)80015-5
Flash T, Hogan N (1985) The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5:1688–1703
Forssberg H, Eliasson A, Kinoshita H, Johansson R, Westling G (1991) Development of human precision grip I: basic coordination of force. Exp Brain Res 85:451–457
Forssberg H, Kinoshita H, Eliasson A, Johansson R, Westling G, Gordon A (1992) Development of human precision grip. Exp Brain Res 90:393–398
Gribble PL, Scott SH (2002) Overlap of internal models in motor cortex for mechanical loads during reaching. Nature 417:938
Gritsenko V, Krouchev NI, Kalaska JF (2007) Afferent input, efference copy, signal noise, and biases in perception of joint angle during active versus passive elbow movements. J Neurophysiol 98:1140–1154
Häger-Ross C, Cole KJ, Johansson RS (1996) Grip-force responses to unanticipated object loading: load direction reveals body-and gravity-referenced intrinsic task variables. Exp Brain Res 110:142–150
Huang FC, Patton JL (2011) Evaluation of negative viscosity as upper extremity training for stroke survivors. 2011 IEEE International Conference on Rehabilitation Robotics. IEEE, pp 1–6
Hwang EJ, Shadmehr R (2005) Internal models of limb dynamics and the encoding of limb state. J Neural Eng 2:S266
Hwang EJ, Donchin O, Smith MA, Shadmehr R (2003) A gain-field encoding of limb position and velocity in the internal model of arm dynamics. PLoS Biol 1:e25
Hwang EJ, Smith MA, Shadmehr R (2006) Adaptation and generalization in acceleration-dependent force fields. Exp Brain Res 169:496–506
Johansson RS, Westling G (1984) Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56:550–564
Johansson R, Westling G (1988) Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res 71:59–71
Johansson RS, Häger C, Riso R (1992) Somatosensory control of precision grip during unpredictable pulling loads. Exp Brain Res 89:192–203
Kawato M (1999) Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9:718–727
Linde RVD, Lammertse P, Frederiksen EB (2002) The HapticMaster, a new high-performance haptic interface. Proc. EuroHaptic, Edinburgh
Lipták BG (2018) Instrument engineers handbook volume two: process control and optimization. CRC Press
Matthews P (1982) Where does Sherrington’s” muscular sense” originate? Muscles, joints, corollary discharges? Annu Rev Neurosci 5:189–218
McIntyre J, Zago M, Berthoz A, Lacquaniti F (2001) Does the brain model Newton’s laws? Nat Neurosci 4:693
Milner TE, Franklin DW (2005) Impedance control and internal model use during the initial stage of adaptation to novel dynamics in humans. J Physiol 567:651–664. https://doi.org/10.1113/jphysiol.2005.090449
Nowak DA, Hermsdörfer J, Topka H (2003) Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol 250:850–860
Pilon J-F, De Serres SJ, Feldman AG (2007) Threshold position control of arm movement with anticipatory increase in grip force. Exp Brain Res 181:49–67
Sherrington C (1906) The integrative action of the nervous system. C. Scribner’s sons, New York
Silva PS, Pereira P, Monteiro P, Silva PA, Vaz R (2013) Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus 35:E7
Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends in Cogn Sci 21:313–332. https://doi.org/10.1016/j.tics.2017.02.005
Stoeckmann TM, Sullivan KJ, Scheidt RA (2009) Elastic, viscous, and mass load effects on poststroke muscle recruitment and co-contraction during reaching: a pilot study. Phys Ther 89:665–678. https://doi.org/10.2522/ptj.20080128
Tomi N, Gouko M, Ito K (2008) Impedance control complements imcomplete internal models under complex external dynamics. 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp 5354–5357
Tseng Y-w, Diedrichsen Jr, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J neurophysiol 98:54–62
Uno Y, Kawato M, Suzuki R (1989) Formation and control of optimal trajectory in human multijoint arm movement. Biol Cybern 61:89–101. https://doi.org/10.1007/BF00204593
Van Der Linde RQ, Lammertse P (2003) HapticMaster-a generic force controlled robot for human interaction. Ind Robot 30:515–524. https://doi.org/10.1108/01439910310506783
Vato A, Szymanski FD, Semprini M, Mussa-Ivaldi FA, Panzeri S (2014) A bidirectional brain-machine interface algorithm that approximates arbitrary force-fields. PLoS ONE. https://doi.org/10.1371/journal.pone.0091677
Vidoni ED, Boyd LA (2009) Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct 5:1–10
Westling G, Johansson R (1984) Factors influencing the force control during precision grip. Exp Brain Res 53:277–284
Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2:338–347. https://doi.org/10.1016/S1364-6613(98)01221-2
Zhang L-Q, Son J, Park H-S, Kang SH, Lee Y, Ren Y (2017) Changes of shoulder, elbow, and wrist stiffness matrix post stroke. IEEE Trans Neural Syst Rehabil Eng 25:844–851
Acknowledgements
This project was supported under grant 90RES5013 from the U.S. Department of Health and Human Services, Administration on Community Living, National Institute on Disability, Independent Living and Rehabilitation Research. This work was also supported by the Korea Institute of Science and Technology (KIST) Institutional Program (Project no. 2E31110).
Funding
This project was supported under grant 90RES5013 from the U.S. Department of Health and Human Services, Administration on Community Living, National Institute on Disability, Independent Living and Rehabilitation Research. This work was also supported by the Korea Institute of Science and Technology (KIST) Institutional Program (Project no. 2E31110).
Author information
Authors and Affiliations
Contributions
All authors were heavily involved in study design, developing experimental apparatus, conducting an experiment, analyzing data, and writing the manuscript. KO carried out the experiments, performed statistical analysis, and drafted the manuscript. WZR designed the study and helped to write the manuscript. JC developed the experimental apparatus, helped to analyze the data, and finalized the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical approval
All subjects signed an informed consent form which was approved by the Northwestern University Institutional Review Board (IRB) before the experiments. This information was also described in the methods section.
Consent for participate
All subjects signed an informed consent form, which was approved by the Northwestern University Institutional Review Board (IRB) before the experiments.
Consent for publication
All images, including subjects, were de-identified and added to this manuscript with subjects’ consent.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oh, K., Rymer, W.Z. & Choi, J. The speed of adaptation is dependent on the load type during target reaching by intact human subjects. Exp Brain Res 239, 3091–3104 (2021). https://doi.org/10.1007/s00221-021-06189-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06189-3