Abstract
This study was designed to explore the effects of intrafusal thixotropy, a property affecting muscle spindle sensitivity, on the sense of force. For this purpose, psychophysical measurements of force perception were performed using an isometric force matching paradigm of elbow flexors consisting of matching different force magnitudes (5, 10 and 20% of subjects’ maximal voluntary force). We investigated participants’ capacity to match these forces after their indicator arm had undergone voluntary isometric conditioning contractions known to alter spindle thixotropy, i.e., contractions performed at long (‘hold long’) or short muscle lengths (‘hold short’). In parallel, their reference arm was conditioned at the intermediate muscle length (‘hold-test’) at which the matchings were performed. The thixotropy hypothesis predicts that estimation errors should only be observed at low force levels (up to 10% of the maximal voluntary force) with overestimation of the forces produced following ‘hold short’ conditioning and underestimation following ‘hold long’ conditioning. We found the complete opposite, especially following ‘hold-short’ conditioning where subjects underestimated the force they generated with similar relative error magnitudes across force levels. In a second experiment, we tested the hypothesis that estimation errors depended on the degree of afferent-induced facilitation using the Kohnstamm phenomenon as a probe of motor pathway excitability. Because the stronger post-effects were observed following ‘hold-short’ conditioning, it appears that the conditioning-induced excitation of spindle afferents leads to force misjudgments by introducing a decoupling between the central effort and the cortical motor outputs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One major property of the human motor system is the nonlinear input–output relationship existing between the different components constituting the motor pathways (Petersen et al. 2010). The gain between the cortical activity intended to implement neural outputs (i.e., intention, effort) and the final muscular response can be influenced by numerous factors and especially by the recent contractile activity of skeletal muscles. For instance, the repetition of fatiguing muscle contractions introduces discrepancies between the effort produced and the cortical motor outputs (Liu et al. 2003) and lowers the responsiveness of muscles to neural drives (Gandevia 2001). In contrast, following short maximal or near-maximal contractions, muscle fatigue can coexist with post-activation potentiation, a phenomenon which enhances subsequent neuromuscular performances, mainly through the enhancement of intramuscular mechanisms (Hodgson et al. 2005).
Since the gains between the different components of the motor pathways are constantly changing, Petersen and collaborators (2010) pointed out the need for movements to be continuously monitored by the proprioceptive signals to adapt the cortical outputs to the changing functional efficacy of the descending systems. Yet, and importantly, the recent contractile history can also affect the strength of muscle afferents and bias proprioceptive representations. For instance, performing conditioning voluntary isometric contractions at short or long muscle lengths modifies the passive stiffness of intrafusal fibers when they return to an intermediate length. This property, which refers to thixotropy, critically affects spindle sensitivity (for reviews, see Proske et al. 1993, 2014). More precisely, during a voluntary contraction, intrafusal and extrafusal fiber lengths evolve in parallel through alpha–gamma neuron co-activation. When a muscle is voluntarily contracted at a given length, stable cross-bridges rapidly form between actin and myosin at the end of the contraction. If the muscle is contracted at a long muscle length and then passively moved to a shorter length (‘hold-long’ conditioning), intrafusal fibers fall slack, leading to decreased background spindle discharges (Wood et al. 1996). In contrast, if the muscle is contracted at a short muscle length and then passively stretched to a longer muscle length (‘hold-short’-conditioning), intrafusal fibers become taut, increasing the sensitivity and the background discharge of muscle spindle sensory endings (Proske et al. 2014). Because the resting firing of spindles informs the central nervous system (CNS) on limb position (Clark et al. 1985), modulation of spindle background activity affects the position sense (Tsay et al. 2014, 2015). More precisely, increased spindle discharges at a given muscle length appear to result in the brain perceiving the muscle as longer than it actually is. In contrast, leaving spindle slack leads the brain to perceive the muscle as shorter than its actual length.
The fusimotor outputs and the spindle activity associated with the achievement of prolonged isometric contractions can also be responsible for the development of involuntary after-contractions known as the Kohnstamm effect (see De Havas et al. 2017 for a comprehensive review). Specifically, when maintaining an isometric contraction representing 30–100% of the maximal voluntary contraction (MVC) for durations of 30–60 s, involuntary activity in the muscle can develop after the voluntary contraction has ceased, resulting in a feeling of lightness and/or a movement of the limb. In a study of Hagbarth and Nordin (1998), the Kohnstamm effect was tested following isometric contractions of the medial deltoid performed at different muscle lengths. As the effect was majored following isometric contractions fulfilling the ‘hold-short’ requirements, it was proposed that the enhanced spindle firing activity provokes a facilitation of the motor circuits at the spinal and cortical stages to finally result in the generation of larger involuntary drives. This is consistent with the observations of Stuart and collaborators (2002) that the size of the motor-evoked potentials increases following the contractions performed with the shorter muscle length. In sum, it appears that the natural variations in the discharge rate of spindle afferents can strongly modulate corticospinal excitability.
Regarding the sense of force, Luu and collaborators (2011) proposed that the thixotropic behavior of spindles may have impacts on force judgements at the thumb. Indeed, these authors proposed that the sense of force arises from the central processing of the reafferent spindle signals produced by the fusimotor outputs (reafferent corollary discharge hypothesis). Based on this hypothesis, modulating spindle sensitivity through thixotropy may also alter force perception. At this point, it is nonetheless important to point out that thixotropy is a property specific to passive and moderately active muscles. That is, voluntary contractions representing 10% of MVC seem sufficient to recruit the majority of intrafusal fibers, thereby removing the existing slack/tautness in spindles (Jahnke et al. 1989; Gregory et al. 1998; Allen et al. 2008). Hence, it seems unlikely that thixotropy could actually affect force perception, except at very low force levels. It is also important to notice that the reafferent corollary discharge hypothesis has little support in the literature. The alternative prevailing theory is that muscle tension is primarily perceived through a sense of effort which arises within the brain (central corollary discharge or efference copy hypothesis). This process would stem from the integration of a copy of the central commands into sensory areas (Christensen et al. 2007). To our knowledge, all the bilateral force matching studies performed at the elbow joint concluded that forces are perceived through a sense of effort (e.g., Carson et al. 2002; Weerakkody et al. 2003; Simon et al. 2009; Brooks et al. 2013). If this theory is true, then thixotropy should not affect the sense of force, except through the modulation of the excitatory inputs spindle afferents provide to the CNS.
In this study, and as encouraged by Proske et al. (2014), we sought to broaden the knowledge relative to the effects of spindle sensitivity manipulation on proprioception, i.e., we directly explored the impact of thixotropy on the sense of force. For this purpose, we used a contralateral isometric force matching paradigm of elbow flexors. Following a control session during which intrafusal stiffness was not manipulated, conditioning maneuvers were used to make either spindles taut or slack in the indicator arm. In these post-conditioning sessions, the contralateral reference limb was also conditioned at a neutral intermediate muscle length (Fig. 1). Three different force magnitudes, representing 5, 10 and 20% of MVC, were tested. Based on the reafferent corollary discharge hypothesis, i.e., if forces are perceived through the intensity of fusimotor-driven afferents, force estimations should depend on the degree of conditioning-induced spindle sensitivity. Yet, as 10% contractions are thought to be sufficient to reset the thixotropic state of the majority of spindles, decreasing errors should be observed as the reference force increases with the larger errors occurring at 5%. At this magnitude, the thixotropy hypothesis predicts (a) an overestimation of the force produced following ‘hold short’ conditioning because of increased spindle responses and (b) an underestimation following ‘hold long’ conditioning because of decreased spindle firing in response to the fusimotor drives. Nonetheless, and as mentioned above, force perception during bilateral matching tasks at the elbow seems to rely on central effort cues. For this reason, we assumed that matching errors would depend on the amount of excitatory inputs received by the CNS during and following the conditioning contractions. According to the works of Hagbarth and Nordin (1998) and Stuart et al. (2002), afferent-induced facilitation of the motor pathways should be majored following ‘hold-short’ conditioning. Therefore, and in total contrast with the thixotropy hypothesis, we hypothesized that ‘hold-short’ conditioning would result in an underestimation of the force generated by modifying the gain between the effort produced and the actual motor outputs. This hypothesis was further tested in a second experiment in which we used the Kohnstamm phenomenon as a probe of the effects of the conditioning maneuvers on sensorimotor pathway excitability.
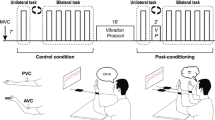
Schematic representation of the protocol (experiment 1). 10 min after the MVC, participants performed three matching conditions (NC, HL and HS) which were spaced fifteen minutes apart. Within each matching condition, three force conditions were tested. Note that the force and the matching conditions, although presented in a defined sequence on the figure, were actually performed in a randomized order. At the bottom of the figures are presented the ways in which the arms were conditioned prior to the matching trials. Please refer to the text for further explanations
Materials and methods
Subjects
This study involved two separate experiments which included 16 healthy and young subjects [mean (SD), 25.1 (2.7) years; 1.76 (0.06) m; 68.5 (4.1) kg]. 15 of them participated in experiment 1 while 11 participated in experiment 2. Subjects were naive to the goal of the study. The study was approved by the local research ethics committee and the subjects’ informed consent was obtained in conformity with the declaration of Helsinki for research on human subjects.
Experiment 1: bilateral force matching task
Apparatus
Subjects (n = 15) were seated on a vertically-adjustable chair with their elbows resting on a table. Webbing straps with extra padding were placed around their wrists. The straps were both connected to force sensors (FUTEK, Model LSB300, Irvine, CA, USA) which were calibrated prior to each experimental session. Force signals were acquired (1000 Hz) using an A/D USB DAQ 6009 National Instrument card (National Instruments, Austin, TX, USA) and saved on a computer for subsequent analyses. Note that subjects had real-time visual feedbacks of the force they generated with their reference arm thanks to a customized LabView interface (LabView 8.2, National Instruments, Austin, TX, USA).
Electromyographic (EMG) data were collected by a Datalog unit (model MWX8, Biometrics Ltd, Newport, UK). EMG signals from two elbow flexors, i.e., the biceps brachii and the brachioradialis, were monitored (1000 Hz) using surface pre-amplified electrodes (type SX230-1000, Biometrics Ltd, Newport, UK) in both the reference and indicator arms. The measured EMG was band-pass filtered (15–450 Hz) close to the recording site. The amplifier had an input impedance of 1015 and a common mode of rejection rate of 110 dB.
Experimental procedures
The chronology of the protocol is depicted in Fig. 1. At their arrival in the laboratory, participants received instructions on the protocol and were acquainted with the experimental task. Their seated position and the placement of their elbows on the table was adjusted so that the upper arm angles relative to the trunk were 80° and the forearms formed a right angle with the table when pulling the straps. After EMG electrode placement and a light warm-up, subjects performed two MVC with each arm in the postural configuration depicted above. These aimed to record maximal forces and EMG. The highest force produced by the indicator arm was used to define the three force levels used for the matching tasks, i.e., 5, 10 and 20% of this peak value. After these tests, subjects relaxed 10 min before the onset of the experimental recordings.
For one-half of the subjects, the indicator arm was the dominant arm (n = 8) while this was the non-dominant for the remaining subjects (n = 7). One matching trial consisted in generating the required force level thanks to the visual feedbacks provided on the screen and to contract the indicator arm until the force produced in both arms felt the same. Subjects were then to signal when they thought the forces were accurately matched. From this point, they were required to maintain the force level as accurately as possible over a 4–5 s period.
Three matching conditions were tested in a random order throughout this experiment (Fig. 1). During one of these conditions, subjects performed matching trials while none of their arms had undergone prior conditioning maneuver (no conditioning, NC). This control condition was intended to check subjects’ ability to accurately match forces when both arms were in a similar thixotropic state or were not under the influence of recent thixotropic-associated changes (e.g., potentiation phenomena). Over the remaining conditions, the two arms were differentially conditioned: while the reference arm was systematically conditioned at a thixotropically neutral angle before the trials (‘hold-test’, HT, elbow angle: 80°), the indicator arm was either conditioned at short muscle length (‘hold-short’ condition, HS, elbow angle: 30–40°) or at long muscle length (‘hold-long’ condition, HL, elbow angle:140–150°). The rationale for conditioning the reference arm at the angle adopted during the matching trials is that force perception during bilateral matching tasks depends on the state of the two limbs. Accordingly, as every isometric contraction potentiates muscle responses (Hutton et al. 1987) and post-contraction spindle discharge regardless of the length at which they are performed (Wilson et al. 1995), the aim of this procedure was to isolate the thixotropic-associated changes from these potential confounding effects.
The force conditions, which included each four consecutive trials, were completed in a random order. As potentiation effects can persist over several minutes (Baudry and Duchateau 2007), and to avoid muscle fatigue development, the matching conditions were spaced 15 min apart. During this period, subjects were instructed to stay still with their forearm lying on the table. More details about the conditioning procedures and the instructions given to subjects are presented in Fig. 2.
Description of the procedures and presentation of typical EMG and force traces recorded during the conditioning and matching contractions (20% here) (experiment 1). In the HS condition, subjects were asked to pull their indicator arm towards their body with half-maximum isometric contraction during 4 s. In the HL condition, the instructions were similar but subjects pulled their indicator arm against the hand of an experimenter who took the care to isometrically block the arm at the defined angle along the contraction. In both conditions, participants contracted their reference arm at the test angle (‘hold-test’) of the matching task by pulling against the webbing straps. At the end of the conditioning contraction, the indicator arm was passively moved by an experimenter and placed at the test angle into the straps. In that regard, the arrows indicate elbow angle evolution between the conditioning and the matching contraction. The horizontal arrow indicate that no angle modifications occurred (reference arm, ‘hold-test’), the arrow pointing up represents an increase in muscle length (indicator arm, ‘hold-short’) and the arrow pointing down indicates a reduction of muscle length (indicator arm, ‘hold-long’). The matching trial started 10 s after this maneuver to minimize post-activation depression differences between ‘hold-long’ and ‘hold-short’ conditionings (Gregory et al. 1998)
Experiment 2: testing the Kohnstamm phenomenon at different muscle lengths
We hypothesized that thixotropy would affect force perception through the degree of spindle excitation resulting from the conditioning contraction. For this reason, in this experiment, we used the Kohnstamm phenomenon as a probe of sensorimotor pathway excitability following the different conditioning maneuvers used in experiment 1 (i.e., HS, HL and HT). Pre-tests were carried out to identify whether the duration and intensity of the contractions performed in experiment 1 (4-s isometric contractions at half-maximum strength) were sufficient to elicit measurable involuntary after-contractions. Six subjects for whom the Kohnstamm phenomenon could be easily generated with optimal contraction conditions (i.e., 35-s isometric contraction at 40% MVC, Duclos et al. 2007) were recruited. As the strength of the after-contraction was significantly lower (n = 4) or absent (n = 2) with the 4-s contraction, the choice was made to study the Kohnstamm phenomenon with the optimal conditioning parameters mentioned above.
Apparatus
As in experiment 1, subjects (n = 11) were seated in the adjustable chair, their forearm placed on a board tilted 45° upward. The height of the seat was adjusted so that the elbow angle was 80°, i.e., the ‘hold-test’ angle (Fig. 3). They were equipped with EMG electrodes at the level of the biceps brachii of their dominant (n = 6) or non-dominant arm (n = 5). A goniometer (type SG150, Biometrics Ltd, Newport, UK) was used to measure (1000 Hz) potential elbow angular displacements during the after-contraction period. Three different adjustable cables connected to webbing straps were fixed to the table or to the adjacent wall to allow the subjects contracting their elbow flexors isometrically at the defined muscle lengths, i.e., short (elbow angle: 30°–40°), test (80°) and long (140°–150°).
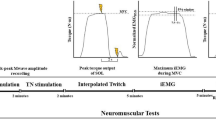
Description of a trial during experiment 2. Subjects contracted their biceps brachii at a strength representing 40% of MVC during 35 s in one of the three elbow configurations (i.e., HS, HT or HL). After 35 s, they were instructed to stop contracting and their forearm was repositioned by an experimenter on a board tilted 45° upward. They were then required to not counteract the development of any involuntary contractions and/or displacements of the forearm. The figure depicts typical EMG and angular displacement signals recorded during a HS trial. The post-effects were quantified as the RMS of the biceps brachii EMG in a 10-s interval ranging from + 10 to + 20 s relative to the end of the conditioning contraction. The angular displacement of the elbow, when present, was measured from the repositioning of the forearm to the end of the EMG computation window
Experimental procedures
In a first time, subjects performed one MVC with EMG recordings intended to define the workload during the conditioning contractions. For this experiment, subjects had to contract their elbow flexors isometrically at a strength representing 40% of their biceps brachii MVC. For this purpose, the rectified smoothed EMG was displayed on a screen in front of the subjects with a horizontal line defining the target intensity. Because EMG amplitude has been shown to be uniform across elbow angles (Leedham and Dowling 1995), this allowed quantifying objectively the neural drives generated towards the biceps brachii and ensured that elbow flexors were conditioned with similar neural outputs in every condition.
To evoke the Kohnstamm effect, each trial consisted of contracting the biceps brachii at the target intensity (40% MVC) during a 35-s period (Fig. 3) with the strap positioned at wrist level. At the end of the contraction, subjects were asked to stop pulling and an experimenter repositioned their forearm on the board. During the after-contraction period, subjects had to close their eyes and to not counteract any involuntary arm contractions and/or displacements.
The three contraction conditions were performed in a randomized order and included each two consecutive trials. To avoid muscle fatigue, trials were interspaced by 3 min and a longer rest period of 5 min was observed between the conditions.
Data processing
Experiment 1 EMG signals were full-wave rectified and expressed in relative expression of the EMG root mean square (RMS) recorded during the MVC tests. Force signals were low-pass filtered with a second order Butterworth filter and a cut-off frequency of 9 Hz. The RMS of these different signals was calculated over a 4-s period from the point when participants indicated that they were satisfied with their match. During the conditioning contractions, EMG signals were recorded and their RMS was computed. Matching errors were expressed as the relative force difference between the indicator and the reference arm (indicator force divided by reference force minus one).
Experiment 2 EMG signals were full-wave rectified and normalized to MVC values. To quantify the Kohnstamm phenomenon, the RMS of the biceps brachii was computed over a 10-s period starting 10 s after the end of the conditioning contraction (Fig. 3). This computation window was chosen to allow the after-contraction initiating as a latent period is usually observed after cessation of the voluntary contraction (Meigal et al. 1996). This also aimed to replicate the time interval used between the conditioning and the matching contraction in experiment 1 (Fig. 2). The signals from the goniometer were low-pass filtered (second order Butterworth filter, cut-off frequency 9 Hz) and the angular displacements were quantified from the repositioning of the forearm to the end of the EMG computing period (Fig. 3).
Statistics
Force and EMG variables were averaged [means (SD)] over the matching conditions for each force level in experiment 1 and over each contraction condition in experiment 2. Afterwards, data were checked for normality using a Shapiro–Wilk test.
Experiment 1 A first step in the analysis was to check subjects’ ability to accurately match the force in the NC condition. For this purpose, we performed a two-factor analysis of variance (ANOVA) on force data [arm (reference vs. indicator) × force (5 vs. 10 vs. 20%)]. In a second session, EMG and force RMS values recorded in the indicator arm were submitted to three matching conditions (NC vs. HS vs. HL) × three force conditions (5 vs. 10 vs. 20%) ANOVA. Matching errors over the HS and HL conditions were compared by means of two-factor ANOVA [matching condition (HL vs. HL) × force (5 vs. 10 vs. 20%)]. To test for differences between EMG during the conditioning contractions, matching condition (HL vs. HL) × arm (reference vs. indicator) ANOVA were used.
Experiment 2 Differences in biceps brachii EMG were tested using a one-way ANOVA (HS vs. HT vs. HL). Regarding forearm displacements, they were not present in all the subjects. For this reason, only the number of ‘responders’ and their mean (SD) angular displacement were reported.
For the two experiments, effect sizes for ANOVA are reported as partial eta-squared (η²) with interpretation thresholds fixed at 0.01, 0.08, 0.26 and 0.50 for, respectively, small, moderate, large and very large effect sizes. Planned comparisons were used for post-hoc tests. For all analyses, statistical level of significance was fixed at P < .05.
Results
Experiment 1
Matching performance without prior conditioning
ANOVA did not reveal a main effect of the arm [F(1,14) = 0.29, P = 0.6, η² = 0.02] nor arm × force interactions [F(2, 28) = 0.31, P = 0.73, η² = 0.02] showing that subjects were able to match the forces with accuracy when their arms did not undergo any conditioning contractions (NC condition). Matching errors, expressed as the percent force difference between the indicator and the reference arm, were 1.5 (5.2) %, 3.4 (5.8) % and − 1.1 (5) % at 5, 10 and 20% of MVC, respectively.
EMG magnitude during the conditioning contractions
During the HS and HL conditions, subjects performed conditioning contractions of their reference and indicator arms before each matching trial. They were required to generate a half-maximum isometric contraction in the direction of elbow flexion during 4 s either at short (HS) or long (HL) muscle lengths with their indicator arm, and at the matching angle (HT) with their reference arm (Figs. 1, 2). On average, during these procedures, EMG amplitudes in the biceps brachii and brachioradialis oscillated between 50 and 60% of MVC EMG. ANOVA did not show any significant differences between arms and conditions for both muscles. As EMG amplitude is uniform across elbow angles (Leedham and Dowling 1995), this result ensures that muscles were conditioned at similar contraction intensities in every condition.
Matching performances following conditioning
The force and EMG recorded in the indicator arm are presented graphically [means (SD)] in Fig. 4. Overall, HS conditioning led subjects to generate excessive force with their indicator arm compared to NC and HL conditions. The ANOVA indicated a main effect of the matching condition [F(2,28) = 9.97, P = 0.001, η² = 0.42] but no conditioning × force effect [F(4, 56) = 0.93, P = 0.45, η² = 0.06]. Post-hoc showed no significant force differences between NC and HL conditions (P = 0.45). In contrast, the forces produced during the HS condition were significantly higher compared to NC and HL conditions (P < 0.004 for both analyses).
Force (a) and EMG values (b) [means (SD)] of the indicator arm during the NC (white bars), HL and HS conditions (gray bars) for each force condition in experiment 1 (n = 15). A. Note that subjects exhibited difficulties in maintaining the low force level imposed by the 5% condition, i.e., they systematically produced more force than required. This explains why the force values at 10% of MVC are not twice that of the 5% condition. In any case, the forces produced by the indicator arm were significantly larger following HS conditioning irrespective of the force condition. b Similar trends were observed regarding EMG magnitudes in the biceps brachii and brachioradialis. *P < 0.05; **P < 0.01; P < 0.001
To further test the effects of reference force magnitude on matching accuracy during the HL and HS conditions, matching errors were compared with an ANOVA. The analysis logically revealed a main effect of the conditioning type [F(1, 14) = 10.23, P = 0.006, η² = 0.42]. In contrast, no main effect of reference force magnitude [F(2, 28) = 1.2, P = 0.32, η² = 0.08] nor interaction effects [F(2, 28) = 1.42, P = 0.26, η² = 0.09] were observed (Fig. 5). For the sake of clarity, this indicates that subjects underestimated the force they produced with their indicator arm following ‘hold-short’ conditioning as, when they thought the force they produced in both arms was similar, they actually produced more force in that limb.
Matching errors following HL (grey bars) and HS (white bars) conditioning in the three force conditions during the matching task (experiment 1). While analyses revealed a main effect of the matching condition, they did not detect any effect of the force condition nor interaction effect. Please refer to the text for the statistical outcomes. Note the large interindividual variability following ‘hold-short’ conditioning compared to the HL condition
EMG magnitude during the matching contractions
EMG data followed similar patterns to those of the matching force. ANOVA relative to the biceps brachii revealed a significant main effect of the matching condition [F(2, 28) = 6.56, P = 0.005, η² = 0.32] as well as a significant interaction [F(4, 56) = 5.07, P = 0.001, η² = 0.27]. Comparable outcomes were obtained for the brachioradialis [main effect of the matching condition: F(2, 28) = 20.87, P < 0.001, η² = 0.6]; interaction: [F(4, 56) = 8.77, P = 0.00001, η² = 0.39]. For both muscles, planned comparisons showed that matching EMG amplitudes were significantly higher following HS conditioning in comparison with the NC and HL conditions (P < 0.004 for all analyses). In contrast, no differences were observed between the NC and HL conditions (P > 0.09 for both muscles). For all force conditions, EMG amplitudes were significantly larger following HS conditioning (except for the biceps brachii at 5% of MVC) compared to HL and NC conditions (Fig. 4).
Experiment 2
During the after-contraction period, EMG signals were systematically present in the biceps brachii albeit sometimes very weak for some subjects. The one-way ANOVA revealed a main effect of the contraction type [F(2, 20) = 6.99, P = 0.005, η² = 0.41] with larger muscle activity following HS compared to HT (P = 0.022) and HL conditioning (P = 0.013) (Fig. 6). No difference between HT and HL contractions was detected (P = 0.15).
Of the 11 subjects who participated in this experiment, only 7 exhibited a displacement of the forearm during the after-contraction period. For two of these seven subjects, displacements were observed only following ‘hold-short’ conditioning. For the remaining subjects (n = 5), angular displacements were present in every condition with more pronounced displacements following HS contractions [33 (26)°] compared to HT [16 (24)°] and HL conditions [13 (21)°].
Discussion
In this study, we tested the effects of conditioning contractions modifying the thixotropic state of muscle spindles on the sense of force. As force perception during bilateral matching tasks depends on the state of the two limbs, the reference arm was also conditioned, but with a contraction performed at the matching angle (‘hold-test’). The aim was to isolate the thixotropic-associated changes from potential confounding potentiation effects which can occur at low force levels (Fukutani et al. 2014) and which result in underestimation of the force produced by the conditioned muscle (Hutton et al. 1987). Moreover, isometric contractions, irrespective of the muscle length at which they are performed, enhance spindle post-contraction activity compared to pre-contraction values (Wilson et al. 1995). Because EMG amplitudes across arms during the conditioning contractions were not significantly different, this allows certifying that the effects observed in the present study were only due to the thixotropic-associated changes in muscle spindles. Overall, while conditioning elbow flexors at long muscle lengths did not alter force sensations, ‘hold-short’ conditioning led to significant underestimations of the force produced by these muscles, irrespective of the reference force magnitude.
Spindles are the sensory organs involved in signaling muscle lengths and joint positions to the brain. Acting on their thixotropic property modifies their background activity and strongly affects the position sense in unloaded conditions (Tsay et al. 2014). Specifically, when the resting discharge is increased by ‘hold-short’ conditioning, position errors are consistent with the brain perceiving muscles as longer than they actually are. In contrast, when the resting discharge decreases following ‘hold-long’ conditioning, error patterns seem to indicate that muscles are perceived as shorter than they actually are. When the limb is loaded, the errors progressively alleviate until fusimotor co-activation results in a complete resetting of the thixotropic state, at approximately 10% of MVC (Allen et al. 2008). Based on the assumption that force sensations arise from the fusimotor-driven spindle discharges (Luu et al. 2011), important force misjudgments may have been observed at 5% compared to 10 and 20% of MVC. Indeed, at 5%, it is likely that most of spindles were in the thixotropic-induced state and that their responsiveness to neural drives were potentiated (HS condition) or depressed (HL condition). However, the present results are far from supporting this hypothesis in that error signs were opposite to those predicted by the thixotropy hypothesis (Fig. 4). Furthermore, relative error magnitudes were rather insensitive to reference force amplitudes (Fig. 5).
‘Hold-short’ conditioning strongly affected force perception in comparison with ‘hold-long’ conditioning. As this particular form of conditioning is known to be particularly effective in increasing spindle firing (Wood et al. 1996), we sought to test the idea that potentiated mechanisms related to the strength of the reafferent signals during the period preceding the matching trial could account for the excessive force produced by the conditioned arm. We thus carried out a second experiment in which we evaluated the magnitude of the Kohnstamm phenomenon following the three types of conditioning used in this study. Because the contraction parameters used in the matching experiment did not result in strong post-activations (pre-tests), we decided to use different ones, which had previously been shown to promote the implementation of the post-effects (Duclos et al. 2007). We reasoned that the stronger the after-contraction, the higher the conditioning-induced facilitation of the motor pathways. Similar to the results of Hagbarth and Nordin (1998), larger involuntary drives were observed following HS conditioning in comparison with the HL and HT conditions.
Group Ia afferent impulses are known to evoke powerful excitatory supports to homonymous spinal motoneurons through oligosynaptic connections (Hagbarth et al. 1985). Thus, it may be suggested that the increased spindle activity elicited by HS conditioning resulted in enhanced autogenic excitatory inflow to motoneurons, increasing the gain of the cortical drives at the spinal level during the matching contractions (experiment 1) and leading to strong after-contraction effects (experiment 2). Yet, following voluntary contractions (Crone and Nielsen 1989) and strong spindle activity (Gregory et al. 1998), H-reflex has been shown to be largely depressed due to a post-activation depletion of neurotransmitters. Following HL conditioning, the size of the H-reflex evoked immediately after returning to a shorter muscle length falls because of the important discharge levels induced by the muscle stretch (Wood et al. 1996). Once at the new position, spindles remain silent, allowing repletion of neurotransmitters and a rapid rise of the H response. In contrast, stretching a muscle after HS conditioning increases spindle firing rate and thus creates a longer depression of the H-reflex as compared to HL conditioning. Still, this phenomenon is transitory and the differences between the two types of conditioning tend to disappear 10 s after the return to the intermediate muscle length (Wood et al. 1996; Gregory et al. 1998). Accordingly, the larger force and EMG in the indicator arm as well as the larger Kohnstamm effect observed following HS conditioning are probably not accounted for by enhanced spinal excitability of the homonymous motoneuron pool.
Other spinal mechanisms, however, may have facilitated the motor outputs in the ‘hold-short’-conditioned arm. Although not evaluated here, a concurrent activation of antagonistic muscles is usually observed during voluntary isometric contractions (e.g. Levenez et al. 2008). In the present study, the greater spindle activity in elbow flexors following ‘hold-short’ conditioning may have resulted in increased reciprocal inhibition of antagonistic motoneurons, reducing co-activation phenomena and promoting elbow flexion torques. Besides, a dual effect of contracting elbow flexors at short muscle lengths is to introduce slack in the extensors (Proske et al. 2014). Assuming that their co-activation was not sufficient during the matching contractions to remove the conditioning-induced slack, decreased reciprocal inhibition of elbow flexor motoneurons may have indirectly contributed to their facilitation.
In addition to the spinal stage, muscle spindle afferents exhibit wide connections with the cortico-motoneurons as evidenced by the contribution of transcortical reflex loops to the long-latency stretch response (Christensen et al. 2000; Pruszynski 2014). In that regard, the proprioceptive inputs received by the brain have been shown to largely modify cortical excitability. For instance, rises in spindle firing induced by tendon vibration (Siggelkow et al. 1999), conditioning voluntary isometric contractions (Stuart et al. 2002) or nerve stimulation (Roy and Gorassini 2008; Devanne et al. 2009) increase the size of the motor-evoked potential elicited by transcranial magnetic stimulation. In an fMRI study on the Kohnstamm phenomenon, Duclos et al. (2007) investigated the cortical areas involved in the after-contraction effects. They found that the brain regions responsible for voluntary motor command generation were strongly activated. Taken together, these data and the present results are evidence that the massive afferent signals generated by ‘hold-short’ conditioning increased cortical excitability. While the conditioning-induced facilitation of the motor pathways during the matching experiment was probably insufficient to generate involuntary drives in all subjects, the excitatory inputs it provided to the brain may have enhanced cortical output generation. Assuming that participants declared a match based on effort cues of purely central origin, the larger afferent transmissions may have increased the gain between the effort produced and the cortical drives, generating additional unperceived forces. Note, however, that this process seems to be subject to large interindividual differences as suggested by the important standard deviations in matching error magnitudes (Fig. 5). The unbiased matching performances observed following HL conditioning are also in line with our effort-based interpretation. Indeed, the excitability of the motor pathways controlling the reference and the indicator arms were apparently not different, as revealed by the non-significant after-contraction difference observed between HT and HL during experiment 2 (Fig. 6). Similar effort thus produced similar motor outputs. Note that we depict our interpretations of the present results in the context of the efference copy theory in Fig. 7, a theory which is strongly supported here.
Schematic representation of the efference copy theory with the influence of increased spindle inflow to the brain. Following maneuvers increasing spindle ending sensitivity, a large amount of excitatory inputs feeds the cortical motor regions. This creates a decoupling between the efference copy and the voluntary drives. Specifically, for a particular effort, the motor output is more important than expected from the efference copy. Note that the thickness of each arrow schematically indicates the strength of each signal to highlight the decoupling which occurs between the different pathways. In a neuroanatomical perspective, this involves that the increased excitatory afferent inputs primarily act downstream the pre-motor areas responsible for efference copy generation. This is in agreement with the fMRI study of Duclos et al. (2007) which showed that the pre-motor structures involved in efference copy generation (especially the supplementary motor area) are not active following enhanced spindle activity. This is also in line with the idea that involuntary contractions do not generate an efference copy (De Havas et al. 2015)
Conclusive remarks and wider implications
Petersen et al. (2010) pointed out the need for movements to be continuously monitored through proprioceptive signals to adapt the cortical outputs to the changing functional efficacy of the descending systems. Nevertheless, proprioceptive inputs can signal erroneous position information to the brain (Tsay et al. 2014). Additionally, and as suggested by the present study, spindle signals can modify the excitability of the sensorimotor loops and, associatively, affect the sense of force by decoupling effort and motor outputs. These changes are critical as they necessarily affect sensorimotor integration processes during normal motor activities. From an applied perspective, while the results of the present study can hardly be extended to ecological and dynamic motor situations, they suggest that the immediate muscular activity and the length at which muscles are contracted can strongly affect proprioceptive representations and potentially sensorimotor control accuracy.
References
Allen TJ, Ansems GE, Proske U (2008) Evidence from proprioception of fusimotor coactivation during voluntary contractions in humans. Exp Physiol 93:391–398. https://doi.org/10.1113/expphysiol.2007.040741
Baudry S, Duchateau J (2007) Postactivation potentiation in a human muscle: effect on the rate of torque development of tetanic and voluntary isometric contractions. J Appl Physiol 102:1394–1401. https://doi.org/10.1152/japplphysiol.01254.2006
Brooks J, Allen TJ, Proske U (2013) The senses of force and heaviness at the human elbow joint. Exp Brain Res 226:617–629. https://doi.org/10.1007/s00221-013-3476-6
Carson RG, Riek S, Shahbazpour N (2002) Central and peripheral mediation of human force sensation following eccentric or concentric contractions. J Physiol 539:913–925. https://doi.org/10.1013/jphysiol.2001.013385
Christensen LOD, Petersen N, Andersen JB et al (2000) Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol 62:251–272
Christensen MS, Lundbye-Jensen J, Geertsen SS et al (2007) Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat Neurosci 10:417–419. https://doi.org/10.1038/nn1873
Clark FJ, Burgess RC, Chapin JW, Lipscomb WT (1985) Role of intramuscular receptors in the awareness of limb position. J Neurophysiol 54:1529–1540
Crone C, Nielsen J (1989) Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res 78:28–32. https://doi.org/10.1007/BF00230683
De Havas J, Ghosh A, Gomi H, Haggard P (2015) Sensorimotor organization of a sustained involuntary movement. Front Behav Neurosci 9:1–16. https://doi.org/10.3389/fnbeh.2015.00185
De Havas J, Gomi H, Haggard P (2017) Experimental investigations of control principles of involuntary movement: a comprehensive review of the Kohnstamm phenomenon. Exp Brain Res 235:1953–1997. https://doi.org/10.1007/s00221-017-4950-3
Devanne H, Degardin A, Tyvaert L et al (2009) Afferent-induced facilitation of primary motor cortex excitability in the region controlling hand muscles in humans. Eur J Neurosci 30:439–448. https://doi.org/10.1111/j.1460-9568.2009.06815.x
Duclos C, Roll R, Kavounoudias A, Roll JP (2007) Cerebral correlates of the “Kohnstamm phenomenon”: An fMRI study. Neuroimage 34:774–783. https://doi.org/10.1016/j.neuroimage.2006.06.050
Fukutani A, Hirata K, Miyamoto N et al (2014) Effect of conditioning contraction intensity on postactivation potentiation is muscle dependent. J Electromyogr Kinesiol 24:240–245. https://doi.org/10.1016/j.jelekin.2014.01.002
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Gregory JE, Wise AK, Wood SA et al (1998) Muscle history, fusimotor activity and the human stretch reflex. J Physiol 513:927–934. https://doi.org/10.1111/j.1469-7793.1998.927ba.x
Hagbarth KE, Nordin M (1998) Postural after-contractions in man attributed to muscle spindle thixotropy. J Physiol 506:875–883. https://doi.org/10.1111/j.1469-7793.1998.875bv.x
Hagbarth K, Hagglund J, Nordin M, Wallin E (1985) Thixotropic behaviour of human finger flexor muscles with accompanying changes in spindle and reflex responses to stretch. J Physiol 368:323–342. https://doi.org/10.1113/jphysiol.1985.sp015860
Hodgson M, Docherty D, Robbins D (2005) Post-activation potentiation motor performance. Sport Med 35:585–595
Hutton RS, Kaiya K, Suzuki S, Watanabe S (1987) Post-contraction errors in human force production are reduced by muscle stretch. JPhysiol 393:247–259. https://doi.org/10.1113/jphysiol.1987.sp016822
Jahnke MT, Proske U, Struppler A (1989) Measurements of muscle stiffness, the electromyogram and activity in single muscle spindles of human flexor muscles following conditioning by passive stretch or contraction. Brain Res 493:103–112. https://doi.org/10.1016/0006-8993(89)91004-4
Leedham JS, Dowling JJ (1995) Force-length, torque-angle and EMG-joint angle relationships of the human in vivo biceps brachii. Eur J Appl Physiol Occup Physiol 70:421–426. https://doi.org/10.1007/BF00618493
Levenez M, Garland SJ, Klass M, Duchateau J (2008) Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99:554–563. https://doi.org/10.1152/jn.00963.2007
Liu JZ, Shan ZY, Zhang LD et al (2003) Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an fMRI study. J Neurophysiol 90:300–312. https://doi.org/10.1152/jn.00821.2002
Luu BL, Day BL, Cole JD, Fitzpatrick RC (2011) The fusimotor and reafferent origin of the sense of force and weight. J Physiol 589:3135–3147. https://doi.org/10.1113/jphysiol.2011.208447
Meigal AY, Lupandin YV, Hänninen O (1996) Influence of cold and hot conditions on postactivation in human skeletal muscles. Pflugers Arch 432:121–125. https://doi.org/10.1007/s004240050113
Petersen NC, Butler JE, Taylor JL, Gandevia SC (2010) Probing the corticospinal link between the motor cortex and motoneurons: some neglected aspects of human motor cortical function. Acta Physiol 198:403–416. https://doi.org/10.1111/j.1748-1716.2009.02066.x
Proske UWE, Morgan L, Gregory JE (1993) Proske U et al. (1993) - Thixotropy in skeletal muscle and in muscle spindles; a review.pdf. https://doi.org/10.1016/0301-0082(93)90032-N
Proske U, Tsay A, Allen T (2014) Muscle thixotropy as a tool in the study of proprioception. Exp Brain Res 232:3397–3412. https://doi.org/10.1007/s00221-014-4088-5
Pruszynski JA (2014) Primary motor cortex and fast feedback responses to mechanical perturbations: a primer on what we know now and some suggestions on what we should find out next. Front Integr Neurosci. https://doi.org/10.3389/fnint.2014.00072
Roy FD, Gorassini MA (2008) Peripheral sensory activation of cortical circuits in the leg motor cortex of man. J Physiol 586:4091–4105. https://doi.org/10.1113/jphysiol.2008.153726
Siggelkow S, Kossev A, Schubert M et al (1999) Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve 22:1544–1548. https://doi.org/10.1002/(SICI)1097-4598(199911)22:11<1544::AID-MUS9>3.0.CO;2-8
Simon AM, Kelly BM, Ferris DP (2009) Sense of effort determines lower limb force production during dynamic movement in individuals with poststroke hemiparesis. Neurorehabil Neural Repair 23:811–818. https://doi.org/10.1177/1545968308331163
Stuart M, Butler JE, Collins DF et al (2002) The history of contraction of the wrist flexors can change cortical excitability. J Physiol 545:731–737. https://doi.org/10.1113/jphysiol.2002.032854
Tsay A, Savage G, Allen TJ, Proske U (2014) Limb position sense, proprioceptive drift and muscle thixotropy at the human elbow joint. J Physiol 592:2679–2794. https://doi.org/10.1113/jphysiol.2013.269365
Tsay A, Allen TJ, Proske U (2015) Position sense at the human forearm after conditioning elbow muscles with isometric contractions. Exp brain Res 233:2635–2643. https://doi.org/10.1007/s00221-015-4334-5
Weerakkody N, Percival P, Morgan DL et al (2003) Matching different levels of isometric torque in elbow flexor muscles after eccentric exercise. Exp Brain Res 149:141–150. https://doi.org/10.1007/s00221-002-1341-0
Wilson LR, Gandevia SC, Burke D (1995) Increased resting discharge of human spindle afferents following voluntary contractions. J Physiol 488:833–840. https://doi.org/10.1016/0304-3940(89)90484-9
Wood SA, Gregory JE, Proske U (1996) The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol 497:279–290
Acknowledgements
The authors wish to thank all the subjects for their cooperation and Maximilien Bowen for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Monjo, F., Forestier, N. Muscle spindle thixotropy affects force perception through afferent-induced facilitation of the motor pathways as revealed by the Kohnstamm effect. Exp Brain Res 236, 1193–1204 (2018). https://doi.org/10.1007/s00221-018-5207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5207-5