Abstract
We explored the origin of the impaired control of action stability in Parkinson’s disease (PD) by testing levodopa-naïve PD patients to disambiguate effects of PD from possible effects of long-term exposure to levodopa. Thirteen levodopa-naïve PD patients and 13 controls performed single- and multi-finger force production tasks, including producing a self-paced quick force pulse into a target. A subgroup of patients (n = 10) was re-tested about 1 h after the first dose of levodopa. Compared to controls, PD patients showed lower maximal forces and synergy indices stabilizing total force (reflecting the higher inter-trial variance component affecting total force). In addition, PD patients showed a trend toward shorter anticipatory synergy adjustments (a drop in the synergy index in preparation to a quick action) and larger non-motor equivalent finger force deviations. Lower maximal force, higher unintentional force production (enslaving) and higher inter-trial variance indices occurred in PD patients after one dosage of levodopa. We conclude that impairment in synergies is present in levodopa-naïve patients, mainly in indices reflecting stability (synergy index), but not agility (anticipatory synergy adjustments). A single dose of levodopa, however, did not improve synergy indices, as it did in PD patients on chronic anti-PD medication, suggesting a different mechanism of action. The results suggest that indices of force-stabilizing synergies may be used as an early behavioral sign of PD, although it may not be sensitive to acute drug effects in drug-naïve patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of multi-finger interaction and coordination have documented significant changes in patients with early-stage Parkinson’s disease (PD), particularly in indices reflecting the neural control of action stability (Park et al. 2012; Jo et al. 2015; reviewed in Latash and Huang 2015). These studies used the framework of the uncontrolled manifold (UCM) hypothesis (Scholz and Schoner 1999) to quantify indices of stability during steady-state multi-finger accurate force production, as well as indices of agility associated with a drop in the stability indices in preparation to a quick action (anticipatory synergy adjustments or ASAs, Olafsdottir et al. 2005). If a person plans to perform a quick action, high stability of the performance variable to be changed is antithetical to the planned change. ASAs reflect a neural mechanism of feed-forward preparation to an action, a mechanism that ensures a drop in the stability index.
According to the UCM hypothesis, the central nervous system organizes commands to individual fingers to stabilize a salient performance variable (such as the total force, FTOT). This leads to two consequences. First, inter-trial variance in the finger force space is higher in directions that do not affect FTOT (within the UCM for FTOT) compared to directions that affect FTOT (orthogonal to the UCM, ORT), resulting in a signature inequality: VUCM > VORT. Second, changes in individual finger forces between two steady states separated by a quick action are large within the UCM (motor equivalent, ME) compared to displacements orthogonal to the UCM (non-motor equivalent, nME) (Mattos et al. 2011, 2015).
Early-stage PD has been shown to lead to smaller differences between VUCM and VORT and shorter and smaller ASAs (reflecting impaired agility). These differences were seen in the apparently unaffected hands of Hoehn and Yahr (1967) stage I PD patients (Park et al. 2012, 2014) and even in healthy persons at high risk for developing parkinsonian signs such as professional welders (Lewis et al. 2016). In PD patients with history of being treated chronically with anti-PD drugs, levodopa, which is the most effective dopamine-replacement medication, has been shown to improve both indices of stability and agility (Park et al. 2014), whereas deep brain stimulation improved the latter, but not the former (Falaki et al. 2018).
The described findings could be direct reflections of changes in the neural circuitry in PD and/or of long-term exposure to dopamine-replacement drugs. To disambiguate the effects of PD from possible effects of drugs, we tested a group of levodopa-naïve PD patients. Based on earlier studies, we expected these levodopa-naïve patients to exhibit lower indices of stability and agility compared to age-matched controls (Hypothesis 1). Testing this hypothesis is important for possible future use of synergy indices as an early behavioral sign of PD.
We also re-tested a subgroup of the PD patients about 1 h after they had taken their first dosage of levodopa. Based on the mentioned studies, we expected medication to improve indices of both stability and agility (Hypothesis 2). This hypothesis is highly non-trivial given that long-term exposure to dopamine-replacement medications is known to lead to major changes in brain circuitry, including changed sensitivity to drugs (Feigin et al. 2002; Hershey et al. 2003; Politis et al. 2017) and even cases of tardive dyskinesia.
We also explored the effects of levodopa-naïve PD and levodopa on indices of performance (such as maximal voluntary force, MVC, and peak rate of force production during the force pulse) and finger interaction (such as enslaving, reflecting unintentional force production by non-instructed fingers, Zatsiorsky et al. 2000). Based on earlier studies, we expected lower MVC force and higher enslaving in PD patients compared to the controls and that levodopa would lead to an increase in MVC force and peak rate of force production, without affecting enslaving (cf. Park et al. 2014).
Methods
Participants
Thirteen levodopa-naïve PD individuals and 13 healthy controls participated in this study. Detailed demographic and clinical information are presented in Table 1. Levodopa-naïve PD subjects were invited to take part if they were not taking any form of levodopa as part of their regular medications. Ten PD participants had not taken any anti-PD medication, one had taken Amantadine (100 mg, t. i. d, Subject 7, Table 1), one had taken Selegiline (5 mg, b. i. d., Subject 8, Table 1), and one had taken both Pramipexole (ER 4.5 mg/day, and 0.25 mg, b. i. d.) and Selegiline (5 mg, b. i. d., Subject 13, Table 1). Controls had no known neurological disorders and were free from musculoskeletal and joint disorders in their upper extremities. A subgroup of 10 PD individuals (first 10 listed in Table 1) was tested twice, with the first evaluation occurring in the morning and the second test ~ 60 min after taking the very first dose of carbidopa/levodopa 25/100 at lunch time. The other three participants in the PD group were assessed only once as they decided against initiating levodopa therapy at the time of study. Prior to their participation, all individuals gave written informed consent according to the protocol approved by the Penn State Hershey Institutional Review Board. After giving consent and before performing the main experiment, the subjects were assessed using the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III, Goetz et al. 2008) and performed the Nine Hole Peg Test (9HPT, Mathiowetz et al. 1985).
Apparatus
Four uniaxial force sensors (Honeywell, Model 31, 25 LBS, Columbus, OH, USA) mounted on slots of a steel frame (140 × 90 mm), 3-cm apart from each other in the medial–lateral direction were used in this study. The sensors measured the downward pressing forces produced by the index, middle, ring, and little fingertips. The top surfaces of the sensors were covered with sandpaper (100-grit) to increase friction.
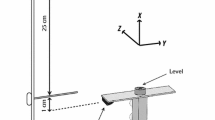
The subjects sat with their forearm in the sagittal plane, parallel to the ground, and placed each fingertip of the hand on the corresponding force sensor. The sensor positions were adjusted in the anterior–posterior position to allow each finger to be curved slightly, making the hand look like a dome. A wooden piece was placed underneath the subject’s palm to maintain a constant hand and finger configuration during the tests. A Velcro strap was placed slightly above the wrist joint of the subject to restrain the forearm to avoid its movement during the tasks. A 19′′ monitor, positioned approximately 0.6 m from the subject’s face provided visual feedback on the total downward force (FTOT) produced by the fingers or by one of the fingers in single-finger ramp task (see later). Figure 1 shows a schematic representation of the experimental setup.
Before each trial, all sensor signals were set at zero when subjects rested their fingertips on the sensor centers and relaxed their hands. Consequently, only active downward forces were measured by the sensors. A customized LabVIEW routine (2014 version, National Instruments, Austin, TX, USA) was used to present visual feedback and acquire the force data. The force data were recorded at 1000 samples per second with a 16-bit resolution (NI PCI-6225, National Instruments) using a desktop computer and stored for offline data analyses.
Procedures
The subjects performed three unimanual multi-finger pressing tasks with each hand. Both hands were tested in a counterbalanced way, with some participants starting with the right hand and some with the left hand. The tasks were: (1) the maximal voluntary contraction (MVC); (2) the single-finger ramp task; and (3) the multi-finger accurate FTOT with quick pulse production task (Fig. 1a). In the MVC task, FTOT was displayed on the monitor screen as a left-to-right running white circle (cursor) on the black background. Participants were asked to press with the four fingertips as hard as they could to reach maximal FTOT within 3–4 s until a command “relax” was given by the experimenter. Two MVC trials were performed with a 30-s interval in between. The trial with the highest FTOT value was used to set the target force levels for the other two tasks.
Immediately after the MVC task, participants performed the ramp task. In this task, subjects were requested to keep all four fingers on the sensors and produce force with an instructed finger (master finger) to match the force profile shown on the monitor. The target force profile started with a 2-s horizontal line set at 10% of the master finger force at the moment of the FTOT MVC, followed by a 6-s ramp line ending at 40% of the master finger MVC and finishing with a 2-s horizontal line at the 40% level (Fig. 1b). Only the force produced by the master finger was displayed on the screen. Participants were told not to pay attention to possible force production by the non-task (enslaved) fingers and keep all of their fingers on the sensors at all times. Eight trials (two for each master finger) were performed. The first trial by each finger was considered familiarization; only the second trial was used in the analysis. On a few occasions, trials were repeated, mainly because subjects lifted one or more fingers during the task execution.
In the multi-finger accurate FTOT with quick pulse production task, a line corresponding to 5% of FTOT MVC was shown as a blue target line on the monitor. The actual FTOT was shown as a left-to-right running white circle. A vertical line was shown such that the cursor would cross it 6 s after trial initiation (see Fig. 1a). Two extra horizontal lines were shown after the vertical line at 20% and 30% of the FTOT MVC to define the target for the following force pulse. The instruction was to press on the sensors with all four fingers and match FTOT with the initial target line (5% MVC) as accurately as possible. Participants were instructed to produce a rapid force pulse into the target at a self-selected time after the FTOT cursor crossed the vertical line. The instruction emphasized speed of the force pulse over accuracy. After producing the force pulse, the participants were asked to “return to the blue horizontal target line” as fast as they could and continue matching this line until the trial stopped about 3 s after the pulse initiation. The subjects performed six practice trials (recorded but not used in data analysis) followed by 24 more trials. The 30 trials were performed in three blocks of ten trials. Each trial lasted for 12 s and 15 s of rest was given in between trials. A more extended rest (≈ 1 min) was given between blocks of trials to prevent fatigue. Participants were encouraged to ask for extra rest as needed; fatigue was never reported.
Data analysis
Customized LabVIEW (2017 version) routines were used to process and analyze the data obtained in the ramp and accurate FTOT production tasks. The force signals were filtered with a fourth-order, zero-lag, low-pass Butterworth filter with a cut-off frequency of 10 Hz.
For the ramp task, the enslaving matrix E and an index reflecting unintentional force production by non-task fingers (index of enslaving, IEN) were calculated. For each trial, the first and last 1-s intervals of the 6-s ramp phase were removed to avoid possible edge effects. Linear regressions were computed between FTOT and the force produced by each individual finger. The regression coefficients (k) were used as entries in the 4 × 4 E matrix:
Here, the first subscript shows the finger producing force, whereas the second subscript shows the task finger (i.e., master finger). The main diagonal of E (highlighted in gray) represents the master fingers and the off-diagonal values represent the unintentional force production by the enslaved fingers. An index of enslaving, IEN (in % of the total force produce by all fingers) was computed as the average of the off-diagonal values of E multiplied by 100.
For the analysis of performance in the accurate FTOT with quick pulse production task, the first step was to detect the pulse initiation (t0) and align all trials by this event. The t0 was defined as the moment in time when the FTOT rate (dFTOT/dt) reached 5% of its peak value in that specific trial. All the t0 values were checked for accuracy, and no trial was rejected based on t0 accuracy criteria. Further, forces produced by each finger were normalized to the task force level, i.e., 5% of FTOT MVC.
Task performance
The consistency of accurate FTOT production was assessed with the coefficient of variation (CV) of FTOT at steady state (CVSS, from − 1.2 to − 1 s, with respect to t0) and during the pulse-approaching phase (CVPA from − 1 to 0 s). The CVSS and CVPA were averaged across trials and used for further analysis. The performance in the pulse production was assessed by the peak FTOT and the peak FTOT rate values measured during the pulse.
UCM-based analysis of variance
The inter-trial variances within the UCM (VUCM) and within the space orthogonal to the UCM (VORT) were computed within the framework of the UCM hypothesis (Scholz and Schoner 1999; for computational detail see the Appendix). VUCM is the variance that did not affect FTOT, whereas VORT is the variance component that affected task performance. The two variance components were computed for each time-sample starting 1.2 s before t0 using the null-space of the Jacobian matrix, J= [1 1 1 1], as an approximation of the UCM in the space of finger modes (for more details see Latash et al. 2001; Park et al. 2012). Finger modes are hypothetical control variables to individual fingers that can be manipulated by the neural controller one at a time (Danion et al. 2003); modes were computed from the finger force data using the individual E matrix (details in Latash et al. 2001). The finger modes were calculated as the product of the inverse of the enslaving matrix E by the forces recorded from each individual finger [m= E−1f] where m and ƒ stand for the finger mode and force vectors.
An index of synergy was calculated for each time-sample: ΔV = (VUCM/3 − VORT/1)/(VTOT/4), where VTOT stands for total variance at a given sample and each variance index is normalized by the dimensionality of the corresponding space (Scholz et al. 2002). Because ΔV is not normally distributed, it was log-transformed taking into account its computational boundaries using a modified Fisher z-transformation: ΔVZ = 0.5 × ln [(ΔV + 4)/(1.33 − ΔV)] (Park et al. 2010). Values of VUCM, VORT, and ΔVZ were averaged within the steady-state time interval [− 1.2: − 1 s] with respect to t0 = 0.
Anticipatory synergy adjustments (ASAs)
ASAs prior to the force pulse initiation were quantified using two indices, the time of ASA initiation (tASA) and the magnitude of the drop of ΔVZ (∆∆VZ), which was quantified from the steady-state level until t0. The tASA was defined as the time when ΔVZ dropped below its average steady-state value by more than two standard deviations and stayed below that level until t0. A drop in ΔVZ starting prior to t0 was reflected in negative values of tASA.
Motor equivalence (ME) analysis
This analysis quantified the magnitude of motion in the finger mode space between two time intervals within each single trial: motion within the UCM (motor equivalent, ME) and orthogonal to the UCM (non-motor equivalent, nME). In each trial, average values of finger modes were computed in 200 ms windows 1 s before t0 [− 1.2; − 1.0 s] and 2 s after t0 [+ 2.0; + 2.2 s]. The second time interval was selected as the time by which all participants returned to the instructed steady force level (according to visual inspection). For each pair of measurements, the difference vectors (∆M) were projected onto the UCM and ORT spaces; the magnitudes of these two projections, normalized by the square root of the space dimensionality, were taken as ME and nME, respectively (Mattos et al. 2015; see the “Appendix”). For each participant, the ME and nME values were averaged across trials and used for further statistical analysis.
Statistical analysis
The statistical analyses were performed in the IBM SPSS Statistics Package (Version 25). The normality of data distribution for each outcome variable was tested using the Shapiro–Wilk test. Outcome variables with non-normal distributions were log-transformed to obtain a normal distribution. Further, parametric analyses were performed for all outcome variables, except for the UPDRS-III.
A series of statistical tests intended to assess the effect of the disease with the factor Group (levodopa-naïve PD vs. controls) on all tested outcome variables. For UPDRS, Mann–Whitney test was performed. Two-way (Group × Hand) analyses of variance (ANOVA) with the factor Hand (dominant vs. non-dominant) treated as repeated measure (RM) were performed for the following outcome variables: 9HPT, FTOT MVC; IEN; peak FTOT, peak FTOT rate, ∆VZ, ∆∆VZ, and tASA obtained from the multi-finger accurate FTOT production task. A three-way (Group × Hand × Time-Interval: at steady state vs. at the pulse-approaching phase) ANOVA, with Hand and Time-Interval as RM, was performed for the variable CV. Finally, two three-way (Group × Hand × Component: UCM vs. ORT) ANOVA, with Hand and Component as RM factors, were performed for the variance (VUCM and VORT) and motor equivalence (ME and nME) indices.
The other series of statistical tests intended to assess the effect of medication on levodopa-naïve PD subjects (n = 10) who were tested twice, before and after the first dose of medication. Note that, even with the relatively small number of patients, the normality assumption was not violated for some of the variables that did not require log transformation (i.e., FTOT MVC, IEN, ∆VZ, and tASA), as well as for other variables after log transformation [i.e., peak FTOT, peak FTOT rate, CV (at steady state and pulse-approaching phase), variance (VUCM and VORT), motor equivalence (ME and nME), and ∆∆VZ] according to the Shapiro–Wilk tests. Therefore, two-way RM ANOVAs (Medication: “on” vs. “off” and Hand) were performed for the following outcome variables: 9HPT, FTOT MVC; IEN; peak FTOT, peak FTOT rate, ∆VZ, ∆∆VZ, and tASA. A three-way (Medication × Hand × Time-Interval) RM ANOVA was performed on CV. Two three-way RM ANOVAs (Medication × Hand × Component) were performed for the indices of variance (VUCM and VORT) and motor equivalence (ME and nME). Pairwise contrasts with Bonferroni corrections were performed as needed. For all statistical tests, the alpha level was set at p = 0.05.
Results
Effects of PD
PD patients were on average 29% slower than controls in the 9HPT (F(1,24) = 27.7, p < 0.001, η2 = 0.54), with no effect of Hand and no Group × Hand interaction (Fig. 2a). Overall, the PD patients showed lower maximum four-finger FTOT (MVC) than controls (on average, by about 35%; F(1,24) = 6.69, p = 0.016, η2 = 0.21, Fig. 2b). No difference between hands and no Group × Hand interaction were found. No effects and interaction were found for the overall index of enslaving, IEN, although the indices in the PD group were, on average, about 20% higher (Fig. 2c).
Across-subject average of the a time to perform the Nine Hole Peg Test (9HPT), bFTOT MVC, and c index of enslaving (IEN) for the PD (white filled bars) and control groups (black filled bars) for the dominant (DH) and non-dominant hands (NDH). Error bars show standard errors. Stars shows significant group effects (p < 0.05)
Task performance
There was no difference in the force pulse characteristics between the groups. Specifically, the peak FTOT and peak FTOT rate were similar between the PD and control groups (Fig. 3a, b). No main effect of Hand and no Group × Hand interaction were found.
Across-subject average of the a peak FTOT, b peak FTOT rate, and c coefficient of variation (CV) for the PD (white and light gray filled bars) and control groups (black and dark/gray filled bars) for the dominant (DH) and non-dominant hand (NDH). c Shows CV at steady state (SS, white and black bars) and during the pulse-approaching (PA, light and dark gray) phase. Error bars show standard errors. Stars shows significant group effects (p < 0.05)
Overall, the coefficient of variation for FTOT (CV) was greater for the PD group (effect of Group: F(1,24) = 15.54, p = 0.001, η2 = 0.39) than controls, with this difference being larger in the steady state compared to the pulse-approaching phase (Group × Time-Interval: F(1,24) = 5.94, p = 0.023, η2 = 0.2). Also, the CV was smaller during the steady-state phase (CVSS) than during the pulse-approaching phase (CVPA), which was confirmed by an effect of Time-Interval (F(1,24) = 226, p < 0.001, η2 = 0.9, Fig. 3c). In addition, CVSS was smaller for the dominant hand than the non-dominant hand, but there was no difference between hands for the CVPA (Time-Interval × Hand: F(1,24) = 77, p < 0.001, η2 = 0.76).
UCM-based analysis of variance
Levodopa-naïve PD subjects showed significantly higher inter-trial variance component in the space of finger modes affecting FTOT (VORT); no such effect was seen for the variance component that did not affect FTOT (VUCM). This was confirmed by ANOVA during steady state (Group × Component: F(1,24) = 7.21, p = 0.013, η2 = 0.23; main effect for VORT: F(1,24) = 17, p < 0.001, η2 = 0.41). Much higher VUCM compared to VORT values were seen in both groups (Fig. 4a). This result was confirmed by the significant effect of Component (F(1,24) = 321, p < 0.001, η2 = 0.93). No effect of Hand and no other interactions were found.
Across-subject average of the a variance not affecting (VUCM) and affecting (VORT) total force, b index of synergy, ΔVZ, c time of ASA initiation (tASA), d drop in ΔVZ (ΔΔVZ) during the ASA, and e outcome variables of the motor equivalence analysis (ME and nME) for the PD (white and light gray filled bars) and control groups (black and dark/gray filled bars) for the dominant (DH) and non-dominant hand (NDH). In panels (a, e), the bars show VUCM and ME (white and black bars) and VORT and nME (PA, light and dark gray). Error bars show standard errors. Stars shows significant group effects (p < 0.05)
As a result of the inequality VUCM > VORT, the synergy index, ∆VZ was consistently positive in both groups (Fig. 4b). Averaged across subjects, time profiles of ∆VZ are shown in Fig. 5. Note the higher values in the controls during steady state (between 1.2 and 1 s before the first vertical dashed line) and the consistent drop in ∆VZ in preparation to the force pulse initiation (second vertical line) reflecting the ASA. Smaller synergy index values, ∆VZ, during steady state in levodopa-naïve PD were confirmed by the effect of Group (F(1,24) = 6.28, p = 0.019, η2 = 0.21). No other effects or interaction were seen.
The time profiles of average and SE of the synergy index, ΔVZ, for the PD (black line) and control groups (dotted line) of the dominant and non-dominant hands during the accurate FTOT with pulse production task. The first vertical dashed line in each plot (at − 1 s) represents the end of the steady-state phase [− 1.2: − 1 s] and the second vertical dashed line in each plot (at 0) represents the time of pulse initiation (t0)
The drop in the synergy index for both groups started, on average, 360 ms before t0. No Group and Hand effects were observed for tASA (Fig. 4c). Whereas no difference between groups was observed for the ΔVZ drop magnitude (∆∆VZ) in the dominant hand, ∆∆VZ was smaller for the non-dominant hand in the PD group compared to controls (Group × Hand; F(1,24) = 5.47, p = 0.028, η2 = 0.18; main effect of Group in the non-dominant hand, F(1,24) = 4.86, p = 0.037, η2 = 0.17) (Fig. 4d).
Motor equivalence analysis
The changes in the finger modes between steady states separated by the force pulse were confined primarily to the UCM for FTOT (Fig. 4e). Consequently, ME was about three times larger than nME (effect of Component: F(1,24) = 231, p < 0.001, η2 = 0.91). No differences between the hands were found. While no group difference was found for ME, levodopa-naïve PD subjects showed higher nME compared to controls reflected in an equivocal Group × Component interaction (F(1,24) = 3.73, p = 0.065, η2 = 0.13) and main effect of Group on nME (F(1,24) = 8.93, p = 0.006, η2 = 0.27).
Effects of the first dose of levodopa
Levodopa-naïve PD patients, on average, showed a small reduction in the UPDRS-III score after taking their first dose of carbidopa/levodopa (25/100 mg) (Z = − 2.196, p = 0.028, see Table 1 for individual data) and were, on average, 18% faster in the 9HPT (F(1,9) = 11.82, p = 0.007, η2 = 0.57), with no effect of Hand and no Medication × Hand interaction (Fig. 6a).
Across-subject average of the a time to perform the 9HPT, bFTOT MVC, and c index of enslaving (IEN) for the PD patients in the “off-medication” (white filled bars) and “on-medication” (black filled bars) states for the dominant (DH) and non-dominant hand (NDH). Error bars show standard errors. Stars shows significant medication effects (p < 0.05)
The first dose of medication had negative effects on both maximal force magnitude (it decreased) and unintentional finger force production (enslaving increased). Compared to the metrics observed in the off-drug state, PD subjects showed, on average, approximately a 17% drop in FTOT MVC (F(1,9) = 13.9, p = 0.005, η2 = 0.61, Fig. 6b). No effect of Hand and no Medication × Hand interaction were seen for FTOT MVC. Additionally, there was an increase in the index of enslaving, IEN, by about 20% (F(1,9) = 7.42, p = 0.023, η2 = 0.45) with no other effects (Fig. 6c).
Task performance
Compared to the off-drug state, in the on-drug state, PD patients showed a 12% increase in the magnitude of peak FTOT (effect of Medication: F(1,9) = 13.9, p = 0.005, η2 = 0.61, Fig. 7a) and a 20% increase in the peak FTOT rate (effect of Medication: F(1,9) = 8.86, p = 0.016, η2 = 0.5, Fig. 7b) during the force pulse. No effect of Hand and Medication ×Hand interaction were observed for these outcome variables.
Across-subject average of the a peak FTOT, b peak FTOT rate, and c coefficient of variation (CV) for PD patients in “off- medication” (white and light gray filled bars) and “on- medication” (black and dark gray filled bars) states for the dominant (DH) and non-dominant hand (NDH). Panel C shows CV at steady state (SS, white and black bars) and during the pulse-approaching (PA, light and dark gray) phase. Error bars show standard errors. Stars shows significant medication effects (p < 0.05)
Overall, CV of FTOT was smaller during the steady-state phase than the CV during the pulse-approaching phase (effect of Time-Interval: F(1,9) = 97.5, p < 0.001, η2 = 0.92, Fig. 7c). In addition, the CV in both phases tended to be higher in the on-drug state (marginal effect of Medication: F(1,9) = 4.02, p = 0.076, η2 = 0.31). No effect of Hand and no interactions were observed.
UCM-based analysis of variance
The inequality VUCM > VORT was valid both for off- and on-drug (effect of Component: F(1,9) = 71.77, p < 0.001, η2 = 0.89) states. In the on-drug state, there was an increase in both variance components (effect of Medication: F(1,9) = 14.87, p = 0.004, η2 = 0.62). No effect of Hand and no interaction were seen (Fig. 8a). As a result of proportional changes in the two variance components, the index of synergy, ∆VZ, was not affected by medication and showed no other significant effects (Fig. 8b). There were no effects of medication on either of the two indices of ASAs, tASA, or ∆ΔVZ (Fig. 8c, d). No effect of Hand and no interactions were seen.
Across-subject average of the (A) variance not affecting (VUCM) and affecting (VORT) performance, (B) index of synergy, ΔVZ, (C) time of ASA initiation (tASA), (D) drop in ΔVZ (ΔΔVZ), and (E) outcome variables of the motor equivalence analysis (ME and nME) for PD patients in “off” (white and light gray filled bars) and “on” (black and dark gray filled bars) medication stage for the dominant (DH) and non-dominant hand (NDH). In panels A and E, the bars show VUCM and ME (white and black bars) and VORT and nME (PA, light and dark gray). Error bars show standard errors. Stars shows significant medication effects (p < 0.05)
Motor equivalence analysis
During both off-drug and on-drug tests, changes in finger modes between steady states separated by the force pulse were confined primarily to the UCM for FTOT (Fig. 8e). This led to ME being about three times larger than nME (effect of Component: F(1,9) = 68.31, p < 0.001, η2 < 0.88). No differences between the hands were found. There were effects of Medication (F(1,9) = 12.42, p = 0.006, η2 = 0.58), which tended to be component specific (Medication × Component: F(1,9) = 3.76, p = 0.084, η2 = 0.30). Specifically, whereas no effect of Medication was seen for nME (p > 0.2), ME increased on-drug (F(1,9) = 10.74, p = 0.01, η2 = 0.54). No other significant effects were observed.
Discussion
Overall, our observations provide support for the first hypothesis formulated in the Introduction, but not for the second one. Indeed, as suggested by Hypothesis-1, levodopa-naïve PD patients showed reduced indices of stability (lower synergy index, ∆V reflecting an increase in nME without a change in ME) compared to controls. These results confirm earlier observations in early-stage PD patients tested in the “on-medication” state (Park et al. 2012; Jo et al. 2015). The results were ambiguous with respect to ASAs: Only one of the indices (drop in the synergy index in the non-dominant hand) showed a significant difference from the values in the control group. As in earlier studies, PD patients showed lower MVC force and a tendency toward higher enslaving values (cf. Park et al. 2012).
In contrast, effects of medication were opposite to our expectations and went against all the predictions of Hypothesis-2. After taking the first dose of levodopa despite better performance in UPDRS-III and 9HPT, the PD patients showed lower MVC, higher enslaving, higher overall variance (without a change in ∆V), and a trend toward smaller ASAs. These observations are mostly in the opposite direction compared to those reported in an earlier study on the effects of levodopa in PD patients who were on chronic anti-PD drugs, including levodopa (Park et al. 2014). These unexpected findings are consistent with the idea that the long-term chronic anti-PD drug exposure in the PD population we previously studied may lead to brain circuity changes; these changes may, in turn, lead to the differences in the acute sensitivity to levodopa between the levodopa-naïve PD population and PD patients on chronic dopamine-replacement therapy.
Stability and agility in early-stage PD
Two aspects of change in motor coordination have been identified in PD and addressed as impaired control of action stability (reviewed in Latash and Huang 2015). These two aspects are related to two groups of seemingly incompatible signs and symptoms in PD. On the one hand, patients with PD show low stability of actions, which leads to loss of stability of posture and gait with disease progression. On the other hand, these patients are unable to destabilize steady states of their bodies leading to hesitations, problems with movement initiation, and even episodes of freezing (e.g., freezing of gait, reviewed in Schaafsma et al. 2003; Morris et al. 2008; Snijders et al. 2016). We address these two groups of signs and symptoms as impaired stability and impaired agility.
Analysis of action stability has been developed within the framework of the UCM hypothesis (Scholz and Schoner 1999); this analysis takes into account that any action involves multiple elements that may or may not be coordinated to ensure stability of a salient performance variable, to which all the elements contribute (Schöner 1995). The concept of task-specific, controlled stability has allowed developing a number of computational approaches to quantify action stability (reviewed in Latash and Zatsiorsky 2016). One of them is based on the idea that trajectories of movements in unstable directions diverge, while they converge in stable directions. As a result, inter-trial variance estimated at a particular action phase is expected to be larger in more unstable (less stable) directions compared to less unstable (more stable) ones. Note that this is a non-trivial step: Using indices of inter-trial variance to reflect stability along each individual trial.
Another method to quantify stability, analysis of motor equivalence, is more direct: Using a perturbation and quantifying deviations of the system of interest. The perturbation can represent a change in external forces (e.g., Wilhelm et al. 2013; Zhou et al. 2014) or a quick action performed by the person (Mattos et al. 2011, 2015). Deviations in less stable directions in response to either type of perturbation are expected to be larger compared to deviations in more stable directions, if the perturbation is transient and the system returns close to its initial state (ME > nME). This method has a clear advantage for clinical studies, because it requires fewer trials. Recently, we noticed that outcome variables from the ME analysis (i.e., ME and nME) require between 4 and 10 trials, whereas outcome variables of the inter-trial variance within the UCM and ORT (VUCM and VORT) space need between 10 and 14 trials for reliable assessment (Freitas et al. 2019). Also, note that both analysis of variance and analysis of motor equivalence produce synergy indices with high reliability (de Freitas et al. 2018).
In our experiment, both methods produced indices suggesting high stability of FTOT (VUCM >> VORT and ME >> nME) in steady states. Controls, however, showed larger differences between VUCM and VORT (larger ∆V) compared to PD subjects. Whereas, there was no difference in ME between the groups, nME was larger in the PD group, also suggesting lower FTOT stability. Given that PD patients were tested before taking their first dose of dopamine-replacement medication, we conclude that the change in stability was due to PD-related changes in brain circuitry and not to levodopa treatment effects.
The differences in the indices of agility (tASA and ∆∆VZ) were more ambiguous. It is possible that problems with action stability emerge early in the neurodegenerative process, whereas problems with agility need more time to develop. Consistent with this idea, an earlier study did not find significant changes in the indices of agility in professional welders who are at high risk for Parkinsonism, while there was a significant reduction in indices of stability (Lewis et al. 2016).
It is undisputable that cortical (e.g., originating from M1) commands directed to motoneurons (Gentner and Classen 2006; Overduin et al. 2012) and spinal interneurons (Takei et al. 2017) via the corticospinal pathways play a significant role in the multi-finger posture and movement control. Interestingly, chronic post-stroke individuals with a cortical lesion showed slower force pulse production than controls during a quick force pulse production task, while showing no change in the index of multi-finger force-stabilizing synergy in either the contralesional or ipsilesional hand (Jo et al. 2016). This latter result is counterintuitive and suggests that cortical areas may be not as important as subcortical structures and loops to ensure the stability of salient performance variables.
Consistent with this general idea, previous studies from our group have shown that subcortical structural damage in the basal ganglia, cerebellum, and brain stem affects multi-finger stability and agility indices. In particular, Park et al. (2012, 2014) have shown that individuals with PD have lower indices of multi-finger synergy during steady-state force control (i.e., impaired stability) and delayed and smaller anticipatory synergy adjustments (i.e., impaired agility) during quick force change compared to healthy age-matched controls. These studies also showed that temporary withdrawal of levodopa negatively affected the indices of stability and agility in PD subjects. In addition, Lewis et al. (2016) showed that welders who have exposure to welding fumes and may represent a subclinical model of basal ganglia dysfunction (Bowler et al. 2006; Dorman et al. 2006) have lower indices of stability than controls, which correlated with microstructural damage in the globus pallidus. Recently, Falaki et al. (2018) have shown that PD subjects who had undergone deep brain stimulation (DBS) directed to the globus pallidus or subthalamic nuclei showed indices of multi-finger synergy as high as healthy controls. Collectively, these results demonstrate the importance of the basal ganglia in the control of multi-finger coordination during tasks requiring force control.
The cerebellum also is important for the organization of motor synergies (Thach et al. 1992; Houk et al. 1996; Brandauer et al. 2011). Specifically, Park et al. (2013) have found that individuals with olivopontocerebellar atrophy (OPCA) show lower indices of multi-finger synergies than controls in an accurate force production task. In addition, OPCA subjects showed smaller anticipatory synergy adjustments (lower agility) immediately prior to the initiation of a force pulse. It is known that PD patients display symptoms associated with cerebellar dysfunction and weakened connections in the striatum-cerebellar pathway (for review see Wu et al. 2011). This tract is related to action initiation. The fact that we found early-stage PD subjects already having reduced indices of stability, but relatively intact anticipatory synergy adjustments (agility), may mean that the striatum-cerebellar pathway is preserved in the initial disease stage. With disease progression, however, this pathway may become affected. This aligns with the clinical scenario in PD, whereby action initiation becomes more challenging (e.g., freezing of gait) and/or balance issues arise (e.g., falls) as the disease progresses. If the striatum-cerebellar pathway indeed is preserved in early-stage PD, this offers an opportunity to develop neuroprotective treatments for preventing the pathway impairment.
Lastly, it is also worth noting that stroke patients presented delayed and smaller anticipatory synergy adjustments (i.e. reduced agility) immediately prior to the quick force pulse initiation, when compared to age-matched controls (Jo et al. 2016). This result indicates that neural circuitry involved in ensuring stability of action may differ from that involved in ensuring action agility. The lack of major ASA differences in our newly diagnosed and drug-naïve PD patients may reflect the lack of involvement of the cortical areas, which are perhaps involved at later stages of PD.
Indices of finger synergies as behavioral biomarkers for early-stage PD
The identification of PD biomarkers that can be used as a predictive and/or diagnostic tool would have tremendous clinical and scientific impact, as neuroprotective treatments could be developed and tested rigorously in individuals at risk for PD. Several behavioral tests have been put forth as predicting PD, including low sense of smell [UPSIT—University of Pennsylvania Smell Identification Test (Hummel 1999; Tissingh et al. 2001) and sleep disorder measures (Howell and Schenck 2015). These metrics, however, are based on non-motor domains. Besides, they may be sensitive but not specific to PD and occur in other neurodegenerative disorders such as Alzheimer’s disease and diffuse Lewy body disease. Diagnostic and predictive PD biomarker candidates based on motor symptoms may add specificity to those non-motor metrics. The UPDRS-III (motor evaluation by a rater) has been used widely to assess motor disability and outcomes in symptomatic trials in PD (Adler et al. 1997; Trenkwalder et al. 2011). This metric, however, is brief (5–10 min) and influenced by factors intrinsic to patients (e.g., circadian, diurnal and daily fluctuations, food, stress, etc.), raters (rater–rater reliability), and/or subject–rater interaction (“white coat” effect). Thus, development of more objective and reliable motor metrics is needed.
Earlier studies have suggested that indices of stability and agility during multi-finger tasks can serve as potential theory-based motor metrics for early PD detection. In particular, typical changes in indices of stability and agility were documented in both hands of Hoehn-Yahr stage I PD patients (Park et al. 2012, 2014) when clinical signs of PD are observed only on one side. In addition, the changes in synergy indices for asymptomatic welders were quantitatively smaller, but qualitatively similar to those in PD (Lewis et al. 2016), suggesting that they may be sensitive to subtle, subclinical neural perturbations. It is known that welders may develop manganism, a Parkinsonian syndrome (Cersosimo and Koller 2006; Guilarte 2013), and synergic metrics have been linked to basal ganglial regions (Lewis et al. 2016). Taken together, these previous studies suggest that changes in indices of multi-finger synergies may be used for early detection of PD-related changes in the basal ganglia. Our study supports this idea by showing that levodopa-naïve PD patients already show detectable changes in indices of stability. We are particularly encouraged by the significant group differences in the nME index. Note that this index can be measured in individual trials and relatively few trials (4–10 trials) are needed to reach statistically reliable results compared to those needed during analysis of inter-trial variance (10–14 trials, Freitas et al. 2019). The approximately twofold reduction in the number of trials makes analysis of motor equivalence attractive for potential clinical use.
It is known that the PD patients’ initial clinical response to levodopa is variable, with some patients demonstrating an improvement of motor symptoms dramatically and others showing no improvement (Marsden and Parkes 1977). The first dose of levodopa in healthy controls increases the blood flow in the left sensorimotor cortex (SMC), probably caused by an increased activity of the thalamocortical projections. It also increases blood flow in the left ventrolateral prefrontal cortex (VLPFC), which could be caused by increased activity in dopaminergic neurons in different brain regions, including midbrain and basal ganglia that send direct inputs to VLPFC (Hershey et al. 2003). Note that VLPFC activity is associated with motor response inhibition (Menon et al. 2001) and object manipulation (Brodmann area 44, Binkofski et al. 1999).
Levodopa has been shown to attenuate the deficits in the communication between cortical and subcortical areas in PD patients (Palmer et al. 2009). Other brain structures seem to compensate for basal ganglia deficits when PD patients are levodopa naïve. However, because some deficits persist even on chronic anti-Parkinsonian medication, it has been argued that the medication is unable to revert this compensatory brain connectivity due to PD (Palmer et al. 2009). This may explain the different effects observed in functional and experimental tasks of our study. Changes after taking levodopa observed only in more functional tasks may be related to the nature of the task, number of body segments involved, and the outcomes used to assess performance.
Earlier studies have suggested that impaired motor synergies during multi-finger action observed in Hoehn-Yahr stages I and II PD patients, even in the apparently unaffected (subclinical) side of the body of stage I patients (Park et al., 2012, 2014), could be one of the earliest motor dysfunctions of PD. In this study, the first dose of levodopa caused similar (“positive”) and opposite (“negative”) effects on outcome variables of the other tasks performed. In terms of positive effects on the clinical tests, there was a small reduction in the UPDRS part III score (~ 15%) and an 18% reduction in the time to perform the 9HPT. The reduction in the UPDRS part III score after the first dose of levodopa is below the cut-off value of a 33% improvement expected for PD suggested earlier (Merello et al. 2011; Schade et al. 2017). Although most of the patients did not improve their UPDRS-III scores beyond the cut-off value, they were responsive to chronic use of levodopa (noted by their treating physicians). The 18% improvement in the 9HPT after taking the first dose of levodopa could be explained by learning/adaptation effects. Participants performed two trials before taking the medication and two more trials after taking the first dose. It is plausible that the improvement could be cause of practice, even though the participants performed the trials after the medication on average 2 h after the first set of trials. The improvement also could be caused by reduction in bradykinesia in proximal muscle groups of the upper limbs after medication. Bradykinesia is a cardinal PD symptom and it is known to be reduced with chronic levodopa use. The increase in the peak FTOT rate during quick force pulse production in the main experimental task also could be seen as a sign of the acute effect of levodopa on bradykinesia.
The different findings observed between the experimental and functional tasks in our study may be also due to differences in the nature of tests. The 9HPT used to assess hand dexterity involves movement of upper limb segments (a more dynamic task), while the main experimental task (used to quantify synergy indices) was an isometric task requiring mainly activation of intrinsic and extrinsic finger flexors. Hence, it seems that a beneficial acute effect may be observed in more dynamic tasks such as arm reaching and gait characteristics (cf. Castiello et al. 2000; Smulders et al. 2016). In addition, while the dexterity task only assesses the final product (performance), the investigated task is related to the motor strategies used to accomplish the task successfully.
Nonetheless, the synergy measures were able to distinguish early PD patients from healthy, control individuals, reproducing early findings in which PD patients on chronic anti-Parkinsonian medication showed lower indices of synergy than controls (Park et al. 2012; Jo et al. 2015). Therefore, the indices of stability and agility quantified in our study may be proposed as biomarkers for early identification of PD process, particularly its impact in basal ganglial structures, although they may not be sensitive to track acute drug effects on functional behaviors.
Effects of long-term medication exposure on drug sensitivity for multi-finger synergies
Motor control studies investigating the acute effect of the first dose of levodopa in de novo PD patients are scarce. Most of the studies tested PD patients “off” medication after an approximately 12 h withdrawal from all dopaminergic medication followed by another evaluation 1 h after ingestion of levodopa in an “on” medication state. A review paper from Smulders et al. (2016) showed that gait parameters such as speed, stride length, duration, and arm swing amplitude and peak velocity increased after levodopa administration in PD patients, as did excursion of the center of pressure just before the gait initiation. Levodopa also affects upper extremity function and control in PD patients. For example, during a reaching-to-grasp task, moving the hand from its initial position to the object is faster, and peak movement velocity and acceleration are higher on levodopa (Castiello et al. 2000). Conversely, the manipulation component of the action is not affected by levodopa as demonstrated by no change in the magnitude of hand aperture or the moment when the maximum hand aperture was reached (Castiello et al. 2000, Kelly et al. 2002: Schettino et al. 2006).
In the current study, the effects of the first dose of levodopa were unexpected and intriguing. Indeed, the acute levodopa results, we found in de novo PD subjects taking their first dose are qualitatively different from similar studies in PD subjects on chronic drug therapy (Nutt and Holford 1996; Michely et al. 2012; Park et al. 2014). In particular, Park et al. (2014) reported increased MVC force and decreased enslaving after levodopa, whereas we observed a decreased MVC force and higher enslaving indices. This previous study also reported longer and larger ASAs, whereas we observed trends (non-significant) of ASAs being smaller and shorter. Synergy indices were unchanged after levodopa in our study (although the variance components increased), whereas they were higher “on” medication in the Park et al. study.
Overall, the quick change in both synergy indices and ASAs were observed after dopaminergic medication administration (Park et al. 2014) matches well with the known positive effects of these drugs on clinical motor metrics related to bradykinesia and rigidity (Adler et al. 1997; Rocchi et al. 2002; Smulders et al. 2016, Trenkwalder et al. 2011). The effects observed in our study, however, showed that levodopa worsened individual control of fingers (higher enslaving index) and led to higher overall variance indices and smaller ASAs. One would expect these effects to lead to poorer performance on functional tests and worse clinical scores, which was not the case (see the UPDRS in Table 1 and 9HPT in Fig. 6a).
There are a number of possible explanations for these unexpected findings: First, the comparison in the previous study used a practically defined “off” medication state (overnight withdrawal of PD medication), whereas in the current study, subjects were tested truly in the “off” medication state, since they had never taken levodopa before. It is possible that residual effects of medication in the previous study influenced the results. On the other hand, such residual effects could be expected to reduce the magnitude of differences between the “on” and “off” states, not to change its sign.
Second, it also is possible that the order of testing affected the outcome. For obvious practical reasons, de novo patients always were tested first in the “off” medication condition, which represented their steady-state condition, followed by “on” medication testing. This means that we explored the effects of added medication. In earlier studies, however, the steady-state condition for PD subjects was “on” medication and data collected after overnight withdrawal represented effects of removed medication (even though the comparisons were made with the “on” medication state after taking the first morning dose).
An intriguing possibility, however, is that the difference between the current and previous results is due primarily to long-term exposure to medication that may modify neural circuits and their acute response to levodopa. Consistent with this idea, PD subjects treated chronically showed larger and more beneficial effects of acute addition of medication. We currently are retesting those patients after 6 months of anti-PD drug treatment and it will be very interesting to observe whether levodopa response changes with treatment. It also remains to be seen whether the described contrasting effects are typical only of finger coordination tasks or also can be seen in other tasks, in particular in multi-muscle coordination of vertical posture.
Abbreviations
- ASA:
-
Anticipatory synergy adjustment
- F TOT :
-
Total force
- HY:
-
Hoehn and Yahr
- MVC:
-
Maximal voluntary contraction
- PD:
-
Parkinson’s disease
- UCM:
-
Uncontrolled manifold
- ME:
-
Motor equivalent
- nME:
-
Non-motor equivalent
References
Adler CH, Sethi KD, Hauser RA, Davis TL, Hammerstad JP, Bertoni J, Ropinirole Study Group (1997) Ropinirole for the treatment of early Parkinson’s disease. Neurology 49:393–399
Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ (1999) A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci 11:3276–3286
Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA (2006) Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 27:315–326
Brandauer B, Hermsdörfer J, Geissendoerfer T, Schoch B, Gizewski ER, Timmann D (2011) Impaired and preserved aspects of independent finger control in patients with cerebellar damage. J Neurophysiol 107:1080–1093
Castiello U, Bennett KMB, Bonfiglioli C, Peppard RF (2000) The reach-to-grasp movement in Parkinson’s disease before and after dopaminergic medication. Neuropsychologia 38:46–59
Cersosimo MG, Koller WC (2006) The diagnosis of manganese-induced parkinsonism. Neurotoxicol 27:340–346
Danion F, Schöner G, Latash ML, Li S, Scholz JP, Zatsiorsky VM (2003) A force mode hypothesis for finger interaction during multi-finger force production tasks. Biol Cybern 88:91–98
de Freitas PB, Freitas SMSF, Lewis MM, Huang X, Latash ML (2018) Stability of steady hand force production explored across spaces and methods of analysis. Exp Brain Res 236:1545–1562
Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA (2006) Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci 92:201–210
Falaki A, Jo HJ, Lewis MM, O’Connell B, De Jesus S, McInerney J, Huang X, Latash ML (2018) Systemic effects of deep brain stimulation on synergic control in Parkinson’s disease. Clin Neurophysiol 129:1320–1332
Feigin A, Ghilardi MF, Fukuda M, Mentis MJ, Dhawan V, Barnes A, Ghez CP, Eidelberg D (2002) Effects of levodopa infusion on motor activation responses in Parkinson’s disease. Neurology 59:220–226
Freitas SMSF, de Freitas PB, Lewis MM, Huang X, Latash ML (2019) Quantitative analysis of multi-element synergies stabilizing performance: comparison of three methods with respect to their use in clinical studies. Exp Brain Res 237:453–465
Gentner R, Classen J (2006) Modular organization of finger movements by the human central nervous system. Neuron 52:731–742
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Dubois B (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Guilarte TR (2013) Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci 5:23
Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS (2003) Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:844–851
Hoehn M, Yahr M (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Houk JC, Buckingham JT, Barto AG (1996) Models of the cerebellum and motor learning. Behav Brain Sci 19:368–383
Howell MJ, Schenck CH (2015) Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol 72:707–712
Hummel T (1999) Olfactory evoked potentials as a tool to measure progression of Parkinson’s disease. In: Chase TN, Bedard P (eds) Focus on medicine Vol 14—new developments in the drug therapy of Parkinson’s disease. Blackwell Science, Oxford, pp 47–53
Jo HJ, Park J, Lewis MM, Huang X, Latash ML (2015) Prehension synergies and hand function in early-stage Parkinson’s disease. Exp Brain Res 233:425–440
Jo HJ, Maenza C, Good DC, Huang X, Park J, Sainburg RL, Latash ML (2016) Effects of unilateral stroke on multi-finger synergies and their feed-forward adjustments. Neuroscience 319:194–205
Kelly VE, Hyngstrom AS, Rundle MM, Bastian AJ (2002) Interaction of levodopa and cues on voluntary reaching in Parkinson’s disease. Mov Disord 17:38–44
Latash ML, Huang X (2015) Neural control of movement stability: lessons from studies of neurological patients. Neurosci 301:39–48
Latash ML, Zatsiorsky VM (2016) Biomechanics and motor control: defining central concepts. Academic Press, New York
Latash ML, Scholz JF, Danion F, Schöner G (2001) Structure of motor variability in marginally redundant multi-finger force production tasks. Exp Brain Res 141:153–165
Lewis MM, Lee E-Y, Jo HJ, Park J, Latash ML, Huang X (2016) Synergy as a new and sensitive marker of basal ganglia dysfunction: a study of asymptomatic welders. Neurotoxicol 56:76–85
Marsden CD, Parkes JD (1977) Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet 309:345–349
Mathiowetz V, Weber K, Kashman N, Volland G (1985) Adult norms for the nine hole peg test of finger dexterity. OTJR (Thorofare NJ) 5:24–38
Mattos DJ, Latash ML, Park E, Kuhl J, Scholz JP (2011) Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol 106:1424–1436
Mattos D, Schöner G, Zatsiorsky VM, Latash ML (2015) Motor equivalence during accurate multi-finger force production. Exp Brain Res 233:487–502
Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001) Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12:131–143
Merello M, Gerschcovich ER, Ballesteros D, Cerquetti D (2011) Correlation between the Movement Disorders Society Unified Parkinson’s Disease rating scale (MDS-UPDRS) and the Unified Parkinson’s Disease rating scale (UPDRS) during L-dopa acute challenge. Parkinsonism Relat Disord 17:705–707
Michely J, Barbe MT, Hoffstaedter F, Timmermann L, Eickhoff SB, Fink GR et al (2012) Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson’s disease. Neuropsychologia 50:2506–2514
Morris ME, Iansek R, Galna B (2008) Gait festination and freezing in Parkinson’s disease: pathogenesis and rehabilitation. Mov Disord 23(Suppl 2):S451–S460
Nutt JG, Holford NH (1996) The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol 39:561–573
Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML (2005) Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosci Lett 381:92–96
Overduin SA, d’Avella A, Carmena JM, Bizzi E (2012) Microstimulation activates a handful of muscle synergies. Neuron 76:1071–1077
Palmer SJ, Eigenraam L, Hoque T, McCaig RG, Troiano A, McKeown MJ (2009) Levodopa-sensitive, dynamic changes in effective connectivity during simultaneous movements in Parkinson’s disease. Neurosci 158:693–704
Park J, Zatsiorsky VM, Latash ML (2010) Optimality vs. variability: an example of multi-finger redundant tasks. Exp Brain Res 207:119–132
Park J, Wu YH, Lewis MM, Huang X, Latash ML (2012) Changes in multifinger interaction and coordination in Parkinson’s disease. J Neurophysiol 108:915–924
Park J, Lewis MM, Huang X, Latash ML (2013) Effects of olivo-ponto-cerebellar atrophy (OPCA) on finger interaction and coordination. Clin Neurophysiol 124(5):991–998
Park J, Lewis MM, Huang X, Latash ML (2014) Dopaminergic modulation of motor coordination in Parkinson’s disease. Parkinsonism Rel Disord 20:64–68
Politis M, Wilson H, Wu K, Brooks DJ, Piccini P (2017) Chronic exposure to dopamine agonists affects the integrity of striatal D2 receptors in Parkinson’s patients. Neuroimage Clin 16:455–460
Rocchi L, Chiari L, Horak FB (2002) Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73:267–274
Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (2003) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol 10:391–398
Schade S, Sixel-Döring F, Ebentheuer J, Schulz X, Trenkwalder C, Mollenhauer B (2017) Acute levodopa challenge test in patients with de novo parkinson’s disease: data from the DeNoPa Cohort. Mov Disord Clin Pract 4:755–762
Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H (2006) Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res 168:186–202
Scholz JP, Schoner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Scholz JP, Danion F, Latash ML, Schöner G (2002) Understanding finger coordination through analysis of the structure of force variability. Biol Cybern 86:29–39
Schöner G (1995) Recent developments and problems in human movement science and their conceptual implications. Ecol Psychol 8:291–314
Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, Horak FB (2016) Pharmacological treatment in Parkinson’s disease: effects on gait. Parkinsonism Relat Disord 31:3–13
Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, Aziz TZ, Papa SM, Factor SA, Hallett M (2016) Physiology of freezing of gait. Ann Neurol 80:644–659
Takei T, Confais J, Tomatsu S, Oya T, Seki K (2017) Neural basis for hand muscle synergies in the primate spinal cord. Proc Natl Acad Sci 114:8643–8648
Thach WT, Goodkin HG, Keating JG (1992) Cerebellum and the adaptive coordination of movement. Ann Rev Neurosci 15:403–442
Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, Wolters EC (2001) Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord 16:41–46
Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, Kassubek J (2011) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26:90–99
Wilhelm L, Zatsiorsky VM, Latash ML (2013) Equifinality and its violations in a redundant system: multi-finger accurate force production. J Neurophysiol 110:1965–1973
Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P (2011) Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 55:204–215
Zatsiorsky VM, Li ZM, Latash ML (2000) Enslaving effects in multi-finger force production. Exp Brain Res 131:187–195
Zhou T, Solnik S, Wu Y-H, Latash ML (2014) Equifinality and its violations in a redundant system: control with referent configurations in a multi-joint positional task. Mot Control 18:405–424
Acknowledgements
We would like to thank all the participants in the study. XH and MML were supported by NIH Grants NS060722, ES019672, and NS082151. MLL, XH, and MML were supported by NIH Grant NS095873.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest are claimed by any of the authors.
Additional information
Communicated by John C. Rothwell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Analysis of the structure of variance and motor equivalence within the UCM-framework
The force data from multiple trials f were converted into a mode vector m using the enslaving matrix, E, where f = [fifmfrfl]T; (T represents a matrix transpose):
Change in total force, FTOT was a function of the changes in modes dm = [dmi dmm dmr dml]T :
The UCM was approximated as a sub-space defined by an orthogonal set of the vectors ei in the m space satisfying:
These vectors were found by computing the null-space of the Jacobian of this transformation ([1 1 1 1]∙E). The mean-free modes were then projected onto these directions and summed to produce:
where n = 4 is the number of finger modes, m, and p = 1 is the number of constraints defined by the performance variable, FTOT. The orthogonal to the null-space projection was computed as:
The amount of variance per degree of freedom within the UCM is:
The amount of variance per degree of freedom orthogonal to the UCM is:
For the analysis of motor equivalence, mean deviation mode vector, ∆m, was projected onto the null and orthogonal spaces of the corresponding J as follows:
where \(\Delta m_{\parallel }\) is the null-space component and \(\Delta m_{ \bot }\), is the orthogonal component of the mean deviation mode vector. Both components are still four-dimensional mode vectors. The magnitude of ME and nME changes in the mode space was assessed by computing the length of these vectors, normalized by the square root of the number of DOF in the corresponding dimension.
Rights and permissions
About this article
Cite this article
de Freitas, P.B., Freitas, S.M.S.F., Reschechtko, S. et al. Synergic control of action in levodopa-naïve Parkinson’s disease patients: I. Multi-finger interaction and coordination. Exp Brain Res 238, 229–245 (2020). https://doi.org/10.1007/s00221-019-05709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05709-6