Abstract
The present study tested whether remapping of visuomotor correspondence alters automatic motor responses induced by visual stimuli. We hypothesized that the congruency effect, an automatic modulation of motor responses based on stimulus–response congruency, changes in accordance with a new visuomotor correspondence acquired through an adaptation task. To induce visuomotor adaptation, participants performed a tracking task with 30° or 150° rotation of the visual feedback. The congruency effect was evaluated multiple times by a visual response task where participants moved their finger left or right. We predicted that the congruency effect, as a measure of automatic responses, would be almost reversed after adaptation to the 150° rotation, because a visual stimulus spatially opposite to the participant’s own action would become a “congruent” stimulus in a 150°-rotated environment but not in a 30°-rotation environment. The results show that visuomotor adaptation to the 150° rotation did modulate the congruency effect in accordance with the acquired visuomotor correspondence, but did not completely reverse the effect. When the effect was assessed after the manipulation, which was assumed to switch an internal model back to its normal state, there was no change in automatic motor responses. Furthermore, we found that after effects developed as the training proceeded but decreased over time. These findings suggest that the visuomotor system subserving automatic modulation in motor responses is based on the currently active internal model and, therefore, highly adaptive. In addition, the mechanism underlying after effects in a visuomotor task is discussed in terms of a switching function of internal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans can acquire various motor skills through repetitive practice, eventually mastering them perfectly, and switch between skills whenever necessary. Skillful actions are characterized as automatic control: appropriate motor responses are elicited by certain sensory stimuli, without attention or effort. The present study tested whether temporary remapping of visuomotor correspondence alters automatic visual stimulus-induced motor responses that are usually inconspicuous but inevitably occur. Studies have shown that our motor behavior is influenced by several components of visually observed movements (Simon and Rudell 1967; Tardy-Gervet et al. 1984; Craighero et al. 1999, 2002; Edwards et al. 2003; Kilner et al. 2003; Bosbach et al. 2005; Bertenthal et al. 2006; Liepelt and Brass 2010; Heyes 2011; Boyer et al. 2012; Kupferberg et al. 2012). Movement direction of a visual stimulus is one of the major factors inevitably interfering with an observer’s movement execution (Brass et al. 2000, 2001; Kilner et al. 2003; Bouquet et al. 2007; Kupferberg et al. 2012; Itaguchi and Kaneko 2018). Such automatic effects are likely based on a visuomotor correspondence that has been acquired through long-term experience in everyday life. It has, however, never been fully clarified how a newly acquired visuomotor correspondence affects the motor responses automatically induced by visual stimuli. To address this question, we experimentally varied visuomotor correspondence by visuomotor adaptation, and assessed the induced changes in the congruency effect, a well-established measure of automatic motor response.
Stimulus–response (S–R) congruency is one of the critical factors automatically and unconsciously modulating observers’ motor responses (Simon and Rudell 1967; Hommel 1996; Brass et al. 2001). When the movement direction of the visual stimulus is inconsistent with that of the planned or ongoing action of the observer, the response time or movement accuracy of the action will be deteriorated (Brass et al. 2000, 2001; Kilner et al. 2003; Bosbach et al. 2005; Bouquet et al. 2007; Kupferberg et al. 2012). That is, movement performance is greater when one observes a movement that is “congruent” than when one observes a movement that is “incongruent” with one’s own. The congruency effect is a fundamental phenomenon that can be elicited by visually recognizing the intention of an action without actual observation of its movement execution (Liepelt et al. 2008; Liepelt and Brass 2010), suggesting that the S–R congruency effect is subserved by overlapping representations between visual input and motor output (Buccino et al. 2004; Itaguchi and Kaneko 2018).
Novel correspondences between visual inputs and motor outputs can be acquired, but they can also decay on a relatively short timescale. Visuomotor adaptation tasks have been used to elicit such temporal alternations in visuomotor correspondence (Krakauer et al. 2004; Mazzoni and Krakauer 2006; Krakauer 2009; Kim et al. 2015); this process is sometimes regarded as skill acquisition for a new tool (Imamizu et al. 2000, 2003). During the task, participants perform a motor task in an environment where the visual feedback of their movements is systematically modified. In such a condition, at first, participants make large errors when visually guiding their body or an apparatus. However, they gradually adapt to the environment through training, and will eventually be able to control their body as in a normal environment. This adaptation develops regardless of whether participants notice or understand the visual perturbation of the feedback (Kagerer et al. 1997; Klassen et al. 2005; Hatada et al. 2006; Taylor et al. 2014).
We hypothesized that the congruency effect changes in accordance with a new environment as visuomotor adaptation proceeds. Specifically, if participants are using a new internal model that represents a visuomotor correspondence opposite to the normal environment, the congruency effect, as a measure of automatic responses, would be “reversed”, because a visual stimulus spatially opposite to their own action becomes a “congruent” stimulus for the new model. When the S–R correspondence has been changed to a new one by visuomotor adaptation, a new type of motor response should be elicited even if an identical stimulus is presented. Given that voluntary motor practice in the visuomotor adaptation task changes involuntary motor responses, this hypothesis is consistent with the general idea that newly acquired skills become automatic through reorganization of the visuomotor system and then require less effort (Woodworth 1899; Doyon et al. 1996; Krebs et al. 1998; Petersen et al. 1998; Floyer-Lea and Matthews 2004; Mazzoni and Krakauer 2006; Dayan and Cohen 2011). We thus predicted that the congruency effect, which is automatically induced by a visual stimulus, would change according to a newly acquired visuomotor correspondence.
We made another prediction about adaptation-induced changes in the congruency effect. It has been suggested that visuomotor adaptation is achieved by acquiring a new visuomotor correspondence sometimes referred to as an internal model (Imamizu and Kawato 2009; Taylor et al. 2014; Shadmehr 2017), which represents the relation of motor commands and their consequences in the central nervous system (Kawato et al. 1987; Wolpert et al. 1995; Miall and Wolpert 1996; Wolpert and Kawato 1998). An internal model for the “normal” environment is not necessarily overwritten by a newly acquired one and, therefore, we can retain multiple internal models for different environments or skills at the same time in the brain (Krakauer et al. 1999; Imamizu et al. 2003, 2004, Osu et al. 2004; Lee and Schweighofer 2009). Based on the idea that multiple internal models are stored in the sensorimotor system, we also have to assume a switching system, such as those incorporated in Mixture-of-expert model and MOSAIC model (Jacobs et al. 1991; Ghahramani and Wolpert 1997; Wolpert and Kawato 1998; Haruno et al. 2001; Imamizu et al. 2004). Several studies have postulated such a switching function and localized it to the prefrontal and parietal areas (Imamizu et al. 2003; Imamizu and Kawato 2008, 2009). In our daily life, the internal model should be appropriately selected as corresponding to the current environment. A switching system of multiple internal models predicts that the congruency effect does not necessarily relate to the “degree” of learning progress of a new visuomotor environment, but that the effect is rather related to the active or inactive state of the new internal model. If an acquired model (or one that is currently being acquired) is activated in the visuomotor system, the model would modulate automatic motor responses; however, if the model is not activated, it would not affect the motor responses regardless of the degree of acquisition.
To examine our hypothesis, we manipulated the time of the evaluation of the congruency effect as well as the rotation angle of visuomotor adaptation. We evaluated congruency effects multiple times during the adaptation process, to illustrate adaptation-dependent changes in the effects. To elicit visuomotor adaptation, we used a tracking task with a 150° rotational transformation applied to the visual feedback of a mouse cursor. During the task, participants tracked a continuously moving target with a mouse cursor (Foulkes and Miall 2000; Imamizu et al. 2000; Tong and Flanagan 2003; Bock 2005; Kaida et al. 2017). Tracking tasks, unlike reaching tasks, require continuous and omnidirectional movements, which have been shown to facilitate implicit learning (Künzell et al. 2016; Ewolds et al. 2017) and to induce spatial generalization of the adaptation (Krakauer et al. 2000). A 150° rotation was selected to create an internal model with a visuomotor correspondence roughly reversed to the normal one. In the main experimental group, the congruency effect was evaluated immediately after a rotation trial, where the new internal model was assumed to be active in the evaluation of the congruency effects. We predicted that the congruency effect would change in proportion to the degree of acquisition of the new model and that the effect would eventually be reversed. In addition to the main experimental group, we used two control groups, in which we manipulated the currently active internal model or the amplitude of visuomotor remapping.
In addition to the examination of our hypothesis, we analyzed after effects to further understand the nature of visuomotor adaptation in the light of a switching system for internal models. When participants are exposed to a normal (no-rotation) environment after they have already adapted themselves to a novel (rotated) environment, movement error temporarily increases even in the familiar environment. This marked error increase is called an after effect, which is characterized by a gradual but relatively fast decay (going back to original performance). In the literature, after effects have often been assumed to reflect acquisition of a new internal model, but not explicit strategies or knowledge of the environment, and used as a measure to assess the result of visuomotor adaptation (Kravitz and Yaffe 1972; Buch et al. 2003; Bock 2005; Gandevia et al. 2006; Mazzoni and Krakauer 2006; Krakauer 2009; Kim et al. 2015). However, based on the model switching account, we predicted that it would be more adequate to regard after effects as a measure of inappropriate use of internal models for the current environment. That is, it is plausible that after effects are a complex measure of switching efficiency and acquisition of an internal model. This notion predicts that after effects are not monotonically related to the development of visuomotor adaptation.
Materials and methods
Forty-eight students (20.8 ± 1.9 years old, male 22, female 26) participated in the experiment (16 students in each experimental group). All of them were right-handed, did not have any sensorimotor disorders, and had never performed a visuomotor rotation task. The participants carried out a tracking task to induce implicit visuomotor adaptation and a response task to assess changes in the congruency effect. The experiment was conducted at Waseda University and took about 3 h to complete. The study and consent procedures were approved by the Ethics Committee on Human Research of Waseda University. All participants provided written informed consent.
Experimental groups
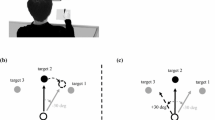
Participants were randomly assigned to one of the three experimental groups, which differed in task parameters (Fig. 1a). First, in the “150R group”, the group of main interest in the present study, the congruency effect was evaluated immediately after a rotation trial. We used a 150° rotation instead of a full 180° rotation because it might be relatively easy for participants to recognize the nature of the latter visuomotor perturbation, which would lead them to use strategic control based on explicit knowledge. Second, in the “150N group”, the congruency effect was evaluated after exposure to a no-rotation trial that worked as a washout. The differences in the task order between the 150R and 150N groups were introduced to change the participants’ internal model used in the response task; we assumed that the newly acquired (or currently being acquired) model was not active anymore after the exposure to a no-rotation trial, but still activated immediately after a rotation trial. Participants in the 150R and 150N groups had the same amount of experience of a novel visuomotor environment. Third, in the “30R group”, participants were trained in a 30°-rotation environment, and the congruency effect was evaluated immediately after a rotation trial, as in the 150R group. The 30R group served as a control regarding visuomotor remapping and to confirm that multiple evaluations of congruency effects and a long-term visuomotor task did not systematically influence congruency effects. Although a 30° visuomotor rotation could create or update a visuomotor correspondence, we assumed that its influence on the congruency effect is relatively small and that it cannot reverse the congruency effect.
a Experimental procedures in the three groups. In the 150R and 150N groups, the visual feedback of the mouse cursor was rotated by 150°; in the 30R group, the visual feedback was rotated by 30°. b Stimulus presentation in the response task. A red dotted arrow on the display indicates the distractor movement, which was not presented in the experiment. c Key positions of the response task. Participants pressed a left or right key with their index finger according to the presented number. d Schematic image of distractor movements and their congruency with the motor response. After complete adaptation to the 150°-rotation environment, we expected S–R congruency to be almost reversed
Tracking task
Participants tracked a ball moving around on a display with a mouse cursor as accurately as possible. The computer display was located in front of the participants at a 60 cm distance. During the rotation trials, a counter-clockwise 150° or 30° rotation was applied to the mouse cursor position. The participants did not receive any instructions about the nature of the transformation of the visual feedback. Instead, they were told that the mouse cursor “moved weirdly”, but that they would get accustomed to it as the experiment proceeded. In the no-rotation trials, the visual feedback of the mouse cursor was normal. Before each trial began, participants were informed whether the mouse cursor movement in the subsequent trial would be “normal” or “weird” (Imamizu and Kawato 2008; Lee and Schweighofer 2009; Taylor et al. 2014) by presenting a color cue; green corresponded to a no-rotation trial (“normal”) and red corresponded to a rotation trial (“weird”). Participants tracked the ball for 20 s in each trial. One session consisted of six consecutive rotation trials with 5 s intervals. One block consisted of five sessions, and participants were allowed to take a rest for about 20 s between sessions. Eight blocks were carried out in total, and a no-rotation trial as well as a response task was conducted before starting the first block and after each following block. In total, participants completed 80 min of rotation trials.
Target ball trajectories were created by summing two undulating trajectories with different frequencies and amplitudes, which were made from sine waves with two different random frequencies each for x and y coordinates (Imamizu et al. 2000; Kaida et al. 2017). The target ball and mouse cursor always started from the center of the display, and participants did thus not have to move the cursor back to the start position after a trial finished. The initial movement direction of the target varied across trials. The trajectory of the ball movement was complicated, and participants could not follow it perfectly. The trajectory changed in every trial, but its moving range, speed, and acceleration were controlled; the moving range of trajectories was 40 mm in both x and y directions on the display, the mean tangential velocity was below 55 mm/s, and the maximum tangential acceleration was below 400 mm/s2. The mean tangential velocity for the whole trials was 44.8 mm/s (SD 4.9). These constraints on target movements allowed an average tracking error (distance between the target and the mouse cursor) to reflect the degree of visuomotor adaptation well. Note that these constraints were not varied across trials and, therefore, the difficulty of the task did not change throughout the experiment.
Response task
Participants pressed a left or right key as quickly and accurately as possible according to a presented number stimulus (1 or 2) on the display. The correspondence between the presented numbers and the keys to be pressed was specified before the experiment and did not change throughout the experiment. The correspondence was balanced across participants; the 1 corresponded to the right key and the 2 to the left key in half of the participants, and the 1 corresponded to the left key and the 2 to the right key in the other half of the participants.
The trial procedure is shown in Fig. 1b. A trial started with a blink of a fixation cross at the center of the display, which was then replaced by a red filled circle with a 1.8-mm diameter. After the circle presentation with a random duration (800‒2400 ms), one of the numbers (1 or 2) was presented in the center of the display. At the same time, the red circle linearly moved toward a certain direction (distractor movement), which lasted 50 ms (three frames), with a travel distance of 30.6 mm. The movement duration was defined based on previous studies (Brass et al. 2000, 2001) and the movement distance and size of the distracter were determined by a preliminary experiment, to induce congruency effects in the current experimental setting.
Participants were instructed to ignore the distractor movement and to focus on the number stimulus and their key responses. Participants used their index finger to press the keys (Fig. 1c). Before starting each trial, they put their index finger on a “home” position that was placed between the left and right key. The surface of the home position was covered with a smooth Teflon tape, and the participants were instructed to slide their finger rather than to lift it to press one of the keys on either side of the home position. In one set of the response task, participants performed 48 trials (8 directions × 2 responses × 3 trials). The response task was conducted before the first block as a baseline and after each block of tracking tasks, and participants thus performed nine sets of the response task in total. Each response task took less than 4 min to complete.
The distractor moved toward one of eight directions (0°, 30°, 90°, 150°, 180°, 210°, 270°, or 330°) to prevent participants from paying exclusive attention to the distracters toward transverse directions (Fig. 1d). For the calculation of the congruency effect, the trials where the distractor moved in 90° and 270° directions were excluded from the analysis, because for these, as the movement direction of the stimuli was orthogonal to the responses, S–R congruency could not be defined in the no-rotational environment. In addition to the 0° and 180° directions, which in the normal environment are perfectly consistent in spatial direction with the finger movements (left and right), we included trials with distracter movements of 30°, 150°, 210°, and 330° in the analysis. This was done to compensate for a 30° deviation in movement direction in S–R congruency in the 150° rotational environment; if visuomotor adaptation for the 150° rotation is complete, distractor movements toward the 330° and 150° directions are congruent with the motor responses toward the left and right directions, respectively. Based on the normal visuomotor environment, we defined a trial as “congruent” when a distractor moved toward directions of 150°, 180°, or 210° on the display for a leftward finger response, and toward directions of 330°, 0°, or 30° on the display for a rightward finger response. Note that, in theory, congruency effects would not be completely reversed if visuomotor adaptation was completely achieved because in the present experimental manipulation, the rotation angle was 150° rather than 180°.
Analysis
For the tracking task, we calculated the tracking error as the distance between the target ball and the mouse cursor, to evaluate improvements in visuomotor adaptation over time. Furthermore, we defined after effects, which are considered to reflect the acquisition of an internal model (Krakauer 2009), as the difference in the tracking error between rotation trials and no-rotation trials in a baseline trial. To test the effect of the factor group (three groups) and the phase (eight blocks) on tracking performance, we conducted a two-way mixed-factor analysis of variance (ANOVA), applying Shaffer’s correction for multiple comparisons. In addition, to test the experimental manipulation, we compared the average tracking error in the last 5 s of a no-rotation trial with the baseline tracking error in the 150N group, again correcting for multiple comparisons using Shaffer’s method; we assumed that the new internal model was no more active at the start of the response task that followed the no-rotation trial.
The congruency effect was calculated as the difference in response times between congruent and incongruent trials. The response time was defined as the time between the stimulus presentation and the key press. When the direction of the expected finger movement was the same as that of the distractor, the trial was classified as a congruent trial; when they were in opposite directions, the trial was called an incongruent trial. Positive values indicate the existence of a conventional effect that is based on S–R congruency. Because of incorrect responses or mechanical errors, 3.9% of the data were excluded from the analysis.
To test the main hypothesis, we conducted t tests to examine whether the effect after the last block was negative (below zero). Furthermore, we performed t tests to verify that the baseline congruency effect was positive (above zero). To characterize the time course of the adaptation-induced changes in the congruency effect, we conducted a mixed-factor two-way ANOVA (3 groups × 9 phases) and applied Shaffer’s correction for multiple comparisons. For a future reference, we also calculated directional error in the initial part of movements in the adaptation task (see Online Resource 1).
Results
Tracking errors in the visuomotor rotation task
Before examining the congruency effect we were mainly interested in, we tested whether the participants successfully adapted to the rotated visuomotor correspondences or not, especially participants in the 150R and 150N groups. The average tracking errors as a function of time course are shown at a trial basis in Fig. 2, revealing a gradual decrease in errors in the 150R and 150N groups but not in the 30R group. After effects were evident in the 150R group, while they were not in the 30R and 150N groups. The following statistical analyses confirmed that a comparable amplitude of adaptation occurred in the two groups where the 150° rotation was applied, while there were statistically significant but rather small changes in tracking error in the 30°-rotation group.
The average tracking error of each session is shown in Fig. 3a. The two-way ANOVA (3 groups × 8 phases) on the average tracking error revealed main effects of group [F(2, 45) = 14.48, p < .001] and phase [F(7, 315) = 44.14, p < .001]. Although the interaction effect of the two factors was significant [F(14, 315) = 8.55, p < .001], the analyses of simple main effects revealed that the main effect of group was significant for all blocks [F(2, 45) = 16.1, 12.7, 11.9, 10.2, 12.3, 12.6, 11.0, and 12.8, for the first to the eighth block, respectively, all p < .001] and that the main effect of phase was significant in all groups [F(7, 105) = 2.74, p < .05 for the 30R group; F(7, 105) = 34.07, p < .001 for the 150R group; F(7, 105) = 18.49, p < .001 for the 150N group]. To examine whether there was a statistical difference in adaptation amplitude between the two 150°-rotation groups, we additionally conducted a two-way ANOVA (2 groups × 8 phases) on the data without the 30R control group. The ANOVA did not reveal an interaction effect between the 150R and 150N groups [F(7, 210) = 0.82, p = .57], while there were significant main effects of group [F(1, 30) = 4.27, p < .05] and phase [F(7, 210) = 43.75, p < .001]. These results confirmed that the amplitude of the decrease in tracking errors did not statistically differ between the two 150°-rotation groups. In contrast, in the 30°-rotation group, a statistical decrease in tracking error was found, but its amplitude was small, thus leading to the significant interaction effect in the original two-way ANOVA.
The average after effects in tracking error are shown in Fig. 3b. The two-way ANOVA on average after effects revealed main effects of group [F(2, 45) = 10.30, p < .001] and phase [F(7, 315) = 20.16, p < .001]. Although the interaction effect was significant [F(14, 315) = 10.00, p < .001], the analyses of simple main effects demonstrated that the main effect of group was significant for all blocks [F(2, 45) = 25.20, 14.92, 14.93, 7.58, 5.73, 5.65, 4.46, and 3.51, for the first to the eighth block, respectively, p < .001 for the fourth to the sixth block, p < .01 for the first to the third block, and p < .05 for the seventh and eighth block]. The simple main effect of phase was significant in the 150R [F(7, 105) = 15.86, p < .001] and 150N groups [F(7, 105) = 3.32, p < .01] but not in the 30R group [F(7, 105) = 1.90, p = .08]. The lack of after effects in the 30R group was not surprising, because the 30° visuomotor rotation was not so different from the normal environment in the tracking task, as evident in the time course of average errors. The detailed results of multiple comparisons for tracking errors and after effects are shown in ESM Tables 1 and 2 (see ESM Appendix). Note that after effects cannot be directly compared between the 150R/30R and 150N groups, because the timing of the evaluation differed between groups.
We then examined the effect of the experimental manipulation in the 150N group, by comparing tracking errors during the last 5 s of the baseline phase (i.e., without any experience of rotation trials) to errors during the last 5 s of the following blocks (i.e., after a certain experience of rotation trials). We assumed that the 20 s of no-rotation trials were enough to switch the internal model back to normal. In other words, we assumed that the no-rotation trials worked as “washout” trials in the 150N group, although they would not induce “complete washout” because their duration was relatively short. Figure 4 shows the time course of the average errors for the last 5 s of the no-rotation trials, and those for the first 5 s, for reference. The average errors of the last 5 s did not vary with the rotation practice, while the errors for the first 5 s increased and decreased over time. Multiple comparisons for average tracking errors for the last 5 s revealed that there were no statistical differences in any pairs between the baseline phase and the following blocks [t(15) = 2.11; t(15) = 1.41; t(15) = 2.11; t(15) = 1.36; t(15) = 0.74; t(15) = 1.68; t(15) = 0.78; t(15) = 0.30]. This result was consistent with our prediction about the experimental manipulation in the 150N group.
Congruency effects at baseline and in the last block
The individual data and group averages of congruency effects in the baseline phase and in the last block are shown in Fig. 5. The average baseline congruency effects were 14.2 (SD 27.8) ms, 15.2 (SD 23.6) ms, and 31.3 (SD 21.8) ms in the 30R, 150R, and 150N groups, respectively. To confirm that those congruency effects were positive, one-sided t tests against zero with Shaffer’s correction were conducted; they revealed that the baseline congruency effects were significantly larger than zero at a significance level of 0.05 in all three groups [t(15) = 1.97 in the 30R group; t(15) = 2.48 in the 150R group; t(15) = 5.57 in the 150N group]. This result confirmed that in all groups, normal congruency effects were induced before visuomotor adaptation.
Individual and group averages of congruency effects in the baseline phase and after the last block. At baseline, congruency effects in all groups were significantly positive at the group level. After the last block, the congruency effects were not significantly negative at the group level in either group. In the three group data sets (on the left), each mark corresponds to one participant’s data, while the data set on the right depicts group averages
We then conducted one-sided t tests against zero with Shaffer’s correction to test the hypothesis that only in the 150R group, congruency effects in the last block were negative. The average congruency effects in the last block were 17.2 (SD 20.5) ms, − 8.8 (SD 23.6) ms, and 11.1 (SD 29.4) ms in the 30R, 150R, and 150N groups, respectively. The t tests revealed that the congruency effects were not significantly negative at a significance level of 0.05 in either of the three groups [t(15) = − 3.24 in the 30R group; t(15) = 1.43 in the 150R group; t(15) = − 1.47 in the 150N group].
Effects of the factors block and group on the congruency effect
Next, to characterize the time course of the changes in congruency effects induced by visuomotor adaptation, we conducted a two-way ANOVA on the congruency effects. The group averages of congruency effects are shown in Fig. 6. The main effects of both group and block were significant [F(2, 45) = 3.77, p < .05; F(8, 360) = 4.91, p < .001]. The interaction effect between the two factors was also significant [F(16, 360) = 2.03, p < .05]. The simple main effect of the factor group was significant for the fifth, sixth, and eighth block [F(2, 45) = 3.40, p < .05; F(2, 45) = 5.54, p < .01; F(2, 45) = 5.00, p < .05], and the simple main effect of the factor block was significant in all groups [F(8, 120) = 2.41, p < .05 in the 30R group; F(8, 120) = 3.75, p < .001 in the 150R group; F(8, 120) = 3.10, p < .01 in the 150N group]. These results confirmed that congruency effects differed across the groups during the later phases.
Congruency effects for the three groups. In the last block, the congruency effect for the 150R group was significantly lower than the effects for the other two groups. The congruency effects in the blocks marked with an asterisk were significantly lower than those at baseline in the 150R and 150N groups (all p < .05). In the 30R group, there were no significant differences in the congruency effects between baseline and other phases, but the congruency effect in the sixth block was significantly lower than that in the fourth block
We then conducted multiple comparisons; important statistical results of these comparisons are indicated with asterisks in Fig. 6. First, we analyzed practice-dependent changes in congruency effects for each group. In the 150R group, the congruency effect at baseline was significantly larger than the effects in the first, third, fifth, seventh, and eighth block (all p < .05). In the 150N group, the congruency effect at baseline was significantly larger than that in the first block. In the 30R group, the congruency effect in the fourth block was significantly larger than that in the sixth block. These results indicate that only in the 150R group, the congruency effect gradually decreased from the baseline phase toward the last block. Regression analysis also confirmed that the congruency effects in the 150R group decreased over time more steeply than other groups; averaged regression coefficients were − 0.51 (SD 1.76), − 2.25 (SD 1.83), and − 1.11 (SD 2.71) for the 30R, 150R, and 150N groups respectively.
Second, we conducted multiple comparisons within each phase. At the fourth block, the congruency effect of the 150R group was significantly lower than that of the 30R group [t(45) = 2.61, p < .05]. The congruency effect of the 150R group was significantly lower than those of the 30R and 150N groups in the fifth [t(45) = 2.53; t(45) = 3.14, p < .05] and eighth block [t(45) = 2.87; t(45) = 2.20, p < .05]. Importantly, the congruency effect in the last block in the 150R group was lower than the effects in the other two groups, while there were no significant differences between the groups at baseline.
To confirm whether congruency effects differently developed among orientations over the adaptation time course, we plotted them separately. These results showed that changes in congruency effect did not differ among orientations in all groups, although we found a bias in the 150R group. Additionally, we also examined the relationship between the change in congruency effects and the tracking performance. We calculated correlation coefficients for a combined “R” group (30R and 150R) because their experimental condition was the same in terms of congruency effect. We obtained a significant correlation only for tracking error in the combined R group (r = − 0.54), indicating that greater improvement in tracking induced greater changes in congruency effect (see Online Resource 1 for details).
Discussion
The present study tested whether visuomotor adaptation induced by rotational visual feedback inverses automatic modulation in motor responses based on S–R congruency. We demonstrate that visuomotor adaptation changed the congruency effect in accord with the acquired visuomotor correspondence. The congruency effects measured immediately after adaptation tasks progressively changed to negative as a function of the amount of adaptation experience. However, the effect was not strong enough to completely reverse the congruency effect, which does not support our hypothesis. Nevertheless, the present results indicate that a relatively short-term experience of visuomotor rotation induces a new type of automatic responses based on the acquired visuomotor correspondence. In addition, we found that even with the identical amount of adaptation experience, the congruency effects measured after the no-rotation trials did not lead to changes in the 150N group, after adaptation was reasonably achieved. Likewise, weak visuomotor adaptation did not modulate the automatic responses in the 30R group. These findings suggest that the visuomotor system subserving automatic modulation in motor output is highly adaptive to a changing environment.
Congruency effects change with visuomotor remapping
The monotonic changes in congruency effects in the 150R group can be attributed to visuomotor adaptation to the 150° rotation of the visual feedback, which was in roughly opposite direction to the normal visuomotor environment. The results, however, do not completely support our hypothesis that the congruency effect is reversed after the completion of visuomotor adaptation; in the 150R group, the congruency effect was not significantly smaller than zero. Nevertheless, the following results are consistent with our predictions. First, the congruency effect in the 150R group continuously decreased as the visuomotor tasks proceeded. Second, the baseline congruency effect (15.2 ms) significantly differed from that at the last measurement (− 8.8 ms) in the 150R group. Third, in the 30R group, the congruency effect did not significantly change from baseline after intensive practice to adapt to the new environment. Since the congruency effect did not reach its theoretical value (15.2 ms × the cosine of 150° = − 13.2 ms) in the 150R group, there might still be room for additional improvement in adaptation that could elicit the complete reversal of the congruency effect. In addition, a 180°-rotation training, instead of the 150°-rotation training performed here, might have led to a clearer effect, since it has been reported that explicit learning of novel visuomotor environments does not interfere with implicit learning (Mazzoni and Krakauer 2006; Taylor et al. 2014; but see also; Day et al. 2016; McDougle et al. 2017). In summary, the present results show that visuomotor remapping almost opposite (150°) to the original mapping systematically modulates congruency effects according to the new environment.
The changes in the congruency effect found here are in agreement with the classic idea that a newly acquired skill becomes automatic through motor learning (Woodworth 1899). It has been shown that participants can learn to accomplish daily activities with prism glasses after long-term wearing, which may change their body image according to the new visuomotor correspondence (Sekiyama et al. 2000). These findings are not surprising, because even the visuomotor correspondence that we consider “normal” has been acquired through experience. For example, we consider it normal to move a computer mouse forward when we want to move a mouse cursor upward on a display standing upright on a desk; these directions are however not consistent in three-dimensional space. It has also been shown that motor practice improves the automaticity of a task (i.e., the task requires less attention) (Floyer-Lea and Matthews 2004), and that implicit learning processes of a novel visuomotor correspondence are likely automatic or unintentional (Mazzoni and Krakauer 2006; Taylor et al. 2014). Although the term “automatic” does not necessarily refer to the same process in each study, one might infer that the automatically acquired correspondence automatically influences our motor responses.
Nevertheless, we cannot exclude the possibility that a cognitive strategy acquired through the visuomotor task indirectly induced the systematic changes in the congruency effect. In the response task, there is no good reason for participants to use the explicit strategy acquired in the tracking task. However, it is possible that the use of the explicit strategy during the tracking task could have unconsciously modulated the motor system and continued to influence it in the response task that was performed after the tracking task. This interpretation is consistent with recent studies that reported a relatively large contribution of explicit strategy in aiming tasks involving visuomotor rotation (Bond and Taylor 2015; Morehead et al. 2017; Kim et al. 2018; Neville and Cressman 2018). Further discussion of the possibility that a cognitive strategy induced the systematic changes in the congruency effect is described in the supplementary discussion (see Online Resource 1).
Congruency effect based on the currently activated model
The present results are also in line with the idea that the currently activated internal model influences automatic motor processing. The participants in the 150R and 150N groups were exposed to the identical amount of the rotational environment and it was expected that they acquired their new internal model to the same degree. However, the results showed that participants in the 150N group did not change their automatic responses at all. In the 150N group, congruency effects were assessed after a no-rotation trial, whereas in the 150R group, the measurement was immediately after a rotation trial. We assumed that performing a no-rotation trial switches the currently active model back to the normal one, and this assumption was supported by the result that the tracking error of the last 5 s was reduced to a level comparable to baseline in the 150N group; that is, the adaptation to the 150° rotation was apparently “washed out” in that group. Although they would not have induced complete washout, it is plausible that being engaged in a no-rotation trial switched the current internal model to that for the no-rotational environment, resulting in the non-significant changes in congruency effects in the 150N group.
Time course of the after effect
In addition to the findings on changes in congruency effects, importantly, we also observed that after effects showed a peak and then decreased along the time course of visuomotor adaptation. We found that the after effects were strongest after the second block and then started to decrease in both the 150R and the 150N group, with the after effects in the 150N group being less evident as those in the 150R group. If after effects solely reflect the implicit components in adaptation learning, they would have continued increasing asymptotically, instead of decreasing, because implicit learning monotonically progresses regardless of explicit components (Taylor et al. 2014). The time course of the after effect with an early peak and a long-lasting decrease does not suggest the involvement of single components; the present results, rather, agree with the possibility that after effects are a complex measure and reflect more than the degree of acquisition of an internal model. Note that after effects evaluated in the present study were not the same as traditional ones; after effects are usually evaluated without notice (i.e., cues) of the change in the environment. In the present study, they were evaluated after participants were given notice. This discrepancy could explain the decreasing after effects observed in the present study.
The additional component contributing to the after effects could be the efficiency of model switching. In the literature, system switching has been assumed to explain our various and flexible sensorimotor skills (Jacobs et al. 1991; Ghahramani and Wolpert 1997; Wolpert and Kawato 1998; Haruno et al. 2001; Imamizu and Kawato 2009). In the MOSAIC model, multiple internal models simultaneously provide predictions about the upcoming situation (Haruno et al. 2001; Imamizu and Kawato 2009). If a prediction from an inappropriate model is used, the accuracy of both voluntary and involuntary motor control would be lost. This process might be repeated until an appropriate model is selected, and inefficient switching of internal models would contribute to the amplitude of after effects, in addition to the prediction inaccuracy of the selected internal model itself. When one has become an expert in the environment, and thus in model switching, after effects would no longer appear.
The independent developments of two components may explain the time course of after effects with an early peak and a following long-lasting decrease. First, the acquisition of an internal model monotonically improves from the start of the practice, and thus has an effect from the first switching onward. Second, switching efficiency, which is inversely related to the after effect, improves from the first switching and influences the second switching. In the present experiment, participants were informed about the next environment by a colored cue before the trial started, which could have helped selecting an appropriate internal model for the next trial (Imamizu et al. 2007; Imamizu and Kawato 2008; Taylor et al. 2014). The first component develops continuously but relatively slowly (Mazzoni and Krakauer 2006; Taylor et al. 2014), whereas the second component is intermittently updated only at switching but acquired quickly, because it relates to explicit knowledge (Imamizu and Kawato 2008; Taylor et al. 2014). This distinction may agree with an earlier proposed model that assumes single fast processes and multiple slow processes for different environments (Lee and Schweighofer 2009), though they do not assume that switching models is triggered by explicit knowledge. We predict that the combination of these two components, implicit acquisition of a new internal model and switching efficiency triggered by explicit knowledge, should be in line with the time course of after effects evaluated at multiple times, which should be tested in future studies.
We cannot clearly explain why the after effects in the 150N group were so weak in the present experiment. Although the tracking errors in the 150R group were lower than those in the 150N group, the amplitude of adaptation did not significantly differ between the groups (i.e., there was no significant interaction). There are three possible explanations for the weak after effects in the 150N group. First, the adaptation was not sufficient, because of relatively poor overall performance. Second, explicit cueing might be more effective for the same type of task compared to a different type of task; in the 150N group, non-rotation trials were executed immediately after rotation trials, which could have elicited a noticeable contrast between the tasks, and participants might thus have switched to a different type of voluntary motor control, compared to the 150R group. It has been reported that an explicit cuing hinders after effects (Imamizu and Kawato 2008; Taylor et al. 2014). Third, the after effects in the 150R group might have simply been strengthened over time and/or following the experience of another task.
Limitations
There are, however, limitations of this study. First, we cannot exclude the possibility that the generally low performance in the tracking task prevented changes in the congruency effect in the 150N group from reaching statistical significance. The tracking errors in the 150N group were consistently higher than those in the 150R group, from the initial block to the last block, while the amplitudes of the error decrease did not significantly differ between the two groups (i.e., there was no interaction effect). It is thus possible that the insufficient decrease in the tracking error caused the lack of constant changes in congruency effects in the 150N group. Second, we should note that the congruency effect in the current experiment was not stable; that is, the experimental stimuli did not induce robust congruency effects at the individual level. Although the congruency effects were significantly positive at the group level, some participants showed negative values at baseline. This individual-level instability might have been caused by the small number of trial repetitions (three times for each type of stimulus) in the response task. In addition, the nature of the distractor stimulus might have played a role in the unstable effect. We used a filled circle as a distractor without any body-associated images or biological velocity profiles. It has, however, been suggested that the influence of non-biological movement observation on an observer’s action is weaker compared to that of biological movements with a body-part image, human-like velocity profile, or human intention (Brass et al. 2000, 2001; Kilner et al. 2003; Bosbach et al. 2005; Bouquet et al. 2007; Liepelt and Brass 2010; Kupferberg et al. 2012; Itaguchi and Kaneko 2018). Third, there is a possibility that the experimental manipulation in the 150N group was not sufficient to switch the internal model back to normal during the response task. As shown in Fig. 5, in four out of 16 participants in the 150N group, the congruency effects largely decreased from baseline to the last phase, where positive effects turned negative. Although this can be explained by the instability of the congruency effect, conditioned visuomotor adaptation might as well have caused the reversal of the effect (Kravitz and Yaffe 1972; Imamizu and Kawato 2008; Lee and Schweighofer 2009; Taylor and Ivry 2013; Taylor et al. 2014); the color of the distractor (red) could have worked as a color cue, and thus switched the internal model to that for the rotational environment, even in the response task. All these limitations should be addressed to further understand the relation between voluntary control and automatic response in terms of motor learning induced by visuomotor remapping.
Conclusion
The present study shows that relatively short-term visuomotor adaptation modulates automatic motor responses in accord with the acquired visuomotor correspondence. This modulation is apparently based on the internal model that is activated at the time of the motor responses. These results suggest that the visuomotor system subserving automatic modulation in motor responses is based on the currently activated internal model. Harmonious changes in two different types of motor control (at a voluntary level and at an automatic level) are important if we assume one internal system. In addition, we characterized the time course of after effects in a visuomotor transformation task by assessing effects at multiple times. The after effects developed as the training proceeded but began to decrease with time, which may reflect not only successful adaptation but also the efficiency of the switching between internal models.
References
Bertenthal BI, Longo MR, Kosobud A (2006) Imitative response tendencies following observation of intransitive actions. J Exp Psychol Hum Percept Perform 32:210
Bock O (2005) Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res 160:259–263
Bond KM, Taylor JA (2015) Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J Neurophysiol 113:3836–3849
Bosbach S, Prinz W, Kerzel D (2005) Movement-based compatibility in simple response tasks. Eur J Cognit Psychol 17:695–707
Bouquet C, Gaurier V, Shipley T, Toussaint L, Blandin Y (2007) Influence of the perception of biological or non-biological motion on movement execution. J Sports Sci 25:519–530
Boyer TW, Longo MR, Bertenthal BI (2012) Is automatic imitation a specialized form of stimulus–response compatibility? Dissociating imitative and spatial compatibilities. Acta Physiol (Oxf) 139:440–448
Brass M, Bekkering H, Wohlschläger A, Prinz W (2000) Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cognit 44:124–143
Brass M, Bekkering H, Prinz W (2001) Movement observation affects movement execution in a simple response task. Acta Psychol 106:3–22
Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund H-J, Rizzolatti G (2004) Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 42:323–334
Buch ER, Young S, Contreras-Vidal JL (2003) Visuomotor adaptation in normal aging. Learn Mem 10:55–63
Craighero L, Fadiga L, Rizzolatti G, Umiltà C (1999) Action for perception: a motor-visual attentional effect. J Exp Psychol Hum Percept Perform 25:1673
Craighero L, Bello A, Fadiga L, Rizzolatti G (2002) Hand action preparation influences the responses to hand pictures. Neuropsychologia 40:492–502
Day KA, Roemmich RT, Taylor JA, Bastian AJ (2016) Visuomotor learning generalizes around the intended movement. eNeuro 3:ENEURO.0005-0016.2016
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72:443–454
Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC (1996) Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci 8:637–648
Edwards MG, Humphreys GW, Castiello U (2003) Motor facilitation following action observation: a behavioural study in prehensile action. Brain Cogn 53:495–502
Ewolds HE, Bröker L, De Oliveira RF, Raab M, Künzell S (2017) Implicit and explicit knowledge both improve dual task performance in a continuous pursuit tracking task. Front Psychol 8:2241
Floyer-Lea A, Matthews P (2004) Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol 92:2405–2412
Foulkes AJM, Miall RC (2000) Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res 131:101–110
Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL (2006) Motor commands contribute to human position sense. J Physiol 571:703–710
Ghahramani Z, Wolpert DM (1997) Modular decomposition in visuomotor learning. Nature 386:392
Haruno M, Wolpert DM, Kawato M (2001) Mosaic model for sensorimotor learning and control. Neural Comput 13:2201–2220
Hatada Y, Miall R, Rossetti Y (2006) Two waves of a long-lasting aftereffect of prism adaptation measured over 7 days. Exp Brain Res 169:417–426
Heyes C (2011) Automatic imitation. Psychol Bull 137:463
Hommel B (1996) SR compatibility effects without response uncertainty. Q J Exp Psychol Sect A 49:546–571
Imamizu H, Kawato M (2008) Neural correlates of predictive and postdictive switching mechanisms for internal models. J Neurosci 28:10751–10765
Imamizu H, Kawato M (2009) Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol Res PRPF 73:527–544
Imamizu H, Miyauchi S, Tamada T et al (2000) Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195
Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M (2003) Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci 100:5461–5466
Imamizu H, Kuroda T, Yoshioka T, Kawato M (2004) Functional magnetic resonance imaging examination of two modular architectures for switching multiple internal models. J Neurosci 24:1173–1181
Imamizu H, Higuchi S, Toda A, Kawato M (2007) Reorganization of brain activity for multiple internal models after short but intensive training. Cortex 43:338–349
Itaguchi Y, Kaneko F (2018) Motor priming by movement observation with contralateral concurrent action execution. Hum Mov Sci 57:94–102
Jacobs RA, Jordan MI, Nowlan SJ, Hinton GE (1991) Adaptive mixtures of local experts. Neural Comput 3:79–87
Kagerer FA, Contreras-Vidal JL, Stelmach GE (1997) Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115:557–561
Kaida K, Itaguchi Y, Iwaki S (2017) Interactive effects of visuomotor perturbation and an afternoon nap on performance and the flow experience. PloS One 12:e0171907
Kawato M, Furukawa K, Suzuki R (1987) A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern 57:169–185
Kilner JM, Paulignan Y, Blakemore SJ (2003) An interference effect of observed biological movement on action. Curr Biol 13:522–525
Kim S, Oh Y, Schweighofer N (2015) Between-trial forgetting due to interference and time in motor adaptation. PloS One 10:e0142963
Kim HE, Morehead JR, Parvin DE, Moazzezi R, Ivry RB (2018) Invariant errors reveal limitations in motor correction rather than constraints on error sensitivity. Commun Biol 1:19
Klassen J, Tong C, Flanagan JR (2005) Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164:250–259
Krakauer JW (2009) Motor learning and consolidation: the case of visuomotor rotation. In: Progress in motor control. Springer, Boston, pp 405–421
Krakauer JW, Ghilardi M-F, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2:1026–1031
Krakauer JW, Pine ZM, Ghilardi M-F, Ghez C (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20:8916–8924
Krakauer JW, Ghilardi M-F, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C (2004) Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol 91:924–933
Kravitz JH, Yaffe F (1972) Conditioned adaptation to prismatic displacement with a tone as the conditional stimulus. Attent Percept Psychophys 12:305–308
Krebs HI, Brashers-Krug T, Rauch SL et al (1998) Robot-aided functional imaging: application to a motor learning study. Hum Brain Mapp 6:59–72
Künzell S, Sießmeir D, Ewolds H (2016) Validation of the continuous tracking paradigm for studying implicit motor learning. Exp Psychol 63:318
Kupferberg A, Huber M, Helfer B, Lenz C, Knoll A, Glasauer S (2012) Moving just like you: motor interference depends on similar motility of agent and observer. PloS One 7:e39637
Lee J-Y, Schweighofer N (2009) Dual adaptation supports a parallel architecture of motor memory. J Neurosci 29:10396–10404
Liepelt R, Brass M (2010) Top-down modulation of motor priming by belief about animacy. Exp Psychol 57:221–227
Liepelt R, Cramon D, Brass M (2008) What is matched in direct matching? Intention attribution modulates motor priming. J Exp Psychol Hum Percept Perform 34:578–591
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645
McDougle SD, Bond KM, Taylor JA (2017) Implications of plan-based generalization in sensorimotor adaptation. J Neurophysiol 118:383–393
Miall RC, Wolpert DM (1996) Forward models for physiological motor control. Neural Netw 9:1265–1279
Morehead JR, Taylor JA, Parvin DE, Ivry RB (2017) Characteristics of implicit sensorimotor adaptation revealed by task-irrelevant clamped feedback. J Cognit Neurosci 29:1061–1074
Neville K-M, Cressman EK (2018) The influence of awareness on explicit and implicit contributions to visuomotor adaptation over time. Exp Brain Res 236:2047–2059
Osu R, Hirai S, Yoshioka T, Kawato M (2004) Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7:111–112
Petersen SE, Van Mier H, Fiez JA, Raichle ME (1998) The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci 95:853–860
Sekiyama K, Miyauchi S, Imaruoka T, Egusa H, Tashiro T (2000) Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature 407:374–377
Shadmehr R (2017) Learning to predict and control the physics of our movements. J Neurosci 37:1663–1671
Simon JR, Rudell AP (1967) Auditory SR compatibility: the effect of an irrelevant cue on information processing. J Appl Psychol 51:300
Tardy-Gervet MF, Gilhodes JC, Roll JP (1984) Perceptual and motor effects elicited by a moving visual stimulus below the forearm: an example of segmentary vection. Behav Brain Res 11:171–184
Taylor JA, Ivry RB (2013) Context-dependent generalization. Front Hum Neurosci 7:171
Taylor JA, Krakauer JW, Ivry RB (2014) Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34:3023–3032
Tong C, Flanagan JR (2003) Task-specific internal models for kinematic transformations. J Neurophysiol 90:578–585
Wolpert DM, Kawato M (1998) Multiple paired forward and inverse models for motor control. Neural Netw 11:1317–1329
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880
Woodworth RS (1899) Accuracy of voluntary movement. Psychol Rev Monogr Suppl 3:1–114
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science, KAKENHI (Grant Numbers 16J00325 and 15K04195).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Itaguchi, Y., Fukuzawa, K. Adaptive changes in automatic motor responses based on acquired visuomotor correspondence. Exp Brain Res 237, 147–159 (2019). https://doi.org/10.1007/s00221-018-5409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5409-x