Abstract
Experimental pain inhibits primary motor cortex (M1) excitability. Attenuating pain-related inhibition of M1 excitability may be useful during rehabilitation in individuals with pain. One strategy to attenuate M1 excitability is to influence prefrontal and premotor cortex activity. Working memory tasks, e.g. the two-back task (TBT), engage prefrontal and premotor cortices and may influence M1 excitability. We hypothesized that performing the TBT during pain would influence pain-related changes in M1 excitability. Participants (n = 28) received rigorous training in the TBT before baseline testing. Experimental pain was induced by injecting hypertonic saline into the first dorsal interosseous (FDI) muscle. Participants rated pain intensity on a 0–10 numerical rating scale (NRS) every second min until pain-resolved (PR) during the performance of the TBT (n = 14) or during REST (n = 14). In the TBT, letters were presented pseudo-randomly, and accuracy and reaction time to identified letters corresponding to letters shown two times back were recorded. M1 excitability was assessed using transcranial magnetic stimulation. Motor-evoked potentials (MEPs) were recorded at baseline, and at PR, PR + 10, PR + 20, and PR + 30 min. Four minutes after hypertonic saline injection, the pain NRS scores were higher in the TBT group than the REST group (p = 0.009). No time × group interaction was found for MEPs (p = 0.73), but a main effect of time (p < 0.0005) revealed a reduction of MEPs at PR up until PR + 30 (p < 0.008). The TBT accuracy improved at PR + 30 in both groups (p = 0.019). In conclusion, the pain-induced reduction in corticomotor excitability was unaffected by performing a working memory task, despite greater pain in the TBT group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musculoskeletal pain remains in the top ten most debilitating conditions worldwide (Vos et al. 2016). It is well established that functional alterations occur in the central nervous system in the presence of musculoskeletal pain. For example, functional alterations can involve changes in the size of muscle representations in the primary motor cortex (M1) when the muscle is in pain (Tsao et al. 2011; Schabrun et al. 2015b, 2017a). Furthermore, there is strong evidence that experimental skin pain (Valeriani et al. 1999, 2001; Farina et al. 2001; Svensson et al. 2003) and intramuscular pain (Le Pera et al. 2001; Schabrun et al. 2017b; Larsen et al. 2018) exert a robust reduction in corticomotor excitability, as assessed by transcranial magnetic stimulation (TMS) motor-evoked potentials (MEPs) (Burns et al. 2016). The reduction in corticomotor excitability continues for up to 25 min after perception of pain disappears (Le Pera et al. 2001; Schabrun et al. 2015a). The functional impact on, e.g., motor function by reversing this reduction in corticomotor excitability during pain is currently unknown.
The experience of pain may persist after an injury to the musculoskeletal system, and be maintained by maladaptive cortical neuroplasticity (Graven-Nielsen and Arendt-Nielsen 2010). Currently, non-invasive approaches to modulate cortical excitability are limited; however, changes in corticomotor excitability occur during acquisition of novel skills, and re-acquisition of motor skills following injury (Gallasch et al. 2009), or through repetitive transcranial magnetic stimulation (rTMS) to the M1 or the premotor cortex (PMC) (Rizzo et al. 2004; Rothkegel et al. 2010). For instance, ballistic motor practice of the hand induces a rapid increase in flexor pollicis brevis excitability (Muellbacher et al. 2001). Yet, the interaction between corticomotor excitability reduction induced by pain and learning of discrete motor skills is controversial. Some studies have demonstrated that motor skill acquisition remains unaffected in the presence of pain (Bouffard et al. 2014; Lamothe et al. 2014), albeit different motor strategies may be involved (Mavromatis et al. 2017), while others show a reduction in the gains in corticomotor excitability that would, otherwise, occur during motor skill acquisition (Boudreau et al. 2007). Simple repetitive movements performed after experimental muscle pain did not attenuate the pain-induced reduction in corticomotor excitability (Schabrun et al. 2017b). This is surprising given that prior studies report that repeated volitional movements increase corticomotor excitability (Muellbacher et al. 2001; Carroll et al. 2008; Gallasch et al. 2009).
This study explores an alternative approach to modulate pain-induced corticomotor excitability reduction through PMC activation. The presence of a neuroanatomical pathway linking the PMC to the M1 provides a means to influence corticomotor excitability through reciprocal connections (Tokuno and Tanji 1993; Takada et al. 1998). In humans, corticomotor excitability can be modulated by PMC stimulation, by applying TMS conditioning stimuli anterior to the M1 (Civardi et al. 2001). Furthermore, inhibiting the PMC with low-frequency rTMS or continuous theta burst stimulation suppresses first dorsal interosseous (FDI) muscle MEPs (Gerschlager et al. 2001; Münchau et al. 2002; Huang et al. 2009). In addition, functional magnetic resonance imaging (fMRI) studies show strong bilateral activity of the PMC when performing working memory tasks, such as the N-back task (Owen et al. 2005).
Therefore, this study aimed to investigate if performing a working memory task during experimental pain influences the magnitude and duration of corticomotor excitability reduction. It was hypothesized that performing the two-back task during pain would (1) attenuate corticomotor excitability reduction immediately after perceived pain disappeared, and (2) reduce the duration of the corticomotor excitability reduction.
Experimental procedures
Participants
Twenty-eight right-handed, pain-free participants (mean age ± SD: 22.1 ± 2.1 years; 15 women) with no history of musculoskeletal or neurological conditions were included. Participants were excluded based on the following criteria: pregnancy; regular use of analgesics; analgesics or alcohol consumption within the last 24 h; drug use/abuse; use of antidepressants, neuroleptics, and anticonvulsants; any recent history of pain (acute or chronic) affecting the upper limbs or torso. Prior to participation, all participants were screened using the TMS screening questionnaire (Rossi et al. 2009, 2011) to avoid contraindicative delivery of magnetic pulses. All participants were right-handed as assessed by the Edinburgh Handedness Inventory (mean laterality quotient ± SD: 0.76 ± 0.21) (Oldfield 1971). Informed consent was obtained from all individual participants included in the study. The study was approved by the local ethics committee (VN-20170006) and conducted in accordance with the Declaration of Helsinki.
Experimental design
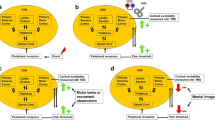
Each participant participated in one session and was randomized into either a two-back task (TBT) group or a rest (REST) group (Fig. 1). Initially, participants were asked to fill out questionnaires including the pain catastrophizing scale (PCS) (Sullivan et al. 1995) and the State-Trait Anxiety Inventory (STAI/S-T) (Spielbeger 1983). They were then seated comfortably in a chair with their right arm resting on a pillow. Elbow flexion was kept at approximately 45° angle flexion. A computer screen was placed immediately in front of them (mid-point of screen at 90 cm distance away from nasion of the participant). All participants underwent a familiarization round consisting of 40 trials (one letter presented = one trial; duration: 3 min) to reach ~ 80% accuracy in all trials. This was done to ensure that the participants focused on the online monitoring and updating of working memory (Owen et al. 2005) while performing the TBT. After the familiarization round, a baseline assessment was performed of the TBT, in which three rounds of 30 trials (6 min) were performed. Subsequently, baseline measures of TMS MEPs were recorded. Experimental pain was induced in the right FDI muscle by injection of hypertonic saline. Pain intensity ratings were recorded every 2 min from 30 s to 10 min after injection, and then every minute until pain-resolve (i.e. first pain rating of 0; PR). The TBT group performed the TBT for 10 min (5 rounds of 30 trials) starting immediately after the first pain rating and then one round until next pain rating, etc. Conversely, the REST group remained at rest between pain ratings for 10 min. After hypertonic saline injection, pain intensity was recorded every other minute (every minute after the first 10 min of rating). At PR, TMS MEPs were recorded again. The mind-wandering scale and effort ratings were recorded immediately after recording MEPs at PR. In both groups, a 10 min break was kept after PR and MEPs were recorded again. This was repeated at 20 and 30 min after PR after which the TBT was re-assessed in both groups (3 rounds of 30 trials). McGill’s Pain Questionnaire was completed at the end of the experiment.
Participants were randomized into a two-back task (TBT) group or a rest (REST) group. Both groups were extensively familiarized with the TBT, and baseline TBT performance and transcranial magnetic stimulation (TMS) motor-evoked potentials (MEPs) were recorded. Hypertonic saline was injected into the first dorsal interosseous (FDI) muscle. Pain intensity ratings were obtained throughout the experiment. The TBT group performed the TBT throughout the pain period, whereas the REST group remained seated quietly. Follow-up MEPs were recorded at pain-resolve (PR), PR + 10 min, PR + 20 min, and PR + 30 min. At PR + 30 min, both groups performed the TBT again
Two-back task
During the TBT, participants were presented with 30 English letters (equal 1 round) including all consonants. The letters were presented on the screen for 3 s, with interstimulus intervals of 500 ms (Vermeij et al. 2012). The participants used their left hand to press a key (i.e., using the numeric keypad 1) whenever the presented letter corresponded to the letter shown two times back (target), or (numeric keypad 2) when it did not match (non-target). Therefore, each letter presented would be accompanied by a button pressed and reaction time and accuracy (i.e., correct or incorrect keypad pressing) were recorded. For each round, 20 non-targets and 10 targets were presented in a random order. Reaction time reflects the time from letter presentation on the screen, to the key press, whereas accuracy is whether the correct button was pressed. The left hand was chosen to avoid on-going ipsilateral motor activity (associated with keypad pressing) of the left M1 affected by pain, which could potentially interfere with the reduction of corticomotor excitability.
Questionnaires
To ensure that the pain reporting was not influenced by cognitive factors such as pain catastrophizing (Turner and Aaron 2001), state and trait anxiety (Tang and Gibson 2005), and mind-wandering (Kucyi et al. 2013), the PCS, STAI, and mind-wandering scale were recorded for each participant. The 13 items of the PCS are categorized into “Rumination” (sum of items 8–11), “Magnification” (sum of items 6, 7, and 13), and “Helplessness” (sum of items 1, 2, 3, 4, 5, and 12) (Sullivan et al. 1995). For each statement, the participants reported the degree to which the sentence corresponded to their own thoughts and feelings when experiencing pain (not at all, to a slight degree, to a moderate degree, to a great degree, and to a large degree). The anxiety score was determined by the STAI (Spielbeger 1983) consisting of 40 questions, half of which are related to state anxiety, and the other half to trait anxiety. Each statement was rated on a 0–3 scale with anchors ‘Almost never’ to ‘Almost always’. The mind-wandering scale was adopted from Kucyi et al. (2013), and consisted of four questions designed to determine the level of mind-wandering occurring during the TBT performance or during rest. Participants were asked “How confident are you that you can accurately assess whether your attention was focused on the task/staying at rest, or on something other than the task/staying at rest; To what degree was your attention on one of the following when not on the task/staying at rest, (1) external/sensory distractions, (2) task-related interferences, and (3) mind-wandering”. Each question was rated on a 0–7 Likert scale ranging from 1 (not confident at all/never) to 7 (extremely confident/always). In addition, the participants were asked for the level of effort which they had to put into the task (TBT) or to staying at rest (REST) during pain (1, no effort; 7, maximum effort). To characterize the experimental pain condition, the short-form McGill Pain Questionnaire-2 (MPQ) was completed at the end of the experiment (Dworkin et al. 2009). The most commonly used words to describe the experimental pain were extracted.
Recording motor-evoked potentials
All TMS methods will be described in accordance with guidelines on reporting TMS methodology (Chipchase et al. 2012). A focal figure-of-eight coil (D702, Magstim Company, UK) was used to deliver monophasic TMS pulses supplied by a magnetic stimulator (Magstim BiStim2, Magstim Company, UK). The coil handle was pointing backwards, laterally, and at a 45° angle to the sagittal plane, inducing a posterior–anterior directed current, to elicit TMS MEPs from the FDI muscle. An interstimulus interval of 6 s was used for all the stimulations. The participants were fitted with a swimming cap and the optimal scalp position was marked on a pre-defined grid (1 × 1 cm squares orientated to vertex) to standardize orientation and location. The optimal scalp position (hotspot) for the FDI muscle was determined using 50% maximum stimulator output and defined as the site yielding consistent and highest peak-to-peak amplitude MEPs in three trials. The intensity needed to evoke MEPs of ~ 1 mV was tested by increasing and decreasing stimulus intensity until ~ 1 mV was consistently evoked in the FDI muscle (in 10 trials). This stimulation intensity was employed for the remaining of the experiment. For each assessment, 15 MEPs were recorded from the right FDI muscle.
Surface electromyography (EMG) was recorded from the muscle belly of the FDI muscle using bipolar Ag/AgCl electrodes (Neuroline 720, Ambu® A/S, DK). Electrodes were placed with an approximate 20 mm interelectrode distance with the reference electrode located at the styloid process. The EMG data were pre-amplified (1000 × gain), analog band-pass filtered (5 Hz–1 kHz), and sampled at 4 kHz by a 16-bit data-acquisition card (National Instruments, NI6122). Peak-to-peak MEPs were shown online by custom-made LabView software (Mr. Kick III, Aalborg University). Peak-to-peak amplitude was extracted for each MEP and averaged across the 15 recorded MEPs at each time point for further analysis.
Experimental muscle pain
The sites of injections were determined by palpation of the contracted FDI muscle, and the skin was cleaned with alcohol. A bolus injection of sterile hypertonic saline (0.2 mL, 5.8% NaCl) was administered into the FDI muscle using a 1 mL syringe with a disposable needle (27 G) (Le Pera et al. 2001; Larsen et al. 2018). The right FDI was chosen due to the body of evidence already available on the effect of pain on FDI M1 excitability (Le Pera et al. 2001; Schabrun et al. 2013; Larsen et al. 2018, 2019). Hypertonic saline excites group III/IV muscle afferents (Cairns et al. 2006) and an earlier study demonstrated that hypertonic saline injection temporally reduces primary somatosensory cortex (S1) excitability before M1 excitability (Schabrun et al. 2013). To assess the intensity of saline-induced pain, participants were asked verbally to rate the pain intensity on a 0–10 numerical rating scale (NRS), with ‘0’ representing ‘no pain’ and ‘10’ representing ‘worst imaginable pain’.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) unless otherwise stated. Normal distribution was tested using Shapiro–Wilk’s test for normality. Participant demographics were compared between groups using independent samples t tests (age, handedness, and threshold1mV) and Chi-square test (gender ratio). The PCS, STAI-S/T, MPQ, and mind-wandering scale data were analyzed using Mann–Whitney U tests. The MEPs were tested in a two-way mixed-model analysis of variance (ANOVA) with group (TBT or REST) as between-groups factor and time (baseline, PR, PR + 10 min, PR + 20 min, and PR + 30 min) as within-subjects factor. NRS scores were analyzed with a two-way mixed-model ANOVA with group (TBT or REST) as between-groups factor and time (30 s after injection and 2–17 min) as within-subjects factor. The TBT performance data were analyzed by two separate repeated-measures multivariate ANOVAs (one for targets and one for non-targets; MANOVA), with two dependent variables (accuracy and reaction time), one within-group factor (baseline versus PR + 30), and one between-group factor (TBT versus REST). The MANOVAs for target and non-target were corrected by familywise error rate correction (0.05/2 = 0.025). Note that the TBT performance data obtained during pain are not included in these analyses, since the REST group did not perform the TBT during pain. Spearman ranked correlation analyses were performed to test whether there were associations between the pain intensity NRS rating and percentage MEP reduction at peak pain for both the REST and the TBT group. Sidak correction was applied where appropriate. All analyses were carried out in Statistical Package for Social Sciences (SPSS; version 25, IBM). A p value < 0.05 was considered significant.
Results
Participants and TMS parameters
The two groups did not differ in age [22.7 ± 0.6 years vs. 22.9 ± 0.6 years, t(26) = − 0.18, p = 0.86], handedness [laterality quotient, TBT group: 0.76 ± 0.06 vs. REST group: 0.75 ± 0.05, t(26) = 0.09, p = 0.93], or gender ratio [9/14 women in TBT group vs. 6/14 in REST group, χ2(1) = 1.29, p = 0.26]. The stimulator output intensity needed to produce MEPs of ~ 1 mV amplitude was 55.3 ± 14.3% in the TBT group which was not statistically different from the 57.5 ± 11.7% in the REST group [t(26) = − 0.43, p = 0.67].
Pain catastrophizing and anxiety questionnaires
The median PCS score for the TBT group was 16.5 [interquartile range (IQR) = 22.75] and 13.5 (IQR = 8.5) for the REST group, which did not differ significantly, U = 92.5, n1 = n2 = 14, z = −0.25, p = 0.8. Similarly, there were no differences between groups for ‘Rumination’ scores [TBT versus REST: 6 (IQR = 9.75) vs. 5 (IQR = 4.5), U = 91, n1 = n2 = 14, z = − 0.32, p = 0.75], ‘Magnification’ [3 (IQR = 6) vs. 3.5 (IQR = 4.5), U = 92, n1 = n2 = 14, z = − 0.28, p = 0.78], or ‘Helplessness’ [4.5 (IQR = 8.5) vs. 6 (IQR = 5.25), U = 89.5, n1 = n2 = 14, z = − 0.39, p = 0.69]. Similarly, no significant differences between groups were found for the STAI-S [TBT versus REST: 31.5 (IQR = 10.5) vs. 36 (IQR = 9); U = 71.5, n1 = n2 = 14, z = − 1.2, p = 0.22] or STAI-T [40.5 (IQR = 16.25) vs. 35 (IQR = 16.5); U = 86, n1 = n2 = 14, z = − 0.6, p = 0.6].
Mind-wandering scale
One participant from the REST group had missing data from the mind-wandering scale and was excluded from the analysis. The groups did not differ in their confidence in assessing their level of attention towards the task/staying at rest (TBT median: 6 versus REST median: 6, U = 64, n1 = 14, n2 = 13, z = − 1.36, p = 0.17), and did not rate sensory or mind-wandering interference with their attention to either task differently (TBT median: 4 and 3 versus REST median: 4 and 4, both U > 74, n1 = 14, n2 = 13, z > − 0.27, p > 0.4). Conversely, the TBT group rated the task-related interferences with their attention to the task higher than the REST group did (TBT median: 2 versus REST median: 0, U = 0.0, n1 = 14, n2 = 13, z = − 4.72, p < 0.005). Furthermore, the TBT group rated their effort higher than the REST group, when performing their respective task (i.e., performing the TBT as opposed to staying at rest for the REST group) (TBT median: 5 versus REST median: 2, U = 26.5, n1 = 14, n2 = 13, z = − 3.17, p = 0.001).
Pain intensity ratings and profile
A significant two-way interaction was found for the pain intensity NRS scores (F12,338 = 2.3, p = 0.008, \(\eta_{\text{partial}}^{2}\) = 0.08). The REST group gave NRS scores lower than the TBT group at 4 min post-injection (p = 0.009; Fig. 2). Pain reduced significantly for both groups at 8 and 10 min compared to immediately after (I.A.) injection, for the group performing the TBT and the REST group, respectively (p < 0.05, Fig. 2). For all pairwise comparisons, refer to Fig. 2.
Mean (+ SEM) numerical rating scale (NRS) scores following injection of hypertonic saline in the two-back-task (TBT) and resting (REST) groups. NRS scores were significantly reduced in both groups from 8–17 or 10–17 min (TBT and REST, respectively) compared to I.A. (p < 0.05). All significant NRS rating reductions as compared to 2–8 min are indicated in the figure. A between-group difference in NRS was found at 4 min (#p < 0.05). PR pain-resolve
The most common words to describe the hypertonic saline-induced pain sensation in both groups were sharp (82%), cramping (85.7%), aching (75%), heavy (67%), and numbness (67%).
Corticomotor excitability
The two-way mixed-model ANOVA of MEPs did not yield a significant group × time interaction (F4,104 = 0.51, p = 0.73, \(\eta_{\text{partial}}^{2}\) = 0.19) or between-group difference (F1,26 = 0.7, p = 0.4, \(\eta_{\text{partial}}^{2}\) = 0.03), but a strong main effect of time (F4,104 = 8.3, p < 0.0005, \(\eta_{\text{partial}}^{2}\) = 0.24). Post hoc tests showed that corticomotor excitability of the FDI muscle was reduced at PR (p = 0.002) as well as PR + 10 min (p = 0.002), PR + 20 min (p = 0.008), and PR + 30 min (p = 0.002) as compared to baseline (Fig. 3).
Two-back task performance
One and two participants in the TBT and REST group, respectively, had missing data during the performance of the TBT. Therefore, their data were omitted during the analysis of the TBT performance (TBT group, n = 13; REST group, n = 12).
The repeated-measures MANOVA did not yield any time × group interactions for non-target accuracy (F1,20 = 0.21, p = 0.65, \(\eta_{\text{partial}}^{2}\) = 0.01; Fig. 4a) or reaction time (F1,20 = 0.2, p = 0.66, \(\eta_{\text{partial}}^{2}\) = 0.01; Fig. 4b). Similarly, target accuracy (F1,20 = 0.01, p = 0.94, \(\eta_{\text{partial}}^{2}\) < 0.001; Fig. 4c) and target reaction time (F1,20 = 0.03, p = 0.86, \(\eta_{\text{partial}}^{2}\) = 0.002; Fig. 4d) did not show any time × group interactions. A main effect of time was found for target accuracy (F1,20 = 6.55, p = 0.019, \(\eta_{\text{partial}}^{2}\) = 0.25), indicating an increase in accuracy between baseline and PR + 30 (Fig. 4c).
Correlation analyses
No significant correlations were found for the percentage change in MEP amplitude and peak NRS scores of pain intensity for the REST group (ρ = − 0.15, p = 0.6) or the TBT group (ρ = − 0.4, p = 0.89).
Discussion
There is growing interest to find approaches capable of attenuating the pain-induced reduction in corticomotor excitability, since it may prove useful during musculoskeletal pain rehabilitation (Pelletier et al. 2015). The present findings confirm earlier findings of a robust reduction in FDI corticomotor excitability following experimental muscle pain. The pain-induced reduction in MEPs was unaffected by the TBT performance. The TBT group rated higher pain during the performance of the TBT as compared to the group that remained at rest.
Corticomotor excitability reduction was unaffected by performance of the two-back task
Contrary to our hypotheses, the pain-induced reduction in corticomotor excitability and the duration of this reduction were unaffected by performing a working memory task during acute experimental pain. As previously reported (Le Pera et al. 2001; Schabrun and Hodges 2012; Larsen et al. 2018), hypertonic saline-induced pain reduced FDI corticomotor excitability, as reflected by MEP amplitudes, even after pain-resolve. This reduction in corticomotor excitability is considered to be mediated through an increase in gamma-aminobutyric acid inhibitory and decreased glutamate-mediated facilitatory (N-methyl-d-aspartate receptor acting on glutamatergic interneurons) intracortical mechanisms (Schabrun and Hodges 2012), and may serve as a protective mechanism for avoiding further injury by splinting the affected limb (Hodges and Tucker 2011; Burns et al. 2016). The current study aimed at targeting the link between PMC and M1, since the TBT is known to engage prefrontal and premotor cortices during performance (Owen et al. 2005). However, our findings suggest that more research is needed to establish if engaging the PMC by performing the TBT affects M1 excitability, given the lack of a pain-free TBT group. Several factors influence the corticomotor excitability response to pain, such as (1) prefrontal and subcortical brain areas (Mink 1996; Owen et al. 2005; Seminowicz and Moayedi 2017); (2) right PMC-to-contralateral M1 inhibition (Mochizuki et al. 2004); or (3) transcallosal M1–M1 inhibition (Ferbert et al. 1992). It is, therefore, not possible to rule out if performing the task with the left hand may have induced either right PMC-to-M1 or M1–M1 inhibition, thereby counteracting any potential facilitation of M1 excitability. However, considerable bilateral PMC activation has been demonstrated by fMRI across several n-back task paradigms (Owen et al. 2005), and PMC-to-M1 inhibition is, therefore, unlikely to be the sole responsible factor for the lack of MEP facilitation. Instead, the human PMC–M1 relationship remains somewhat elusive, and the notion that PMC may drive M1 excitability is based on empirical evidence, suggesting that rTMS to PMC alters M1 excitability (Gerschlager et al. 2001; Mochizuki et al. 2004; Rizzo et al. 2004). In addition, since M1 receives multiple inputs from, e.g., the S1 (Hatsopoulos and Suminski 2011), it is possible that MEPs are not only reflecting changes at the level of M1. This notion is supported by the finding that S1 excitability changes occur before that of M1 excitability (Schabrun et al. 2013).
Another possibility is that the PMC activation observed during performance of a two-back task is mainly related to attention (Owen et al. 2005). Yet, given the strong motor preparation component of the TBT, prefrontal areas such as the dorsolateral prefrontal cortex (DLPFC), the PMC, and M1 are likely involved (Carlson et al. 1998; Neige et al. 2018), and being purely attention-related activation is unlikely to be the main factor in the lack of M1 excitability change during pain. Based on the current findings, we are unable to infer whether performance of the working memory task influences corticomotor excitability. It is possible that the familiarization and baseline testing on the TBT had already increased MEPs during baseline TMS testing. However, the numerical values of the MEPs were similar to those reported in earlier research utilizing 120% RMT (Schabrun and Hodges 2012; Larsen et al. 2018). Furthermore, the pain-induced reduction in MEPs was similar to those earlier reported (Le Pera et al. 2001; Schabrun and Hodges 2012; Burns et al. 2016; Larsen et al. 2018). Therefore, the familiarization and baseline TBT testing are unlikely to have had any major impact on the MEPs.
Modulating corticomotor excitability through the PMC is an interesting approach, as shown in the other areas of research on action observation (Fadiga et al. 1995; Strafella and Paus 2000) and motor imagery (Stinear and Byblow 2003). Perhaps, these approaches may be more effective for normalizing pain-induced corticomotor excitability reduction.
Pain intensity ratings differed between groups
In this study, those performing the working memory task rated their perceived pain intensity higher than the resting group during pain. In contrast, pain intensities are rated lower during performance of an attention task (Bushnell et al. 1985; Miron et al. 1989), a Stroop task (Bantick et al. 2002; Seminowicz and Davis 2007a), and a three-back task (Buhle and Wager 2010) in previous studies. A reduction in pain intensity during task performance is considered to be mediated through attenuation of activation of pain-related brain regions such as the S1, S2, and the posterior insula, and the ipsilateral (to the pain) anterior insula (Petrovic et al. 2000; Bantick et al. 2002; Seminowicz et al. 2004; Wiech et al. 2005; Bingel et al. 2007). The discrepancy between the current and previous studies in pain ratings may depend on the choice of experimental pain model. Phasic short-lasting (0.8–7 s application) heat pain models applied to the face, the hand, and the left forearm (Bushnell et al. 1985; Bantick et al. 2002; Buhle and Wager 2010) yield moderate pain intensities (4–7) scored on a visual analog scale, whereas hypertonic saline-induced pain in the hand is rated at ~ 6 NRS in this study and earlier work (Larsen et al. 2018). It is, therefore, unlikely that pain intensity alone can explain the discrepancy. Alternatively, performing the task during pain may have shifted the attention towards pain. However, we find this unlikely since (1) the two groups did not rate external/sensory distractions differently with respect to task execution and (2) earlier evidence suggests that task performance remain unaffected when performed during pain (Seminowicz and Davis 2007b). Instead, the difference in quality of the painful sensation, i.e., tonic muscle pain versus phasic skin pain, may impact pain perception during the working memory task performance. Indeed, capsaicin application to the skin before painful heat stimulation (heat allodynia) enhances forebrain responses related to areas such as the anterior insula and dorsolateral prefrontal areas, when compared to similar pain intensity heat stimulation alone (Lorenz et al. 2002). It could be argued that the two groups were different because of interindividual susceptibility to pain perception, given the sizeable heterogeneity in pain ratings amongst healthy volunteers to standardized nociceptive input (Coghill 2010). Although we cannot rule out the possibility of group differences in pain susceptibility, the groups did not differ in age, gender, PCS, or STAI-S/T scores.
Accuracy in the two-back task increased at PR + 30 as compared to baseline
The performance increase in target accuracy was similar for the TBT and REST group. This verifies that no additional learning occurred at PR + 30 for the TBT group compared to the REST group, despite having performed the task for an additional 10 min. This supports the notion that the TBT during pain can be considered a working memory task. The similar increase in target accuracy is plausibly due to the familiarization performance, where the two groups achieved a similar level of target accuracy during the 40 trials. The aim of the current study was to employ the TBT to influence M1 excitability indirectly by engaging the PMC and DLPFC (Owen et al. 2005) and not induce learning per se. Since earlier research showed performance gains in N-back tasks (accuracy and reaction time) in response to facilitated DLPFC excitability by high-frequency rTMS [for review, see Brunoni and Vanderhasselt (2014)], the present findings support that a working memory task that engages the DLPFC, improves accuracy on targets, but corticomotor excitability remains unchanged. The increased accuracy from baseline to PR + 30 is in agreement with earlier lines of evidence on motor learning, which demonstrated that performance still increases when motor practice is performed during pain (Boudreau et al. 2007; Lamothe et al. 2014; Bouffard et al. 2016; Mavromatis et al. 2017).
The lack of facilitation of M1 could be explained by the effort needed to perform the working memory task during pain. It is known that, for instance, motor tasks need to be at a certain level of difficulty for learning to be optimal and engage M1 (Boudreau et al. 2010). Participants in the TBT group consistently rated the effort to complete the TBT higher during pain, but effort is not a direct measure of task difficulty. It, therefore, remains unclear if the task was able to modulate corticomotor excitability, since a TBT group without pain was not included. Nonetheless, it is known that increased task difficulty enhances corticomotor excitability to a greater extent during performance of, e.g., motor imagery (Roosink and Zijdewind 2010), and task difficulty in the current study should therefore be considered.
Limitations
The lack of a TBT group with no pain during performance makes it difficult to assess if the TBT influences corticomotor excitability when performed during pain. Earlier studies where pain-free participants were tested have consistently demonstrated that isotonic saline and no pain perception do not interfere with the magnitude of MEPs (Le Pera et al. 2001; Svensson et al. 2003; Schabrun et al. 2015a), but there are no equivalent data on the TBT alone. The lack of a pre-familiarization TMS baseline does not allow for the relative impact of the familiarization/baseline n-back task performance on MEPs to be assessed.
This is the first study to explore if performing a working memory task modulates the pain-induced reduction in corticomotor excitability. Pain-induced reduction in FDI corticomotor excitability was unaffected by engaging in a working memory task during pain, despite an enhanced pain perception.
References
Bantick SJ, Wise RG, Ploghaus A et al (2002) Imaging how attention modulates pain in humans using functional MRI. Brain 125:310–319. https://doi.org/10.1093/brain/awf022
Bingel U, Rose M, Gläscher J, Büchel C (2007) fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron 55:157–167. https://doi.org/10.1016/j.neuron.2007.05.032
Boudreau S, Romaniello A, Wang K et al (2007) The effects of intra-oral pain on motor cortex neuroplasticity associated with short-term novel tongue-protrusion training in humans. Pain 132:169–178. https://doi.org/10.1016/j.pain.2007.07.019
Boudreau SA, Farina D, Falla D (2010) The role of motor learning and neuroplasticity in designing rehabilitation approaches for musculoskeletal pain disorders. Man Ther 15:410–414. https://doi.org/10.1016/j.math.2010.05.008
Bouffard J, Bouyer LJ, Roy JS, Mercier C (2014) Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. J Neurosci 34:9190–9195. https://doi.org/10.1523/JNEUROSCI.5303-13.2014
Bouffard J, Bouyer LJ, Roy JS, Mercier C (2016) Pain induced during both the acquisition and retention phases of locomotor adaptation does not interfere with improvements in motor performance. Neural Plast. https://doi.org/10.1155/2016/8539096
Brunoni AR, Vanderhasselt MA (2014) Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 86:1–9. https://doi.org/10.1016/j.bandc.2014.01.008
Buhle J, Wager TD (2010) Performance-dependent inhibition of pain by an executive working memory task. Pain 149:19–26. https://doi.org/10.1016/j.pain.2009.10.027
Burns E, Chipchase LS, Schabrun SM (2016) Primary sensory and motor cortex function in response to acute muscle pain: a systematic review and meta-analysis. Eur J Pain 20:1203–1213. https://doi.org/10.1002/ejp.859
Bushnell MC, Duncan GH, Dubner R et al (1985) Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J Neurosci 5:1103–1110. https://doi.org/10.1016/0304-3959(86)90138-7
Cairns BE, Svensson P, Wang K et al (2006) Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 90:2098–2105. https://doi.org/10.1152/jn.00353.2003
Carlson S, Martinkauppi S, Rämä P et al (1998) Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex 8:743–752. https://doi.org/10.1093/cercor/8.8.743
Carroll TJ, Lee M, Hsu M, Sayde J (2008) Unilateral practice of a ballistic movement causes bilateral increases in performance and corticospinal excitability. J Appl Physiol 104:1656–1664. https://doi.org/10.1152/japplphysiol.01351.2007
Chipchase L, Schabrun S, Cohen L et al (2012) A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin Neurophysiol 123:1698–1704. https://doi.org/10.1016/j.clinph.2012.05.003
Civardi C, Cantello R, Asselman P, Rothwell JC (2001) Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14:1444–1453. https://doi.org/10.1006/nimg.2001.0918
Coghill RC (2010) Individual differences in the subjective experience of pain: new insights into mechanisms and models. Headache J Head Face Pain 50:1531–1535. https://doi.org/10.1111/j.1526-4610.2010.01763.x
Dworkin RH, Turk DC, Revicki DA et al (2009) Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 144:35–42. https://doi.org/10.1016/j.pain.2009.02.007
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (1995) Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73:2608–2611. https://doi.org/10.1152/jn.1995.73.6.2608
Farina S, Valeriani M, Rosso T et al (2001) Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neurosci Lett 314:97–101. https://doi.org/10.1016/S0304-3940(01)02297-2
Ferbert A, Priori A, Rothwell JC et al (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546. https://doi.org/10.1113/jphysiol.1992.sp019243
Gallasch E, Christova M, Krenn M et al (2009) Changes in motor cortex excitability following training of a novel goal-directed motor task. Eur J Appl Physiol 105:47–54. https://doi.org/10.1007/s00421-008-0871-y
Gerschlager W, Siebner HR, Rothwell JC (2001) Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57:449–455. https://doi.org/10.1212/WNL.57.3.449
Graven-Nielsen T, Arendt-Nielsen L (2010) Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 6:599–606. https://doi.org/10.1038/nrrheum.2010.107
Hatsopoulos NG, Suminski AJ (2011) Sensing with the motor cortex. Neuron 72:477–487. https://doi.org/10.1016/j.neuron.2011.10.020
Hodges PW, Tucker K (2011) Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152:S90–S98. https://doi.org/10.1016/j.pain.2010.10.020
Huang YZ, Rothwell JC, Lu CS et al (2009) The effect of continuous theta burst stimulation over premotor cortex on circuits in primary motor cortex and spinal cord. Clin Neurophysiol 120:796–801. https://doi.org/10.1016/j.clinph.2009.01.003
Kucyi A, Salomons TV, Davis KD (2013) Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci 110:18692–18697
Lamothe M, Roy J-SS, Bouffard J et al (2014) Effect of tonic pain on motor acquisition and retention while learning to reach in a force field. PLoS One 9:e99159. https://doi.org/10.1371/journal.pone.0099159
Larsen DB, Graven-nielsen T, Hirata RP, Boudreau SA (2018) Differential corticomotor excitability responses to hypertonic saline-induced muscle pain in forearm and hand muscles. Neural Plast 2018:1–9. https://doi.org/10.1155/2018/7589601
Larsen DB, Graven-Nielsen T, Boudreau SA (2019) Pain-induced reduction in corticomotor excitability is counteracted by combined action-observation and motor imagery. J Pain. https://doi.org/10.1016/j.jpain.2019.05.001
Le Pera D, Graven-Nielsen T, Valeriani M et al (2001) Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 112:1633–1641. https://doi.org/10.1016/S1388-2457(01)00631-9
Lorenz J, Cross DJ, Minoshima S et al (2002) A unique representation of heat allodynia in the human brain. Neuron 35:383–393. https://doi.org/10.1016/S0896-6273(02)00767-5
Mavromatis N, Neige C, Gagné M et al (2017) Effect of experimental hand pain on training-induced changes in motor performance and corticospinal excitability. Brain Sci 7:15. https://doi.org/10.3390/brainsci7020015
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425. https://doi.org/10.1016/S0301-0082(96)00042-1
Miron D, Duncan GH, Bushnell MC (1989) Effects of attention on the unpleasantness and intensity of thermal pain. Pain 39:345–352
Mochizuki H, Huang Y-Z, Rothwell JC (2004) Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol 561:331–338. https://doi.org/10.1113/jphysiol.2004.072843
Muellbacher W, Ziemann U, Boroojerdi B et al (2001) Role of the human motor cortex in rapid motor learning. Exp Brain Res 136:431–438. https://doi.org/10.1007/s002210000614
Münchau A, Bloem BR, Irlbacher K et al (2002) Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22:554–561 (pii: 22/2/554)
Neige C, Massé-Alarie H, Mercier C (2018) Stimulating the healthy brain to investigate neural correlates of motor preparation: a systematic review. Neural Plast 2018:1–14. https://doi.org/10.1155/2018/5846096
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Owen AM, McMillan KM, Laird AR, Bullmore E (2005) N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. https://doi.org/10.1002/hbm.20131
Pelletier R, Higgins J, Bourbonnais D (2015) Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet Disord 16:25. https://doi.org/10.1186/s12891-015-0480-y
Petrovic P, Petersson KM, Ghatan PH et al (2000) Pain-related cerebral activation is altered by a distracting cognitive task. Pain 85:19–30. https://doi.org/10.1016/S0304-3959(99)00232-8
Rizzo V, Siebner HR, Modugno N et al (2004) Shaping the excitability of human motor cortex with premotor rTMS. J Physiol 554:483–495. https://doi.org/10.1113/jphysiol.2003.048777
Roosink M, Zijdewind I (2010) Corticospinal excitability during observation and imagery of simple and complex hand tasks: implications for motor rehabilitation. Behav Brain Res 213:35–41. https://doi.org/10.1016/j.bbr.2010.04.027
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686. https://doi.org/10.1016/j.clinph.2010.12.037
Rothkegel H, Sommer M, Paulus W (2010) Breaks during 5 Hz rTMS are essential for facilitatory after effects. Clin Neurophysiol 121:426–430. https://doi.org/10.1016/j.clinph.2009.11.016
Schabrun SM, Hodges PW (2012) Muscle pain differentially modulates short interval intracortical inhibition and intracortical facilitation in primary motor cortex. J Pain 13:187–194. https://doi.org/10.1016/j.jpain.2011.10.013
Schabrun SM, Jones E, Kloster J, Hodges PW (2013) Temporal association between changes in primary sensory cortex and corticomotor output during muscle pain. Neuroscience 235:159–164. https://doi.org/10.1016/j.neuroscience.2012.12.072
Schabrun SM, Burns E, Hodges PW (2015a) New insight into the time-course of motor and sensory system changes in pain. PLoS One 10:e0142857. https://doi.org/10.1371/journal.pone.0142857
Schabrun SM, Hodges PW, Vicenzino B et al (2015b) Novel adaptations in motor cortical maps: the relation to persistent elbow pain. Med Sci Sports Exerc 47(4):681–690
Schabrun SM, Elgueta-Cancino EL, Hodges PW (2017a) Smudging of the motor cortex is related to the severity of low back pain. Spine (Phila Pa 1976) 42:1172–1178. https://doi.org/10.1097/brs.0000000000000938
Schabrun SM, Palsson TS, Thapa T, Graven-Nielsen T (2017b) Movement does not promote recovery of motor output following acute experimental muscle pain. Pain Med. https://doi.org/10.1093/pm/pnx099
Seminowicz DA, Davis KD (2007a) A re-examination of pain-cognition interactions: implications for neuroimaging. Pain 130:8–13. https://doi.org/10.1016/j.pain.2007.03.036
Seminowicz DA, Davis KD (2007b) Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex 17:1412–1422. https://doi.org/10.1093/cercor/bhl052
Seminowicz DA, Moayedi M (2017) The dorsolateral prefrontal cortex in acute and chronic pain. J Pain 18:1027–1035. https://doi.org/10.1016/j.jpain.2017.03.008
Seminowicz DA, Mikulis DJ, Davis KD (2004) Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain 112:48–58. https://doi.org/10.1016/j.pain.2004.07.027
Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (Form Y). Consulting Psychologists Press, Palo Alto, CA
Stinear CM, Byblow WD (2003) Motor imagery of phasic thumb abduction temporally and spatially modulates corticospinal excitability. Clin Neurophysiol 114:909–914. https://doi.org/10.1016/S1388-2457(02)00373-5
Strafella AP, Paus T (2000) Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. NeuroReport 11:2289–2292. https://doi.org/10.1097/00001756-200007140-00044
Sullivan MJL, Bishop SR, Pivik J (1995) The pain catastrophizing scale: development and validation. Psychol Assess 7:524–532. https://doi.org/10.1037/1040-3590.7.4.524
Svensson P, Miles TS, McKay D, Ridding MC (2003) Suppression of motor evoked potentials in a hand muscle following prolonged painful stimulation. Eur J Pain 7:55–62. https://doi.org/10.1016/S1090-3801(02)00050-2
Takada M, Tokuno H, Nambu A, Inase M (1998) Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: Segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res 120:114–128. https://doi.org/10.1007/s002210050384
Tang J, Gibson SJ (2005) A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain 6:612–619. https://doi.org/10.1016/j.jpain.2005.03.009
Tokuno H, Tanji J (1993) Input organization of the distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde labeling study. J Comp Neurol 333:199–209
Tsao H, Danneels LA, Hodges PW (2011) ISSLS Prize Winner: smudging the motor brain in young adults with recurrent low back pain. Spine (Phila Pa 1976) 36:1721–1727. https://doi.org/10.1097/brs.0b013e31821c4267
Turner JA, Aaron LA (2001) Pain-related catastrophizing: what is it? Clin J Pain 17:65–71. https://doi.org/10.1097/00002508-200103000-00009
Valeriani M, Restuccia D, Di VL et al (1999) Inhibition of the human primary motor area by painful heat stimulation of the skin 135. Clin Neurophysiol 110:1475–1480
Valeriani M, Restuccia D, Di Lazzaro V et al (2001) Inhibition of biceps brachii muscle motor area by painful heat stimulation of the skin. Exp Brain Res 139:168–172
Vermeij A, van Beek AHEA, Olde Rikkert MGM et al (2012) Effects of aging on cerebral oxygenation during working-memory performance: a functional near-infrared spectroscopy study. PLoS One 7:e46210. https://doi.org/10.1371/journal.pone.0046210
Vos T, Allen C, Arora M et al (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602. https://doi.org/10.1016/S0140-6736(16)31678-6
Wiech K, Seymour B, Kalisch R et al (2005) Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage 27:59–69. https://doi.org/10.1016/j.neuroimage.2005.03.044
Acknowledgements
Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121). SMS receives salary support from The National Health and Medical Research Council of Australia (#1105040).
Author information
Authors and Affiliations
Contributions
TG-N, RPH, DS, SS, SAB, and DBL contributed to the conceptual development of the study. Data collection, analysis and the preliminary draft were performed by DBL. All authors interpreted and discussed the results. The manuscript draft was critically revised by RPH, TG-N, and SAB, and edited by all authors. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Larsen, D.B., Graven-Nielsen, T., Hirata, R.P. et al. Corticomotor excitability reduction induced by experimental pain remains unaffected by performing a working memory task as compared to staying at rest. Exp Brain Res 237, 2205–2215 (2019). https://doi.org/10.1007/s00221-019-05587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05587-y