Abstract
Ageing is accompanied by neuromuscular changes which may alter fatigue in older adults. These changes may include changes in corticospinal excitatory and inhibitory processes. Previous research has suggested that single joint fatiguing exercise decreases short-(SICI) and long-(LICI) interval intracortical inhibition in young adults. However, this is yet to be established in older adults. In 19 young (23 ± 4 years) and 18 older (69 ± 5 years) adults, SICI (2 ms interstimulus interval; ISI) and LICI (100 ms ISI) were measured in a resting first dorsal interosseous (FDI) muscle using transcranial magnetic stimulation (TMS) before and after a 15 min sustained submaximal contraction at 25% of their maximum EMG. Subsequent ten 2-min contractions held at 25% EMG were also performed to sustain fatigue for a total of 30 min, while SICI and LICI were taken immediately after each contraction. There was no change in SICI post-fatiguing exercise compared to baseline in both young and older adults (P = 0.4). Although there was no change in LICI post-fatiguing exercise in younger adults (P = 1.0), LICI was attenuated in older adults immediately post-fatiguing exercise and remained attenuated post-fatigue (PF)1 and PF2 (P < 0.05). Contrary to previous studies, the lack of change in SICI and LICI in young adults following a sustained submaximal EMG contraction suggests that GABA modulation may be dependent on the type of fatiguing task performed. The reduction in LICI in older adults post-fatiguing exercise suggests an age-related decrease in GABAB-mediated activity with sustained submaximal fatiguing exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular fatigue is defined as an exercise-induced reduction in the ability of a muscle or muscle group to produce muscle force (Taylor et al. 2000). Older adults are predisposed to differing magnitude and duration of fatigue due to age-related changes within the neuromuscular system. For instance, age-related slowing of the muscle mediated by a shift in muscle fibre type to a greater proportion of type I fibres (Andersen 2003; Lexell et al. 1983) and reduced calcium regulation (Hunter et al. 1999) increases fatigue resistance in older adults compared to young adults during isometric contractions (Bilodeau et al. 2001; Chung et al. 2007; Hunter et al. 2008; Yoon et al. 2012, 2013). On the other hand, this advantage is absent and both age groups tend to present with a similar degree of fatigue during low to moderate speed dynamic contractions, whilst older adults fatigue faster during high-speed dynamic contractions (Callahan and Kent-Braun 2011; Dalton et al. 2012; Yoon et al. 2013).
Transcranial magnetic stimulation (TMS) studies have shown that cortical modifications may mediate some of the fatigability differences between young versus older adults during isometric contractions. For instance, reduced modulation of corticospinal excitability and intracortical inhibition, evidenced by a smaller increase in the motor-evoked potential response (MEP) and silent period (SP) duration following fatiguing exercise, is observed in older adults when compared to the young adults (Yoon et al. 2012). SP (an index of intracortical inhibition) is the latency of suppression in the EMG signal during a voluntary contraction following a single pulse TMS. Since it is thought to be influenced by both spinal (< 50 ms) and intracortical γ-aminobutyric acid (GABAB)-mediated inhibitory mechanisms (> 100 ms) (Inghilleri et al. 1993), SP is typically not considered the most appropriate marker of intracortical inhibition. As a result, paired pulse TMS paradigms such as long-interval cortical inhibition (LICI) (Benwell et al. 2007; McNeil et al. 2011b; Valls-Sole et al. 1992) and short-interval cortical inhibition (SICI) (Benwell et al. 2006; Kujirai et al. 1993; Valls-Sole et al. 1992) are increasingly used. LICI, a reflection of GABAB-mediated inhibition, is measured with the application of a suprathreshold conditioning stimulus that attenuates the amplitude of a suprathreshold TMS-evoked test response at an interstimulus interval (ISI) of 50–200 ms (McDonnell et al. 2006); while SICI, a reflection of GABAA-mediated inhibition, is measured with the application of a subthreshold conditioning stimulus applied at an ISI of 1–5 ms (Kujirai et al. 1993). Contrary to SP, both SICI and LICI decline when measured in a resting muscle in the presence of fatigue in young adults; suggesting a decrease in GABAA and GABAB-mediated inhibition (Benwell et al. 2006, 2007; Maruyama et al. 2006). Using single-pulse TMS-evoked potentials (TEP) measured with electroencephalography (TMS-EEG) (Casarotto et al. 2010), we have recently demonstrated age-related differences in GABA modulation in the presence of fatigue (Opie et al. 2020). The TEP contains a series of peaks and troughs that last up to 300 ms (Bonato et al. 2006), the amplitudes of which reflect the excitability of the underlying cortical network. For example, pharmacological studies have shown that the N45 peak reflects GABAA-mediated activity (Ferrarelli et al. 2010), while the N100 peak reflects GABAB-mediated activity (Premoli et al. 2014). Our findings demonstrated a greater reduction in the N45 peak following fatiguing exercise in older adults suggesting a greater reduction in GABAA inhibitory activity; while younger adults displayed a greater reduction in the N100 peak, suggesting a greater reduction in GABAB-mediated inhibitory activity compared to older adults post-fatiguing exercise (Opie et al. 2020). Although there is some pharmacological and behavioural evidence of a relationship between TMS-EEG and TMS-EMG measures of inhibition (Bender et al. 2005; Bonnard et al. 2009; Premoli et al. 2014), it should also be acknowledged that it is not entirely clear as to whether paired pulse MEPs and TEPs are mediated by the same mechanisms (Biabani et al. 2019). In any case, the age-related effect of fatiguing exercise on SICI and LICI measured with TMS-EMG remains undetermined in older adults.
The aim of the current study is to determine age-related differences in SICI and LICI with sustained isometric fatiguing contractions. The current study used single joint fatiguing exercise of a hand muscle for a number of scientific and functional reasons, including the fact that there is a relatively large representation of the hand muscle in the motor cortex and that SICI and LICI can be reliably measured from the hand muscle in both young and older adults (Wassermann et al. 1992). On a functional level, hand muscles play a significant role in day-to-day activities including gardening, cleaning, holding shopping bags and are pivotal to independent living in older adults. We used a sustained contraction held at a constant submaximal EMG to fatigue the muscle because it allowed for the measurement of the development of fatigue during the contraction per se (via reduction in absolute muscle force and with no interruptions from performance of MVC). SICI and LICI were assessed at rest (Benwell et al. 2006, 2007) in order to obtain a sufficient amount of inhibition at baseline; since magnitude of inhibition is attenuated during activity (Ridding et al. 1995). We hypothesised that LICI and SICI would decline in both young and older adults as a consequence of fatiguing exercise. In addition, we hypothesised that younger adults would display a greater decline in LICI compared to older adults, while a greater decline in SICI would be observed in older adults compared to the young with fatiguing exercise.
Methods
We recruited nineteen young and eighteen older healthy participants from the university community, community centres and social media for participation in this study (Table 1). Any ongoing use of psychoactive medication (e.g. sedative, antipsychotics and antidepressants) or history of neurological and/or psychiatric disease excluded participants from the study. Physical activity assessment (work index, sport index, leisure-time index) was performed in both groups via an activity questionnaire (adapted from Baecke et al. 1982). The Edinburgh Handedness inventory was used to evaluate hand preference which confirmed that all participants were right-handed (Table 1). All procedures were performed in accordance with the ethical standards of the University of Adelaide Human Research Ethics Committee, which complies with the 1964 Declaration of Helsinki. Written, informed consent was provided by each participant prior to participation.
Experimental set-up and EMG recordings
Participants were seated upright with their right arm fixed onto a horizontal surface and index finger abducted against a force transducer (MLP 100 Transducer Techniques, Temecula California, USA) as previously described (Otieno et al. 2019). The elbow was flexed at approximately 90°, with the forearm and wrist restrained in a custom-designed manipulandum. Responses evoked from the right first dorsal interosseous (FDI) muscle were recorded using surface electromyography (EMG) with two Ag–AgCl electrodes placed over the muscle in a belly-tendon montage (8 mm diameter; 16 mm between electrodes; 3M Red Dot, Canada). Two grounding straps were also attached around the forearm. EMG was amplified (1000 times) and band-pass filtered (20 Hz high pass, 1 kHz low pass) using CED1902 hardware (Cambridge Electronics Design, Cambridge, UK) before being digitized at 2 kHz with a 1401 interface (Cambridge Electronics Design, Cambridge, UK) and stored offline for analysis.
Experimental protocol
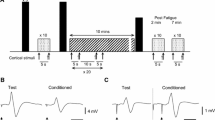
The study was comprised of two sessions (SICI, LICI) with two age groups (young, older adults) and repeated measures to monitor the effects of muscular fatigue over time. Figure 1 shows the experimental protocol carried out in each session. Prior to the fatiguing task, participants received twenty TMS pulses (ten single pulses, ten paired pulses, in five blocks of four stimulations) and two peripheral nerve stimulations (PNS) at rest. Maximum force and EMG were determined by calculating the average value of three, 3–5 s maximum voluntary contractions (MVCs) (separated by 30 s) performed by participants via abduction of the index finger. Participants then performed a submaximal contraction at 25% of their maximum EMG for a total of 15 min. A submaximal EMG, rather than force contraction, was selected in order to document the development of fatigue during the exercise per se, which is typically seen as a reduction in force magnitude during the contraction (Hunter et al. 2016; McNeil et al. 2011a). A verbal cue was provided which indicated when participants should begin exercise or rest. Participants were instructed to refrain from using other hand muscles during the exercise. Visual feedback of EMG output (smoothed using a 500 ms time constant) was displayed on a computer screen in front of the participant, and verbal encouragement was provided throughout the protocol. Immediately post-exercise, participants performed two brief 5 s MVCs with a 10 s rest period in between contractions. Following this, a set of stimulations consisting of six single TMS, six paired-pulse TMS and two PNS was delivered. The order of all single and paired pulse TMS was randomised, with the two PNS always delivered at the end. This was to ensure that the muscle contraction from the nerve stimulation did not impact the MEP response.
Experimental protocol schematic. Before fatiguing exercise, participants received ten single and ten paired pulse transcranial magnetic stimulation (TMS) as well as twoeripheral nerve stimulations (PNS) at rest (solid arrow). Force during three maximum voluntary contractions (MVCs) was measured during a 3- to 5-s index finger abduction before exercise. Participants then performed a sustained submaximal contraction at 25% of the maximum EMGrms of the first dorsal interosseous muscle via index finger abduction for a total of 15 min. Mean force measurements were calculated every 3 min during the fatiguing contraction (F1-F5). Immediately after the fatiguing exercise, participants performed two brief (5 s) MVCs with a 10 s rest period in between, followed immediately by six single and six paired pulse TMS as well as two PNS at rest (dotted arrow). Participants were then instructed to perform a 2 min contraction at 25% EMG in order to sustain fatigue (SF) followed by two brief (5 s) MVCs with a 10-s rest period in between. six single and six paired pulse TMSs were then given at rest. These contractions (SF1- SF10) and measurements were repeated ten times. Measurements taken during two consecutive SF contractions were pooled for post fatigue (PF) measurements (PF1-PF5). A 10-min recovery period was then provided followed by ten single TMS, ten paired pulse TMS and two PNS. Participants then performed one more 2-min contraction at 25% EMG immediately followed by two brief (5 s) MVCs with a 10-s rest period in between
In order to sustain fatigue, participants were instructed to perform a series of contractions that consisted of a two min submaximal contraction at 25% EMG followed by two MVCs and a set of stimulations (as detailed above post-exercise; also see Fig. 1). This was repeated ten times (sustained fatigue: SF1-SF10). A 10 min recovery period was then provided before a final set of twenty TMS pulses (ten single, ten double, in five blocks of four stimulations) and two PNS pulses. To conclude the session, participants were instructed to perform a final 2 min submaximal contraction at 25% EMG followed by two MVCs (Fig. 1). The difference in number of stimulations given between post-fatiguing contractions time points (six single and six paired TMS) versus baseline and recovery time points (ten single and ten paired TMS) is due to the fact that fatigue recovery is known to be fast (Carroll et al. 2017; Gandevia 2001; Kennedy et al. 2014), and a higher number of stimulations at the post-fatigue time points would have required too much time to measure. The protocol was designed to allow for 1-min rest period in between each of the SF1–SF10 contractions, and in this timeframe, only six single TMS, six paired-pulse TMS and two PNS could be feasibility delivered.
Peripheral nerve stimulation
Bipolar surface electrodes with conducting gel were placed at the wrist with the cathode positioned distal to the forearm. The location that produced the largest M-wave response in FDI at a current of approximately 12 mA (1 ms duration) using a constant-current stimulator (DS7A, Digitimer, UK) was marked as the site of stimulation. Stimulation intensity was gradually increased in increments of 5 mA until no further change in M-wave was observed to determine the maximum compound muscle action potential (Mmax). Mmax intensity was then set at 120% of the intensity required to produce the largest M-wave response at rest (Table 1).
Transcranial magnetic stimulation
TMS was applied to the left primary motor cortex using a figure-of-eight coil (external wing diameter 9 cm) with two monophasic Magstim 2002 magnetic stimulators connected through a Bistim (Magstim, Dyfed, UK). The coil was positioned at an angle of 45° to the sagittal plane, tangentially to the scalp, with the handle pointing laterally and backwards, creating an anteriorly directed current flow in the brain. The region that evoked the largest response in the relaxed FDI muscle at a fixed stimulator intensity (60–65% maximum stimulator output) was marked as the site of stimulation. This location was marked on the scalp using a pen, and the coil position was checked continually throughout the experiment. TMS was delivered at a rate of 0.2 Hz with a 10% variance in time between trials (Otieno et al. 2019).
Resting motor threshold (RMT) was defined as the lowest stimulus intensity that produced a response amplitude ≥ 50 µv in at least five out of ten trials in resting FDI muscle (Carroll et al. 2001). Active motor threshold (AMT) was defined as the lowest stimulus intensity to produce a visible MEP response relative to background EMG in at least five out of ten trials during a 5% MVC finger abduction contraction (Ortu et al. 2008).
Intracortical inhibition
SICI and LICI were assessed in a resting muscle as previously documented with fatigue (Benwell et al. 2006, 2007; Maruyama et al. 2006; Vucic et al. 2011). In SICI, subthreshold conditioning intensity (i.e. 70, 80 and 90% AMT) that evoked closest to 50% inhibition of the unconditioned test MEP response (set to 1 mV) at an ISI of 2 ms (Ortu et al. 2008) was selected for experimentation (Table 1). In LICI, suprathreshold conditioning intensity (110, 120, 130 and 140% RMT) that evoked closest to 50% inhibition of the unconditioned test MEP response (set to 1 mV) at an ISI of 100 ms (Benwell et al. 2007) was selected for experimentation (Table 1). SICI and LICI were measured in two separate sessions, separated by at least 48 h. Eleven young and eleven older adults participated in both sessions. The rest of the participants were not able to participate in both sessions due to withdrawals from the study because of personal circumstances (one young and two older participants) and inability to obtain inhibition at baseline (three young and three older participants).
Data analysis
All analyses were completed offline using Spike 2 software and custom-written scripts. Traces showing background voluntary EMG activity exceeding 10 µV in amplitude 100 ms prior to stimulation were removed from analysis (< 0.1% trials), to ensure the muscle was in a complete rested state, while measurements were taken (Otieno et al. 2019). Voluntary EMG (measured as root mean squared EMG; EMGrms) during each 25% EMG was calculated during the plateau in force of the 25% EMG contractions (~ 15 min or ~ 2 min for the 25% EMG contractions). Force amplitude during each 25% EMG contraction and MVC was also calculated during the plateau in force of the MVCs and 25% EMG contractions (~ 5 s for MVC and ~ 15 min or ~ 2 min for the 25% EMG contractions). MEP and Mmax amplitudes from each trial were measured as peak-to-peak in mV. Unconditioned MEPs were expressed as a percentage of Mmax to account for muscle-dependant changes (Samii et al. 1997; Todd et al. 2007). SICI and LICI were calculated as the ratio (expressed as a percentage) between the peak-to-peak amplitudes of the conditioned and unconditioned MEPs (conditioned/unconditioned × 100) (Opie and Semmler 2014). Therefore, an increase in SICI and LICI ratio in the study reflects less inhibition and vice versa (Fig. 2). Since there were age-related differences in SICI and MEP measures at baseline, (Tables 2 and 3) all variables were expressed as a percentage of baseline. EMGrms and force during the 15 min 25% EMG fatiguing exercise were binned and averaged into five time points (Fatigue 1–5; F1-F5). Force measured after the 15 min 25% EMG contraction (MVC); and during and after each of the 2 min 25% EMG contractions was represented as individual time points (Post, sustained Fatigue 1–10; SF1–SF10). MEP and Mmax measurements collected during the 1 min rest intervals after each of the 2 min 25% EMG sustained fatigue contractions were pooled across two sets and averaged into five bins (Post-fatigue 1–5; PF1–PF5; see Fig. 1).
Representative raw traces of conditioned and unconditioned MEP responses at pre, post and post fatigue (PF1) in both young and older adults during the LICI session. CS represents conditioning stimulus and TS represents test stimulus. There was a reduction in unconditioned MEP post fatigue in both age groups, an increase in conditioned MEP post fatigue in the older adult and an attenuation in conditioned MEP in the young adults
Statistical analysis
Linear mixed model with repeated measures (sensitive to analyses with varying data within each time point) was used to compare the effect of time and age on the magnitude of MVC, force, EMGrms, SICI, LICI, MEP and Mmax independently. Significant main effects were further investigated via Bonferroni’s post hoc tests corrected for multiple comparisons. For all variables, assumption of normality was violated as demonstrated with Shapiro–Wilk test (P < 0.05). As a result, all data sets were log transformed prior to statistical analysis. Statistical significance was set at P < 0.05, and we interpreted this as the level at which a significant main effect or interaction was observed in the statistical analysis. All data in figures are presented as means (exponentiated for all log transformed data) and 95% confidence interval of the mean. All data in text are presented as estimated mean differences (EMD) (exponentiated for all log transformed data) and 95% confidence interval for the estimate, providing a non-standardised measure of effect size. All data in tables are presented as means ± SE (Tables 2 and 3).
Results
Baseline measures
All participants completed the experiment in full and without any adverse reactions. No differences in RMT and AMT were found between groups (Table 1). Older adults had less SICI and greater MEP (%Mmax) compared to the young at baseline (Table 1). Older adults also demonstrated lower Mmax and MVC at baseline compared to the young during the LICI session (Table 1). Finally, older adults had a greater Leisure-time index compared to young adults in the SICI session (Table 1).
SICI session
SICI did not change across time (F7,755 = 1.4, P = 0.2), and no interaction between time and age (F7,773 = 1, P = 0.4) was observed. However, conditioned MEP size (% unconditioned MEP) was greater in younger adults compared to older adults post-fatigue (age effect: F1,499 = 10.9, P < 0.01) with an EMD of 119% (95% CI [107,132], P < 0.05; Fig. 3a). While there was no interaction between time and age (F7,729 = 1.2, P = 0.29), unconditioned MEP was reduced post-fatigue and gradually recovered across time compared to baseline in both populations (time effect: F7,738 = 15.1, P < 0.05) with EMD ranging from 277% (95% CI [199,384], P < 0.05) post-fatigue to 147% (95% CI [105,205],P < 0.05; Fig. 3b) at PF5. Unconditioned MEP was also greater in older adults compared to young adults (age effect: F1,365 = 11.7, P < 0.05) with EMD of 82% (95% CI [72,92], P < 0.05; Fig. 3b). Mmax did not change across time (F12,625 = 0.8, P = 0.63), and no interaction between time and age (F12,669 = 1.4, P = 0.12) was observed for Mmax. Mmax was, however, greater in the young compared to old (age effect: F1,139 = 4.9, P < 0.05) with an EMD of 119% (95% CI [102,139], P < 0.05).
Short interval intracortical (SICI) session. Effect of age and fatigue on exponentiated data. SICI (a), unconditioned MEP amplitude (b), MVC force (c) and force during 25% EMG (d) normalised to baseline in young (black bars and dark circles) and older (grey bars and clear circles) adults. #P < 0.05 young vs. older adults; *P < 0.05 compared to baseline. PF Post Fatigue; SF Sustained Fatigue
MVC changed across time (F12,423 = 11.7, P < 0.001) with a significant decline in maximum force observed immediately post-exercise (EMD, 132% (95% CI [118, 146], P < 0.05)) to recovery (EMD, 158% (95% CI [133,188], P < 0.05)). However, older adults showed less decrement in their MVC relative to baseline (age effect: F1,55 = 62.3, P < 0.05) with an EMD of 142% (95% CI[130,155], P < 0.05; Fig. 3c) between young and old. No interaction between time and age was observed for MVC (F12,389 = 1, P = 0.4; Fig. 3c). Force during 25% EMG (Fig. 3d) contraction also changed across time (F15,380 = 5.6, P < 0.05; Fig. 3d) with a significant decline in force from F2 (EMD, 139% (95% CI [117,166], P < 0.05)) to recovery (EMD, 180% (95% CI [115,283], P < 0.05)). However, no main effect of age (F1,88 = 1.2, P = 0.27), or interaction between time and age (F15, 388 = 1.4, P = 0.13) was observed on force during 25% EMG. EMGrms during the 25% EMG contraction did not change across time (F15,404 = 0.5, P = 0.9) nor was there any effect of age (F1,208 = 3, P = 0.08). No interaction between time and age (F15,417 = 0.2, P = 1.0) was observed either.
LICI session
LICI was reduced across time (F3,425 = 11.8, P < 0.05) with a significant age effect (F1,160 = 6.5, P < 0.05) and interaction between time and age (F3,398 = 3.1, P < 0.05; Fig. 4a). An increase in conditioned MEP (% unconditioned MEP) was evident immediately post-exercise (EMD, 252% (95% CI [148,427], P < 0.05)) to PF2 (EMD, 215% (95% CI [123,378], P < 0.05)) in the older adults relative to baseline, demonstrating a decrease in inhibition (see. Figs. 2 and 4a). However, no changes were seen in the younger adults across time. LICI was also reduced to a greater extent in older adults compared to the younger adults at PF1 (EMD, 203% (95% CI [138,299], P < 0.05)). Unconditioned MEP modulated across time (F7,789 = 6.3, P < 0.05; Fig. 3b), with an attenuation observed immediately post-fatigue that gradually recovered across the PF time points; with EMD ranging from 211% (95% CI [143,312], P < 0.05) at immediately post to 166% (95% CI [105,260], P < 0.01) at PF3). However, there was no main effect of age (F1,229 = 0.7; P = 0.4) or interaction between time and age (F7,765 = 1.4, P = 0.18) on unconditioned MEP (Fig. 4b). Mmax did not change across time (F12,616 = 0.9, P = 0.54) and no interaction was seen between time and age (F12,662 = 1.0, P = 0.46). Younger adults, however, displayed higher Mmax values throughout the exercise compared to older adults (age effect: F1,247 = 118.5, P < 0.05) with EMD at 244% (95% CI [208,288], P < 0.05).
Long interval intracortical (LICI) session. Effect of age and fatigue on exponentiated data. LICI (A), unconditioned MEP amplitude (B), MVC force (C) and force during 25% EMG (D) normalised to baseline in young (black bars and dark circles) and older (grey bars and clear circles) adults. #P < 0.05 young vs. older adults; *P < 0.05 compared to baseline. PF Post Fatigue; SF Sustained Fatigue
MVC changed across time (F12,333 = 7.0, P < 0.05) with a main effect of age (F1,64 = 61.8, P < 0.05; Fig. 4c) and interaction between time and age (F12,325 = 2.1, P < 0.05; Fig. 3c). Magnitude of force declined in the young (P < 0.05) with EMD ranging from 145% (95% CI [123, 170], P < 0.05) immediately post to 149% (95% CI [116, 191], P < 0.05) at recovery. However, older adults showed no decrement in their MVC relative to baseline throughout the protocol (P = 1.0). Force during 25% EMG contraction changed across time (F15,366 = 6.9, P < 0.05) with an interaction between time and age (F15,363 = 2.5, P < 0.05) observed. However, no main effect of age (F1,72 = 2.4, P = 0.12) was evident(Fig. 4d). A decline in force compared to F1 was seen in the young adults throughout the protocol (P < 0.05) with EMD ranging from 179% (95% CI [138, 232], P < 0.05) immediately post to 205% (95% CI [103, 413], P < 0.05) at recovery. On the other hand, older adults displayed a decline in force ranging from F3 (EMD, 145% (95% CI [101, 208], P < 0.05)) to SF4 (EMD, 188% (95% CI [104, 338], P < 0.05)) when compared to F1 (Fig. 4d). EMGrms during the 25% EMG contraction did not change across time (F15,403 = 0.8; P = 0.65) nor was there any age effect observed (F1,337 = 3; P = 0.08). No interaction between time and age (F15,406 = 0.5, P = 0.92) on EMGrms was observed either.
Discussion
Main findings
This aim of the current study was to determine whether there were age-related changes in GABA modulation following a sustained submaximal isometric contraction. While young adults were characterised by no change in SICI (Fig. 3a) or LICI (Fig. 4a), we provide evidence of an attenuation in LICI and no change in SICI with fatiguing exercise in older adults. The outcomes of the study suggest an age-related decrease in GABAB-mediated inhibition in the presence of fatigue, but no change in GABAA-mediated inhibition with fatigue in both young and old adults.
Fatigability in young and older adults
Neuromuscular fatigue has two origins—peripheral (distal to neuromuscular junction, e.g. due to impairment in the contraction coupling process) (Spriet et al. 1987) and central (proximal to neuromuscular junction) (Gandevia 2001). Central fatigue occurs when motor unit recruitment is insufficient and/or motor unit firing rates remain suboptimal due to variations in spinal reflex circuits and/or descending motor pathways (Sidhu et al. 2013). During a sustained submaximal force contraction, the active muscle fibres progressively fatigue leading to the recruitment of additional motor units to sustain the required force. This is usually seen as an increase in EMG during a fatiguing task (Fuglevand et al. 1993; Riley et al. 2008). However, in the current study, motoneuron output was ‘clamped’ by maintaining a constant 25% EMG output during the sustained contraction (Hunter et al. 2016; McNeil et al. 2011a). The implementation of an EMG contraction, rather than a force contraction to induce fatigue allowed us to document the development of fatigue during the course of the contraction by measuring the reduction in the magnitude of absolute force held by the participant, without having to interrupt the exercise to perform an MVC. As expected, force generating capacity of the muscle during the 25% EMG contraction was substantially reduced in both young and older adults during both sessions, illustrating the development of fatigue (Hunter et al. 2016). Although MVC force of the hand muscle was attenuated throughout the exercise protocol in young adults, older adults did not experience a similar decrease in maximum muscle force, albeit only during the LICI session, suggesting that the reduction in force during the 25% EMG contraction was insufficient to induce a change in maximum muscle force during this session. The absence of a reduction in MVC force in the LICI session in older adults may be attributed to participant variability, as four older participants were not matched between the two sessions. Indeed, the eleven older adults who were matched between the two sessions showed a decrease in MVC with the fatiguing exercise in both sessions. The fact that force was attenuated during the 25% EMG contraction but not during MVCs in some older adults may also be partially related to the differences in muscle fibre recruitment during the 25% EMG contraction versus MVC. For instance, during low level contractions, type I fibres (slow twitch, smaller in size) are largely recruited; whereas during MVCs, both type I and type II fibres (fast twitch, bigger in size) are recruited (Vollestad and Blom 1985). The discrepancy in fatigue measured between the two contraction types may be further colluded by the fact that the older adults are characterised by greater fatigue resistance (Callahan and Kent-Braun 2011; Yoon et al. 2012, 2013), mediated via the loss of the type II muscle fibres and a concomitant increase in type I muscle fibre composition (Andersen 2003; Lexell et al. 1988).
Change in MEP measures of corticospinal excitability
MEPs reflect the responsiveness of the corticospinal pathway (Gandevia et al. 1996). An increase in MEP size measured in a resting muscle during short-duration intermittent fatiguing contractions (Benwell et al. 2006, 2007; Otieno et al. 2019) suggests an increase in corticospinal excitability in the presence of single-joint exercise fatigue. However, similar to the outcomes reported following sustained submaximal contractions (Brasil-Neto et al. 1993; Sacco et al. 2000; Samii et al. 1997), we demonstrated a decrease in corticospinal excitability in both age groups following a 15 min submaximal EMG contraction and throughout the sustained fatiguing contractions. This post-exercise MEP depression has previously been attributed to intracortical inhibition, since centrally evoked responses by transcranial electrical stimulation (to directly activate spinal motoneurons) remained unchanged after exhaustive weightlifting repetitions of the wrist flexors (Brasil-Neto et al. 1993). Contrary to this, McNeil et al. (2011a) have shown a similar degree of suppression in responses evoked by TMS and spinal stimulations during the silent period of a 10 min sustained 25% EMG contraction, suggesting that a reduction in motoneuron excitability (possibly related to repetitive motoneuron activation during a sustained contraction), rather than a cortical excitability, may be responsible for the impairment in MEP. Our study was designed to investigate the presence of fatigue on intracortical mechanisms measured with respect to a resting muscle. Indeed, given the task- and context-dependant influence of fatigue on corticospinal responses (Taylor et al. 2016), parallels between studies that measure central responsiveness with short intermittent maximum versus sustained submaximal contractions, as well as during muscle activity (McNeil et al. 2009, 2011a) versus resting muscle (Benwell et al. 2006, 2007) may not necessarily be drawn. One consideration for the interpretation of the MEP data is the fact that while the absolute MEP amplitude was kept consistent between participants (1 mV), MEPs were larger in older adults when normalised to Mmax at baseline since older adults are characterised by smaller Mmax in the FDI (Opie and Semmler 2014). Therefore, it is possible that the amount of motoneuron activation influenced the baseline magnitude of inhibition, as in the case of SICI. However, it may also be argued otherwise since the increase in the amount of motoneuron activation did not influence baseline LICI. In any case, to account for these baseline differences, and to address the primary aim of age-mediated influence of fatigue on central responsiveness, we normalised all the post-fatigue data to the baseline values.
SICI and LICI post-fatiguing exercise in a resting muscle
Paired pulse paradigms including SICI and LICI reflect the suppression of later indirect waves (I-waves) such as I3, which follow the corticospinal short latency direct wave (D wave) (Di Lazzaro et al. 1998, 2002; Reis et al. 2008). SICI is known to be mediated by GABAA (Di Lazzaro et al. 2000) while LICI is thought to be mediated by GABAB inhibitory interneurons (McDonnell et al. 2006). When measured at rest, a decrease in SICI and LICI following intermittent fatiguing maximum contractions (Benwell et al. 2006, 2007; Maruyama et al. 2006; Vucic et al. 2011) and after a 2 min maximum fatiguing contraction (Maruyama et al. 2006) has been reported in young healthy individuals, suggesting a decrease in GABAA and GABAB mediated inhibition, respectively. A similar decline in SICI has been reported with sustained submaximal isometric contraction when measured in an active muscle (15–25% MVC) (Hunter et al. 2016; Williams et al. 2014). In the current study, we measured inhibition in a resting muscle instead of active muscle immediately after sustained fatiguing contractions and observed no change in both SICI and LICI in the young adults. This lack of change in SICI and LICI may be attributed to the different exercise modalities used between studies (short duration intermittent exercise versus longer duration sustained exercise), suggesting that the modulation of GABA inhibitory processes with fatiguing exercise is likely task dependant. Interestingly, McNeil and colleagues have identified a contradictory increase in GABAB-mediated inhibition during fatiguing maximum and submaximal isometric exercise in young adults (McNeil et al. 2009, 2011a). However, in addition to the fact that they measured inhibition during an active contraction, they also demonstrated that impaired motoneuron responsiveness played a predominant role in the increase in GABAB inhibition.
With no change in SICI, older adults showed a decrease in LICI. Contrary to our recent TMS-EEG data showing an age-related decrease in GABAA-mediated inhibitory activity with intermittent maximal fatiguing exercise (Opie et al. 2020), the current outcomes demonstrate an age-related decrease in GABAB-mediated inhibitory activity with sustained submaximal EMG contraction; suggesting a task-dependant GABAB modulation in the presence of fatigue in older adults. Even though there is pharmacological and behavioural data demonstrating that TME-EEG data are sensitive to changes in intracortical inhibition (Bender et al. 2005; Bonnard et al. 2009; Premoli et al. 2014), there is some recent data that questions the relationship between LICI elicited with paired pulse MEPs and TEPs (Biabani et al. 2019), which may partly explain the contradictory observations between our previous work using TMS-EEG (Opie et al. 2020) and the current outcomes with TMS-EMG. In addition, even though we are not able to exclude the role of spinal mechanisms in the modulation of GABAB mediated inhibition in older adults (McNeil et al. 2011a), there is evidence from TMS-EEG data to show that GABAB modulation (as measured with LICI) is cortically mediated (Opie et al. 2017). Furthermore, while fatigue was sustained for a prolonged period, its effect on GABA modulation did not persist during maintenance of fatigue in older adults. This implies that GABA modulation may play a role in the initial stages of fatigue but not when fatigue is sustained for a longer period in older adults.
Methodological considerations
While it may be argued that measurements during the fatiguing task per se or during muscle activity provide greater functional representation (Sidhu et al. 2014, 2018), measurement of intracortical inhibition during muscle activity also presents challenges. For example, muscle activity attenuates the magnitude of inhibition (Ridding et al. 1995), making it difficult to obtain sufficient magnitude of inhibition at baseline (i.e. ~ 20–30% during a 25% muscle contraction). Interestingly, we were not able to get sufficient inhibition in ~ 13% of the recruited participants even in a resting FDI muscle. These participants were excluded from data collection. We targeted baseline SICI and LICI that was closest to 50% in all participants to ensure that there was neither a peak nor a trough at baseline to allow for fatigue-related modulations in either direction. Furthermore, our experimental design of measuring inhibition in a rested muscle allows for better comparison of outcomes with recent studies from our group investigating cortical inhibition using TMS-EEG, whereby measurements have to be implemented at rest to avoid confounding influences of movement artefacts on the EEG recordings (Opie et al. 2020; Otieno et al. 2019). An additional consideration is that there are sex differences in fatigability whereby females are typically characterised by greater fatigue resistance due to greater oxygen availability during exercise (Ansdell et al. 2019a); and fatigability and SICI vary across the menstrual cycle (Ansdell et al. 2019b). We did not control for the menstrual cycle phase of the young females in the current study, and this forms a limitation of this work. However, given that most previous work has focused on hormonal influences of fatigue in large locomotor muscles, the evidence for sex differences in fatigability during small muscle exercise remains equivocal. Indeed, in the current study, we did not observe any differences in FDI muscle fatigability between males versus females. Finally, there is some evidence to show that averaging less than 20 simultaneous MEP responses results in increased variability (Biabani et al. 2018). Whilst we were limited with the number of stimulations delivered post-fatigue due to the quick recovery of fatigue, the low number of stimulations (10–12 at each time point including baseline) should be acknowledged.
Conclusion
The current findings suggest that older adults modulate LICI with a submaximal fatiguing contraction by attenuating GABAB-mediated inhibition. Nonetheless, further investigation into the age-related effects of fatigue on LICI and SICI via implementation of exercise models that induce a similar degree of fatigue in both groups forms an important expansion of the present investigation. Investigating GABA mechanisms in larger muscle groups of the upper and lower limbs may also provide a more functional representation of the age-related modulation of GABA inhibitory processes with fatiguing exercise. The discovery of these components may contribute to the development of interventions and training that effectively improve work efficiency, improve exercise tolerance and preserve function in older adults.
Abbreviations
- AMT:
-
Active motor threshold
- CS:
-
Conditioning Stimulus
- EMG:
-
Electromyography
- FDI:
-
First dorsal interosseous
- GABAA :
-
Gamma aminobutyric acid type A receptor
- GABAB :
-
Gamma aminobutyric acid type B receptor
- ISI:
-
Interstimulus interval
- LICI:
-
Long-interval cortical inhibition
- SICI:
-
Short-interval cortical inhibition
- MEP:
-
Motor-evoked potential
- M max :
-
Maximum compound muscle action potential
- MVC:
-
Maximum voluntary contraction
- PNS:
-
Peripheral nerve stimulation
- RMT:
-
Resting motor threshold
- TMS:
-
Transcranial magnetic stimulation
- TS:
-
Test Stimulus
References
Andersen JL (2003) Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13:40–47
Ansdell P, Brownstein CG, Skarabot J, Hicks KM, Howatson G, Thomas K, Hunter SK, Goodall S (2019) Sex differences in fatigability and recovery relative to the intensity-duration relationship. J Physiol 597:5577–5595
Ansdell P, Brownstein CG, Skarabot J, Hicks KM, Simoes DCM, Thomas K, Howatson G, Hunter SK, Goodall S (2019) Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol (1985) 126:1701–1712
Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Bender S, Basseler K, Sebastian I, Resch F, Kammer T, Oelkers-Ax R, Weisbrod M (2005) Electroencephalographic response to transcranial magnetic stimulation in children: evidence for giant inhibitory potentials. Ann Neurol 58:58–67
Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW (2006) Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res 170:191–198
Benwell NM, Mastaglia FL, Thickbrrom GW (2007) Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res 179:255–262
Biabani M, Farrell M, Zoghi M, Egan G, Jaberzadeh S (2018) The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci Lett 674:94–100
Biabani M, Fornito A, Coxon JP, Fulcher BD, and Rogasch NC (2019) Do TMS evoked responses measured from scalp and hand represent the same cortical mechanisms? . https://wwwbiorxivorg/content/101101/765875v1.
Bilodeau M, Henderson TK, Nolta BE, Pursley PJ, Sandfort GL (2001) Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol (1985) 91:2654–2664
Bonato C, Miniussi C, Rossini PM (2006) Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol 117:1699–1707
Bonnard M, Spieser L, Meziane HB, de Graaf JB, Pailhous J (2009) Prior intention can locally tune inhibitory processes in the primary motor cortex: direct evidence from combined TMS-EEG. Eur J Neurosci 30:913–923
Brasil-Neto JP, Pascual-Leone A, Valls-Sole J, Cammarota A, Cohen LG, Hallett M (1993) Postexercise depression of motor evoked potentials: a measure of central nervous system fatigue. Exp Brain Res 93:181–184
Callahan DM, Kent-Braun JA (2011) Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol (1985) 111:1345–1352
Carroll TJ, Riek S, Carson RG (2001) Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods 112:193–202
Carroll TJ, Taylor JL, Gandevia SC (2017) Recovery of central and peripheral neuromuscular fatigue after exercise. J Appl Physiol (1985) 122:1068–1076
Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, Defendi S, Mariotti M, Massimini M (2010) EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS ONE 5:e10281
Chung LH, Callahan DM, Kent-Braun JA (2007) Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol (1985) 103:1628–1635
Dalton BH, Power GA, Vandervoort AA, Rice CL (2012) The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47:85–92
Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC (1998) Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119:265–268
Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC (2000) Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol 111:794–799
Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC (2002) Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol 113:1673–1679
Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G, Pearce RA (2010) Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A 107:2681–2686
Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM (1993) Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460:549–572
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Gandevia SC, Allen GM, Butler JE, Taylor JL (1996) Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490(Pt 2):529–536
Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD (1999) Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol (1985) 86:1858–1865
Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL (2008) Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol (1985) 105:1199–1209
Hunter SK, McNeil CJ, Butler JE, Gandevia SC, Taylor JL (2016) Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res 234:2541–2551
Inghilleri M, Berardelli A, Cruccu G, Manfredi M (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466:521–534
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2014) Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol (1985) 116:385–394
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M (1983) Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6:588–595
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Maruyama A, Matsunaga K, Tanaka N, Rothwell JC (2006) Muscle fatigue decreases short-interval intracortical inhibition after exhaustive intermittent tasks. Clin Neurophysiol 117:864–870
McDonnell MN, Orekhov Y, Ziemann U (2006) The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173:86–93
McNeil CJ, Martin PG, Gandevia SC, Taylor JL (2009) The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587:5601–5612
McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL (2011) Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589:3533–3544
McNeil CJ, Martin PG, Gandevia SC, Taylor JL (2011) Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res 209:287–297
Opie GM, Semmler JG (2014) Age-related differences in short- and long-interval intracortical inhibition in a human hand muscle. Brain Stimul 7:665–672
Opie GM, Sidhu SK, Rogasch NC, Ridding MC, and Semmler JG.(2017) Cortical inhibition assessed using paired-pulse TMS-EEG is increased in older adults. Brain Stimul.
Opie GM, Otieno LA, Pourmajidian M, Semmler JG, Sidhu SK (2020) Older adults differentially modulate transcranial magnetic stimulation-electroencephalography measures of cortical inhibition during maximal single-joint exercise. Neuroscience 425:181–193
Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC (2008) Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586:5147–5159
Otieno LA, Opie GM, Semmler JG, Ridding MC, Sidhu SK (2019) Intermittent single-joint fatiguing exercise reduces TMS-EEG measures of cortical inhibition. J Neurophysiol 121:471–479
Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, Espenhahn S, Heidegger T, Muller-Dahlhaus F, Ziemann U (2014) TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 34:5603–5612
Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG (2008) Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586:325–351
Ridding MC, Taylor JL, Rothwell JC (1995) The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol 487(Pt 2):541–548
Riley ZA, Maerz AH, Litsey JC, Enoka RM (2008) Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586:2183–2193
Sacco P, Thickbroom GW, Byrnes ML, Mastaglia FL (2000) Changes in corticomotor excitability after fatiguing muscle contractions. Muscle Nerve 23:1840–1846
Samii A, Wassermann EM, Hallett M (1997) Post-exercise depression of motor evoked potentials as a function of exercise duration. Electroencephalogr Clin Neurophysiol 105:352–356
Sidhu SK, Cresswell AG, Carroll TJ (2013) Corticospinal responses to sustained locomotor exercises: moving beyond single-joint studies of central fatigue. Sports Med 43:437–449
Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M (2014) Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592:5011–5024
Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ, Amann M (2018) Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596:4789–4801
Spriet LL, Soderlund K, Bergstrom M, Hultman E (1987) Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J Appl Physiol (1985) 62:616–621
Taylor JL, Allen GM, Butler JE, Gandevia SC (2000) Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol (1985) 89:305–313
Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL (2016) Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48:2294–2306
Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC (2007) Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol (1985) 102:1756–1766
Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85:355–364
Vollestad NK, Blom PC (1985) Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand 125:395–405
Vucic S, Cheah BC, Kiernan MC (2011) Dissecting the mechanisms underlying short-interval intracortical inhibition using exercise. Cereb Cortex 21:1639–1644
Wassermann EM, McShane LM, Hallett M, Cohen LG (1992) Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85:1–8
Williams PS, Hoffman RL, Clark BC (2014) Cortical and spinal mechanisms of task failure of sustained submaximal fatiguing contractions. PLoS ONE 9:e93284
Yoon T, Schlinder-Delap B, Keller ML, Hunter SK (2012) Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. J Appl Physiol (1985) 112:849–858
Yoon T, Schlinder-Delap B, Hunter SK (2013) Fatigability and recovery of arm muscles with advanced age for dynamic and isometric contractions. Exp Gerontol 48:259–268
Funding
Ms Lavender Otieno is funded by the Adelaide Graduate Research Scholarships Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Communicated by Winston D Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Otieno, L.A., Semmler, J.G. & Sidhu, S.K. Single joint fatiguing exercise decreases long but not short–interval intracortical inhibition in older adults. Exp Brain Res 239, 47–58 (2021). https://doi.org/10.1007/s00221-020-05958-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05958-w