Abstract
We investigated the influence of the ageing process on the performance of the motor control system accuracy during a challenging motor task throughout the analysis of force output oscillations. The force signal of the first dorsal interosseous during linearly varying static contraction, 0–100–0% of the maximal volitional abduction in 15 s, was studied in 11 young and older adults. The relative error between the target and the actual force as well as several parameters of the force oscillations (corrections) were estimated. To understand the experimental results, we analyzed the force output generated by a set of computational simulations of a pool of motor units controlled by a proportional–integral–derivative system. Compared to young adults the older subjects presented larger errors and a lower number of corrections with longer duration and larger relative amplitude. The motor control system modelling varied the error update frequency (UF) of the controller (from 1 to 2.5 Hz) as well as the range of contraction time (CT) of the recruited motor unit (30–90 ms and 60–120 ms reflecting young and old ranges, respectively). The simulation generated force profiles with parameters similar to experimental recordings in young (UF = 1.5; CT 30–90 ms) and older (UF = 1; CT 60–120 ms) adults. Interestingly, the results of the simulations suggested that the improvement in the error update frequency of the controller was not able to compensate for the contractile changes in the motor unit twitches. In conclusion, the peripheral contractile changes with age can influence motor unit control strategies and represent a crucial phenomenon in the generation of larger force oscillations in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The force fluctuations during isometric contractions and the precision of the motor output may be influenced by several factors: the investigated muscle group, type, speed, intensity of contraction and the motor skills of the subject (Enoka et al. 2003; Christou and Tracy 2005). In general, older adults (O) have lower precision of motor control compared to young (Y) adults (Oomen and Van Dieën 2017). Reduced motor control performance has been first attributed to the greater variability of motor unit (MU) inter-spike intervals (Christou and Tracy 2006; Moritz et al. 2005) and the more variable level of MU recruitment (Jesunathadas et al. 2010). Later investigations, however, could not confirm this hypothesis. For instance, it has been shown that the variability in the inter-spike intervals of individual motor units recorded in older adults was similar to young adults (Barry et al. 2007). Recently, the debate has been partly addressed showing that the variability of force oscillations are reflected in the low-frequency components of the neural drive to muscle (Negro et al. 2009; Dideriksen et al. 2012; Farina and Negro 2015). These oscillations are generated by the shared synaptic inputs to the motor neurons that originate in the spinal and supraspinal centers and are responsible for the error corrections during voluntary control of precision forces (Negro et al. 2016).
Few papers have investigated the influence of the ageing process on the level of force oscillation throughout the entire range of volitional static effort contractions of small hand muscles. In the literature, it can be found data from steady short contractions (Christou and Tracy 2006; Barry et al. 2007; Castronovo et al. 2018) or increasing decreasing challenging ramps up to the maximal voluntary contraction (MVC) (Orizio et al. 2010). Jesunathadas et al. (2010) studied force variability in old subjects during up-going/down-going ramp, but only up to 20% MVC. These studies evaluated the unsteadiness of the force output in respect to the requested target and the EMG properties, but did not focus on the identification of the motor control system components that can change with age and affect the stability of force. Simple models exist to describe the motor control system action on muscle mechanical output as the result of the comparison between the expected target tension and the actual transduced force presented at the same time on a computer screen. The visual information about the instantaneous force error triggers changes in the synaptic input to motor neurons (low-frequency oscillations) and consequent variations of the neural drive to muscle (Fuchs et al. 1989). In this simplified model, two parameters may have a profound influence on the precision of the force output: the visual processing time (central factor) that modifies the update frequency (UF) of the control signal (synaptic input) to the motor neuron pool and the MU twitches (peripheral factor) that influence the low-pass filtering properties of the muscle. It is well known that both visual processing delay and duration of MU twitches increase with age (Oomen and van Dieën 2017; Orizio et al. 2016). In this study, we aimed to understand the relative role of these two factors in generating the greater inaccuracy of the muscle output tension in old subjects. Specifically, the study will focus on:
-
1.
the characterization of the relative error between the expected target and the actual force in terms of force signal oscillations (number, amplitude, and duration), underpinning the motor control compensations in old and young adults. The variables will be extracted from a challenging up-going/down-going ramp up to 100% MVC.
-
2.
the comparison of the variables extracted from the experimental force profiles with the ones obtained from a proportional–integral–derivative controller (PID) (Dideriksen et al. 2017). The force signal for O and Y will be modelled using different values of the updating frequency, MU twitch contraction time and MU number to mimic the known central and peripheral changes in the motor system of older adults and understand the contributions of these two factors in the generation of accurate ramp contractions.
Methods
Eleven young (23.90 ± 3.72 years) and eleven older adults (69.63 ± 4.34 years) without neurological or orthopedic diseases, gave their informed consent to participate in the study after being given a full explanation of the experimental procedure according to the Declaration of Helsinki (1964) and its amends. The local Ethical Research Committee approved the proposed experimental design (CEIOC authorization: 17/2011).
Procedure
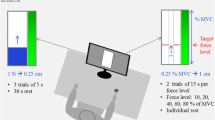
The investigated muscle was the first dorsal interosseous (FDI). The experimental set-up is represented in Fig. 1. The forearm of the subject was positioned halfway between pronation and supination in a custom designed brace and fixed, at the last three digits of the hand, by straps to the rigid frame. The index finger and thumb were at an angle of 90° and the tension during static abduction of the second digit was recorded through a load cell (SM-50N, Interface, Arizona, USA) coupled to the second phalanx. Given the adopted posture, the recorded tension was almost due to FDI activity. The signal was low pass filtered at 128 Hz before storing. The signal was A/D converted (LabVIEW 2010 10.0, National Instrument, Austin, TX, USA) and stored at a sample rate of 1024 Hz in a personal computer for off-line processing. The maximal voluntary contraction (MVC) force was determined as the highest of three consecutive efforts lasting 3 s, with 1 min interval in between. Subsequently, the subject performed one triangular isometric contraction, 0–100–0% MVC (see Fig. 2 panel a). The rate of tension increase/decrease during the up-going and down-going ramps were 13.3% and − 13.3% MVC/s, respectively. The slope of the force was selected to produce static tension above 50% MVC during the triangular task for no more than 7.5 s, and limit muscle fatigue. The participants received visual feedback of the normalized exerted force. The target was shown as a triangular trajectory with two lines above and below showing the required tolerance (+/− 5% MVC). Before starting with the actual experimental session, a period of training was granted to familiarize subjects with the triangular static contraction (0–100–0% MCV) until each of the participants was able to perform the task correctly. Verbal encouragement was provided to all subjects during the entire duration of the experiment.
Schematic representation of the ergometer for the recording of the first dorsal interosseous (FDI) muscle tension generated during static abduction in the vertical plane of the first digit. The relative position between the load cell and the index finger is shown. The angle between the first and the second digit was 90°. See text for details
Panel a: the force target and the actual force produced by the static contraction of the FDI are reported. The grey areas indicate the ramp periods that were discarded to avoid the analysis of transients related with initial and final part of the target dynamics. Panel b: The de-trended force signal is represented
Simulations
The simulations performed in the present study were based on a model developed for the FDI muscle which comprised 120 motor neurons (leaky integrate-and-fire) as suggested by the histological findings (Feinstein et al. 1955). The input resistance of the motor neurons ranged from 0.6 to 1.6 MΩ and their time constants from 5.9 to 20.4 ms. The threshold was set to 8 mV for all motor neurons. The discharge of each individual motor neuron generated a motor unit twitch force profile according to the Fuglevand model (Fuglevand et al. 1993). Therefore, the model combined the motor units mechanical contribution to the output tension summating MU twitches with an exponential variation of the peak twitch amplitude (in the range 1–100 arbitrary units) and of its contraction time (CT) (in the range 30–90 ms).
To simulate the force tracking task performed in the experimental recordings, the model was driven by a proportional-integral-derivative controller (Dideriksen et al. 2017). The input to the motor neurons was a linear summation of the synaptic motor command generated by the proportional–integral controller and Gaussian noises (0–50 Hz). The motor command was a shared synaptic input to all the motor neurons with a bandwidth defined by the UF of the controller. UF determined the time interval between two consecutive estimations of the tracking error. On the basis of the estimated error, the neural activation signal to the motor neurons was adapted. Additionally, a constant visuomotor delay of 135 ms was included in all simulations (Carlton 1992). The shared motor command simulates the slow common drive typically present in motor unit spike trains recorded in experimental recordings (Negro et al. 2009, 2016; De Luca 1985). The motor units spike trains were designed using an inter-spike interval variability of approximately 15% (Dideriksen et al. 2012; Maltenfort et al. 1998). The range of discharge rate of the individual motor neurons was fixed between 8 and 35 pps. The motor neuron parameters (input resistance and membrane time constant) were similar to the ones previously published (Cisi and Kohn 2008; Elias and Kohn 2013; Negro and Farina 2011, 2012) and selected according to an exponential distribution over the pool of motor neurons (Fuglevand et al. 1993; Negro et al. 2016). At 50% of the maximal synaptic input (Burke et al. 1974; Milner-Brown et al. 1973) all the motor units were recruited.
To mimic the behavior of young and older adults during ramp force generation, simulations were performed with seven values of the UF of the controller (1, 1.25, 1.5, 1.75, 2, 2.25, 2.5 Hz). Similarly, the range of time to peak of the motor unit twitches was also shifted from the original 30–90 ms to 60–120 ms with steps of 10 ms. Additionally, the number of MU was decreased to 70 to simulate a decline in MU number due to aging and the peak force of the individual motor unit twitches were adjusted to obtain a potentiation of 50% compared to the original model (Doherty and Brown 1997; Semmler et al. 2000). The parameters were selected to simulate the slowing of the processing/reaction time and the modified contractile properties of the older adults (Orizio et al. 2016).
Analysis
The up-going ramp (UGR) is the first part of the exercise from 0 to 100% of the MVC, down going ramp (DGR) is the second part of the exercise from 100 to 0% of the MVC. The first part (0–1.25 s) and the last part (13.75–15 s) were not considered in the analysis to avoid transients related with the onset and the offset of the target dynamics (Orizio et al. 2010).
Error analysis
From the force signal (digitally filtered 0–20 Hz with OT BioLab 2.0 software) relative error (%Err) was calculated sample by sample as the absolute difference between the actual output tension and the target tension scaled to the target tension itself. The average of the obtained values was calculated for UGR, DGR and for UGR + DGR.
Spike shape analysis (SSA)
Gabriel (2007) described a method to quantify the oscillations (spikes) in the surface EMG signal. Each spike was identified as the composition of two subsequent upward–downward deflections of the signal across the isoelectric baseline. This approach was applied to the force signal in our investigation after band-pass filtering. An example of the force signal obtained after digital filtering (0.2–20 Hz with OT BioLab 2.0) to be used for SSA is reported in Fig. 2 panel b. In Fig. 3 the spikes identified by the SSA algorithm (Gabriel et al. 2007) are shown. The outcome of SSA focused mainly on number of spikes (NoS), average spikes duration (ASD) and average spikes amplitude (ASA). The relative average spike amplitude (RASA) was calculated using the formula (ASA/MVC*100). Eventually, NoS describes the number of force corrections performed by the subject to keep the torque output as close as possible to the required target. ASD, ASA, and RASA are qualitative parameters describing the shape of spikes similarly as described by Gabriel (2007) for the EMG.
Statistical analysis
To compare Y and O MVC values, %Err and the SSA parameters from whole contraction (UGR + DGR) a t Test was used. To compare the %Err and SSA parameters in UGR and DGR (factor 1) between Y and O (factor 2), two-way analysis of variance was used after test for normality and equal variance of the data were passed.
For the simulation force signal, Kruskal–Wallis one-way analysis of variance on ranks was used to compare SSA parameters obtained at different UF of the controller and with different contraction time ranges. The whole statistical analysis was performed using Sigmaplot 12.0. The cutoff for statistical significance was set at P < 0.05. Pearson’s correlation was checked between %Err and NS, ASA, ASD, and RASA of the subjects obtained through the whole task. The data of Y and O were merged.
Results
A statistically significant difference in MVC values between Y (2.77 ± 0.62 Kg) and O (2.10 ± 0.58 Kg) was found (P = 0.014).
Experimental results: %Err
Two representative trials of an O and a Y adult are shown in Fig. 4. The black area reflects the amount of %Err. It can be noted that the black area is wider in O than in Y adults.
The %Err during contraction was significantly greater in the older than in the younger group. A statistical significant difference in %Err between Y (9.52 ± 1.85) and O (16.81 ± 4.95) for the whole contraction (UGR + DGR) was found (P < 0.001). The %Err were not different, within each group, during UGR and DGR (P = 0.908), but a statistically significant difference was found between Y (9.98 ± 2.77) and O (16.15 ± 7.06) for the UGR and between Y (9.06 ± 1.69) and O (17.47 ± 7.93) for the DGR (P < 0.001).
Experimental results: SSA
The SSA parameters were not different, within each group, during UGR and DGR. NoS (related to the number of force corrections), ASD and RASA resulted significantly different in O adults than in Y (Table 1). On the contrary, the absolute value of ASA never reached a significant difference between O and Y.
Pearson’s correlation test was used to estimate the possible relationship between SSA parameters and %Err: %Err tends to decrease as higher as NoS is (correlation coefficient =− 0.620; P < 0.05); %Err increased with ASD (correlation coefficient = 0.639; P < 0.05) and RASA (correlation coefficient = 0.551; P < 0.05). No correlation between %Err and ASA was found. Similar results for the %Err and the SSA analysis were obtained by filtering the force signal between 0 and 5 Hz. This suggests that the oscillations of the force signal are mostly due to voluntary adjustments triggered by the error between the target and the actual force and not by physiological tremor oscillations.
Simulation results
In Fig. 5 the force profiles generated by the PID model using the 30–90 and 60–120 ms MU CT ranges are reported. The force profiles reported in the figure are related to a frequency of error checking between the actual force and the target set at 2.5 Hz. It is evident that the higher range of CTs determines a lower number of spikes (corrections).
Force signals obtained from the PID model using the same updating frequency, but different ranges for the contraction time: 30–90 ms (typical for young subjects) and 60–120 ms (typical for old subjects). It can be noted that the behavior of the force profiles is similar to the ones shown in Fig. 4
In Fig. 6 the averaged results of the simulations for the number of force oscillations are shown. In particular, the number of force oscillations during the whole contraction (UGR + DGR) as a function of the UF of the controller was represented for both contraction time ranges. It can be noted that increasing the UF of the controller, the number of force corrections increases as well. Interestingly, we found a statistically significant effect of the MU twitch CT range (30–90 or 60–120 ms) on the NoS (P < 0.001). The same trend was found for the other SSA variables. In fact, both ASA and ASD were significantly higher in the CT range 60–120 ms compared to the 30–90 ms CT range (P < 0.001). Similar results were found comparing intermediate variations of CT (40–100 and 50–110 ms), but with smaller differences compared to the normal case (30–90 ms). Small deviations were observed simulating a significant decrease in the number of simulated MU and an increase in the averaged peak amplitude of the motor unit twitches, but they did not show statistical differenced compared to the previously reported averaged results. For example, the simulation of a motor pool of 70 MUs with small variations in average discharge rate (± 10%) did not show a statistical difference compared to the original case (120 MUs), for all simulations (P = 0.34). Moreover, the inclusion of motor unit twitch potentiation (+ 50% compared to the original model) showed the tendency to increase the NoS (18 ± 3 vs 20 ± 3), but the difference was not statistically significant (P = 0.051). No difference was observed in the other parameters.
Influence of the update frequency and the contraction time in the simulations. Both parameters were positively influencing the number of force corrections. It is evident that the range of CT, 30–90 or 60–120 ms, has a more profound effect on the extracted variables compared to the update frequency of the controller. At the same update frequency, lower CT range (30–90 ms) provide a significant higher number of force spikes and this allows the system to act on a smaller cumulated relative error
Discussion
We investigated the variability in the control of force during isometric ramp contractions up to 100% MVC in young and older adults. The main findings were that the older subjects presented larger errors in the generation of the isometric ramp contraction compared to the young adults, with a lower number of corrections having a longer duration and relatively larger amplitude.
Motor output error in old and young subjects
During linearly varying static effort, the %Err resulted always larger in O than in Y when the whole contraction (UGR + DGR) was considered. An important factor contributing to this increased instability of the FDI motor output with ageing (Barry et al. 2007; Semmler et al. 2003; Laidlaw et al. 2000) is likely the larger variability of the MU discharge rates (Tracy et al. 2005; Barry et al. 2007; Laidlaw et al. 2000) due to the larger fluctuations of the low-frequency common synaptic input to motor neurons (Feeney et al. 2018; Castronovo et al. 2018). These oscillations are mainly related to the control of synaptic input that provides the error correction signal to the motor neuron pools (Negro et al. 2009, 2016). Another important phenomenon that can influence the steadiness of the force output is related to the MU remodeling, i.e., the process of re-innervation of the orphan muscle fibers of the lost motoneurons, which creates larger MU with increased difference in the recruitment tension between successively recruited motor units in old adults (Jesunathadas et al. 2010). The larger %Err in O subjects is likely the result of the combination of these two phenomena which would obviously affect the performance of the control system to correct errors and follow a predefined force trajectory. Our experimental and simulation results (see below) indicate that in O subjects these factors can have an influence in the performance of the motor task not only during the increasing, but also decreasing of the isometric force task.
Spike shape analysis
Gabriel (2007) described a processing technique that identifies and characterizes spikes in the surface EMG signal. In this study, the same criteria were used to identify force oscillations due to the force control system commands aimed to reduce the distance between the expected and the actually exerted force. In other words, the force spikes reveal the underpinned correction procedure.
Similarly, to the %Err results, within the same group, number, amplitude, and duration of the force signal spikes were not different between UGR and DGR. Otherwise, in the older adults, the NoS and the ASD of the corrections throughout the whole force profile (UGR + DGR) were significantly lower and longer than in the young subjects. The ASA was also larger in O subjects but not significantly. Indeed, when the RASA (spike amplitude scaled to individual MVC) was considered, the results were always larger in O (about 12%) than in Y (lower than 8%). The difference was statistically significant (see Table 1) and supported the indication that the outcome of the control force strategies is more conveniently evaluated on the basis of the normalized contraction intensity (De Luca et al. 1982; Milner-Brown et al. 1973; Seki and Narusawa 1996).
The data from the force spike shape analysis clearly suggest that the larger %Err values in O could be due to multiple peripheral and central factors determining fewer, slower, and larger (when scaled to MVC) corrections. The peripheral factors can be ascribed to the above-cited ageing process resulting in fewer, slower and larger MUs in the muscle. In these conditions, the motor commands from CNS, aimed to correct the desired tension production, have to compensate for these peripheral changes and create larger (in duration and amplitude) force spikes compared to Y subjects. In a similar way, according to Oomen and van Dieën, another important physiological factor influencing force unsteadiness should be identified with an age-related longer processing time of visual information (Sosnoff and Newell 2007), affecting, in particular, the low-frequency bandwidth (0–2 Hz) of force control. The NoS was nearly double in the Y than O suggesting that younger subjects control system had to compensate for smaller errors cumulated between two closer in time corrections.
Simulation
The results of the computer simulations suggested that both the elongation of the force twitches (peripheral factor) and the increase in the visual processing time (central factor) may determine a lower number of corrections with longer durations and relative larger amplitudes in O subjects. The changes in number, duration, and amplitude of the corrections were mainly related to the shift in the twitch duration ranges (from 30 to 90 to 60–120 ms). In particular, even increasing the central processing speed, the number of the corrections obtained with the longer twitches contraction range (60–120 ms) could not match the corresponding values obtained using the faster twitches contraction range (30–90 ms). The same phenomenon was evident for intermediate changes in twitch contraction duration (40–100 and 50–110 ms). In other words, the simulation results showed that the peripheral changes of the motor units may not be compensated by a faster and more efficient CNS control signal to the motor neurons. On the other hand, a decrease in the simulated number of motor units and the potentiation of the motor unit twitch amplitudes show small changes (not statistically significant) in the estimated parameters, in agreement with previous investigations (Enoka et al. 2003). However, we cannot exclude that larger variations and/or non-uniform changes of the twitch amplitude across the motor pool may have a bigger impact on the overall force control.
It is important to underline that the oscillations in force analyzed in the present study are related to the voluntary corrections and not to other involuntary neural mechanisms as physiological tremor (Christakos et al. 2006). Physiological tremor is likely the result of the Ia afferent reflex acting on MU activation pattern (Lippold 1970; Nagamori et al. 2018), but these oscillations ranged normally between 0.1 and 1% MVC, much smaller compared to the oscillations in force observed in the experimental recordings of the present study. In fact, the RASA in our study was about 10 times larger. Therefore, the frequency of the observed mechanical oscillations during physiological tremor is normally 10 times higher compared to the number of spikes/s we have found. To confirm this hypothesis, we calculated the same parameters filtering the original force signal in a narrower frequency band (0–5 Hz), excluding the physiological tremor oscillations, and the overall results showed minimal variations. For these reasons, our attention about possible factors influencing force unsteadiness during linearly varying static effort was focused mainly on the interaction between the central processing of the visual information and the changes in MU twitches properties. Similar considerations were made by Jesunathadas (2010) suggesting that the larger force variability in O could be related to the MU remodeling and the consequent altered shared synaptic input to the motor neurons pools.
Eventually, the twitch elongation with age may represent the underpinning crucial phenomenon in the generation of larger force oscillations and the greater relative errors in O vs Y subjects. Future studies using more complex models including structural changes influencing the tension transmission through muscles having larger relative content of connective tissue and changes in passive stiffness (Gajdosik et al. 2005) will contribute to a deeper insight about the adaptation of the force generation process and control which will include peripheral and central factors.
Conclusions
The results of the present study obtained from the analysis of the muscular mechanical output unsteadiness lead to the conclusion that the lower performance of the motor control system in aged subjects during challenging tasks, consecutive up-going/down-going static linear ramps in the whole range of tension modulation, can be attributed mainly to changes in MU peripheral properties. In fact, the simulation results suggested that improvement of the CNS data processing time is not able to compensate for the elongation of the MU twitches contraction time.
References
Barry BK, Pascoe MA, Jesunathadas M, Enoka RM (2007) Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97:3206–3218. https://doi.org/10.1152/jn.01280.2006
Burke RE, Levine DN, Salcman M, Tsairis P (1974) Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol 238:503–514. https://doi.org/10.1113/jphysiol.1974.sp010540
Carlton LG (1992) Chap. 1 visual processing time and the control of movement. Adv Psychol 85:3–31. https://doi.org/10.1016/S0166-4115(08)62008-7
Castronovo AM, Mrachacz-Kersting N, Stevenson AJT et al (2018) The decrease in force steadiness with aging is associated with increased power of the common but not independent input to motor neurons. J Neurophysiol jn. https://doi.org/10.1152/jn.00093.2018 00093.2018.
Christakos CN, Papadimitriou NA, Erimaki S (2006) Parallel neuronal mechanisms underlying physiological force tremor in steady muscle contractions of humans. J Neurophysiol 95:53–66. https://doi.org/10.1152/jn.00051.2005
Christou EA, Tracy BL (2006) Aging and variability in motor output. In: Davids K, Bennett S, Newell K (eds) Movement system variability. The Human Kinetics, Champaign, pp 199–216
Cisi RRL, Kohn AF (2008) Simulation system of spinal cord motor nuclei and associated nerves and muscles, in a Web-based architecture. J Comput Neurosci 25:520–542. https://doi.org/10.1007/s10827-008-0092-8
De Luca CJ (1985) Control properties of motor units. J Exp Biol 115:125–136
de Luca CJ, LeFever RS, McCue MP, Xenakis AP (1982) Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329:113–128. https://doi.org/10.1113/jphysiol.1982.sp014293
Dideriksen JL, Negro F, Enoka RM, Farina D (2012) Motor unit recruitment strategies and muscle properties determine the influence of synaptic noise on force steadiness. J Neurophysiol 107:3357–3369. https://doi.org/10.1152/jn.00938.2011
Dideriksen JL, Feeney DF, Almuklass AM, Enoka RM (2017) Control of force during rapid visuomotor force-matching tasks can be described by discrete time PID control algorithms. Exp Brain Res 235:2561–2573. https://doi.org/10.1007/s00221-017-4995-3
Doherty TJ, Brown WF (1997) Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol 82:93–101. https://doi.org/10.1152/jappl.1997.82.1.93
Elias LA, Kohn AF (2013) Individual and collective properties of computationally efficient motoneuron models of types S and F with active dendrites. Neurocomputing 99:521–533. https://doi.org/10.1016/J.NEUCOM.2012.06.038
Enoka RM, Christou EA, Hunter SK et al (2003) Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13:1–12. https://doi.org/10.1016/S1050-6411(02)00084-6
Farina D, Negro F (2015) Common synaptic input to motor neurons, motor unit synchronization, and force control. Exerc Sport Sci Rev 43:23–33. https://doi.org/10.1249/JES.0000000000000032
Feeney DF, Mani D, Enoka RM (2018) Variability in common synaptic input to motor neurons modulates both force steadiness and pegboard time in young and older adults. J Physiol. https://doi.org/10.1113/JP275658
Feinstein B, Lindegård B, Nyman E, Wohlfart G (1955) Morphologic studies of motor units in normal human myscles. Cells Tissues Organs 23:127–142. https://doi.org/10.1159/000140989
Fuchs AF, Anderson ME, Binder MD, Fetz EE (1989) The neural control of movement, 21st edn. Saunders Company, Philadelphia
Fuglevand AJ, Winter DA, Patla AE (1993) Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70:2470–2488. https://doi.org/10.1152/jn.1993.70.6.2470
Gabriel DA, Lester SM, Lenhardt SA, Cambridge EDJ (2007) Analysis of surface EMG spike shape across different levels of isometric force. J Neurosci Methods 159:146–152. https://doi.org/10.1016/j.jneumeth.2006.07.004
Gajdosik RL, Vander Linden DW, McNair PJ et al (2005) Viscoelastic properties of short calf muscle-tendon units of older women: Effects of slow and fast passive dorsiflexion stretches in vivo. Eur J Appl Physiol 95:131–139. https://doi.org/10.1007/s00421-005-1394-4
Jesunathadas M, Marmon AR, Gibb JM, Enoka RM (2010) Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108:1659–1667. https://doi.org/10.1152/japplphysiol.00807.2009
Laidlaw DH, Bilodeau M, Enoka RM (2000) Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23(200004):600–612 doi: https://doi.org/10.1002/(SICI)1097-4598(200004)23:4%3C600::AID-MUS20%3E3.0.CO;2-D
Lippold OCJ (1970) Oscillation in the stretch reflex arc and the origin of the rhythmical, 8–12 c/s component of physiological tremor. J Physiol 206:359–382. https://doi.org/10.1113/jphysiol.1970.sp009018
Maltenfort MG, Heckman CJ, Rymer WZ (1998) Decorrelating actions of Renshaw interneurons on the firing of spinal motoneurons within a motor nucleus: a simulation study. J Neurophysiol 80:309–323. https://doi.org/10.1152/jn.1998.80.1.309
Milner-Brown HS, Stein RB, Yemm R (1973) Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol 230:371–390. https://doi.org/10.1113/jphysiol.1973.sp010193
Moritz CT, Barry BK, Pascoe MA, Enoka RM (2005) Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93:2449–2459. https://doi.org/10.1152/jn.01122.2004
Nagamori A, Laine CM, Valero-Cuevas FJ (2018) Cardinal features of involuntary force variability can arise from the closed-loop control of viscoelastic afferented muscles. PLOS Comput Biol 14:e1005884. https://doi.org/10.1371/journal.pcbi.1005884
Negro F, Farina D (2011) Decorrelation of cortical inputs and motoneuron output. J Neurophysiol 106:2688–2697. https://doi.org/10.1152/jn.00336.2011
Negro F, Farina D (2012) Factors influencing the estimates of correlation between motor unit activities in humans. PLoS One 7:e44894. https://doi.org/10.1371/journal.pone.0044894
Negro F, Holobar A, Farina D (2009) Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587:5925–5938. https://doi.org/10.1113/jphysiol.2009.178509
Negro F, Yavuz U, Farina D (2016) The human motor neuron pools receive a dominant slow-varying common synaptic input. J Physiol 594:5491–5505. https://doi.org/10.1113/JP271748
Oomen NMCW, Van Dieën JH (2017) Effects of age on force steadiness: a literature review and meta-analysis. Ageing Res Rev 35:312–321. https://doi.org/10.1016/j.arr.2016.11.004
Orizio C, Baruzzi E, Gaffurini P et al (2010) Electromyogram and force fluctuation during different linearly varying isometric motor tasks. J Electromyogr Kinesiol 20:732–741. https://doi.org/10.1016/j.jelekin.2010.03.005
Orizio C, Cogliati M, Bissolotti L et al (2016) The age related slow and fast contributions to the overall changes in tibialis anterior contractile features disclosed by maximal single twitch scan. Arch Gerontol Geriatr 66:1–6. https://doi.org/10.1016/j.archger.2016.05.003
Seki K, Narusawa M (1996) Firing rate modulation of human motor units in different muscles during isometric contraction with various forces. Brain Res 719:1–7. https://doi.org/10.1016/0006-8993(95)01432-2
Semmler JG, Steege JW, Kornatz KW, Enoka RM (2000) Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol 84:358–366. https://doi.org/10.1152/jn.2000.84.1.358
Semmler JG, Kornatz KW, Enoka RM (2003) Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol 90:1346–1349. https://doi.org/10.1152/jn.00941.2002
Sosnoff JJ, Newell KM (2007) Are visual feedback delays responsible for aging-related increases in force variability? Exp Aging Res 33:399–415. https://doi.org/10.1080/03610730701525311
Tracy BL, Maluf KS, Stephenson JL et al (2005) Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 32:533–540. https://doi.org/10.1002/mus.20392
Acknowledgements
The Authors thank the “Teresa Camplani Foundation” in Brescia (I) where the recordings were made (LARIN laboratory) and the innovation programme under the Marie Skłodowska-Curie grant agreement No 702491 (NeuralCon).
Author information
Authors and Affiliations
Contributions
Itemized list of how each author contributed to the study: CO, MC, AC conceived and designed research. PG and LMB provided lab settings and software for signals harvesting. MC, AC conducted experiments. FN planned and carried out the simulations. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cogliati, M., Cudicio, A., Negro, F. et al. Influence of age on motor control accuracy during static ramp contractions. Exp Brain Res 237, 1889–1897 (2019). https://doi.org/10.1007/s00221-019-05524-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05524-z