Abstract
Direct and indirect corticospinal pathways to finger muscles may play a different role in control of the upper extremity. We used transcranial magnetic stimulation (TMS) and coherence analysis to characterize the corticospinal drive to the first dorsal interosseous (FDI) and abductor pollicis brevis (APB) when active during a precision and power grip task. In experiment 1, single motor units were recorded during precision grip and power grip in 20 adults (25.2 ± 7.1 years). Post-stimulus time histograms (PSTH) were obtained following TMS. In experiment 2, coherence and cross-correlation analysis of the FDI and APB surface EMG were used to investigate the temporal organization of corticospinal drive during precision grip and power grip in 15 adults (27.4 ± 8.1 years). We found no significant differences in PSTH peak onset (26.6 ± 1.9 vs. 26.7 ± 2.0 ms, p = 0.75), maximal peak (27.4 ± 1.9 vs. 27.4 ± 1.9 ms, p = 1.0) or peak duration (2.3 ± 1.1 vs. 2.3 ± 1.0 ms, p = 0.75) for the 11 recovered motor units during precision grip and power grip. Also, no significant difference in coherence or the width of the synchronization peaks during precision grip (7.2 ± 3.7 ms) and power grip (7.9 ± 3.1 ms) could be observed (p = 0.59). The short duration of peaks elicited in the PSTH of single motor units following TMS and central synchronization peaks of voluntarily activated motor units during precision and power grip suggests that the direct corticospinal pathway (the corticomotoneuronal system) is equally involved in the control of both tasks. The data do not support that indirect pathways would make a larger contribution to power grip.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to perform well-coordinated, accurate and independent finger movements is a natural and important part of human life as it enables us to work on a computer or play musical instruments. The neural circuitries involved have developed over millions of years as independent finger movements are also seen in other primates that are evolutionarily close to humans (Lemon 2008). Primates that are evolutionarily more distant from humans and other mammals such as cats and rodents have in comparison only limited ability to perform independent movements of their claws/fingers/toes (Lemon and Griffiths 2005).
It has been proposed that the appearance of direct monosynaptic connections from the motor cortex to spinal motoneurones—corticomotoneuronal (CM) cells—in primates is tightly linked to the evolution of independent finger movements (Lemon et al. 2012; Muir and Lemon 1983; Rathelot and Strick 2009). CM cells are thus seen in old world monkeys, and most predominantly in humans and chimpanzees (Lemon and Griffiths 2005; Rathelot and Strick 2009) whereas they are weak in new world monkeys (Maier et al. 1997) and absent in cats and rodents (Lemon and Griffiths 2005). The most direct connections from the motor cortex to spinal motoneurones in these species are disynaptic, e.g. through C3–C4 propriospinal neurones (PNs) (Alstermark et al. 2007). This system of C3–C4 PNs has also been demonstrated in old world monkeys (Alstermark et al. 2007) and in humans (Pierrot-Deseilligny 1996) and thus exists in these species in parallel to the CM system (Lemon 2008; Rathelot and Strick 2009). However, it is still debated to what extent the system is involved in mediating motor commands from the motor cortex during movements (Alstermark et al. 2007; Lemon 2008). Some have argued that the system is redundant and that the CM system has effectively replaced the disynaptic corticospinal pathway through the C3–C4 PN system for most functional movements (Lemon et al. 2004). Others have provided evidence that go against this and instead argue that the C3–C4 PN system plays an important functional role in humans and other non-human primates in parallel to the CM system (Burke et al. 1994; Marchand-Pauvert et al. 1998, 2000). Lesion studies in monkeys indicate that the C3–C4 PN system contributes to the recovery of fractionated finger movements when CM connections are lost (Isa et al. 2013; Sasaki et al. 2004), but that the system cannot fully take over the function of the direct CM system (Isa et al. 2013).
Other pathways might also be of relevance to hand movement. Recent evidence suggests that the indirect reticulospinal system contributes during power grip and to a lesser extent during precision grip in healthy humans (Baker and Perez 2017; Tazoe and Perez 2017). With this complexity in mind, it will be outside the scope of this article to conclude differences in input from the multiple possible indirect pathways, but instead the aim is to make the distinction between contributions from the monosynaptic CM system and the various indirect pathways described here.
One hypothesis about the CM system states that it may have evolved to improve fractionated finger movement, whereas the older disynaptic C3–C4 system may primarily be responsible for hand movements that involve less precise control of the individual fingers such as a whole hand grasp of larger objects (Rathelot and Strick 2009). If the hypothesis is correct, it should be possible to obtain evidence of differences in the timing of synaptic drive to the spinal motoneurones during these two tasks. This may be done either by investigating the common synaptic drive to pairs of motor units or by investigating the responses of individual motor units to transcranial magnetic stimulation (TMS) during the two tasks (Huesler et al. 1998; Nakajima et al. 2017; Semmler et al. 2006). Direct monosynaptic effects are associated with short-lasting central peaks of synchronization between motor unit activities whereas disynaptic (or polysynaptic effects) usually have a less tight broader synchronization peak (Datta and Stephens 1990; Semmler et al. 2006; Vaughan and Kirkwood 1997). Responses to TMS through a monosynaptic pathway would also be expected to have a shorter latency and a shorter duration than responses through a disynaptic (or polysynaptic) pathway. It was therefore the purpose of the present study to investigate whether responses of single finger muscle motor units to TMS and synchronization of pairs of finger muscle motor units differ in their temporal aspects during precision grip and power grip in human subjects.
Methods
Two series of experiments were performed using precision grip and power grip with tasks performed in a similar way to the methods described in Hasegawa et al. (2001) and Flament et al. (1993). The precision grip was performed by pinching with the thumb and the index finger on a force transducer with a diameter of 37 mm while the power grip involved the coordinated use of all fingers grasping a 58 mm diameter plastic bottle. Both tasks were performed with a neutral hand position. Examples of the hand positions of both tasks can be seen in Fig. 1a.
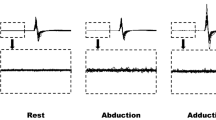
Experimental setup. Experiment 1 (a–c): single voluntarily activated FDI motor units (SMU) were recorded during precision grip and power grip (a) and post-stimulus time histograms (PSTH) were constructed in relation to transcranial magnetic stimulation. Examples of two PSTH from the same SMU during precision grip (b) and power grip (c). In this subject, the peak onset was 26 ms after stimulation during precision grip and 27 ms during power grip, maximal peak latency was 27 ms in both tasks and peak duration was 2 ms during precision grip and 1 ms during power grip. Experiment 2 (d–g): cross-correlation analysis of paired EMG recordings from APB and FDI in a single subject obtained during precision grip (d, f) and power grip (e, g). d–e The coherence analysis. The dashed line represents the 95% confidence interval. f, g The cumulant density. The dashed line represents the expected value of zero for uncorrelated EMG signals and the fully drawn lines show the upper and lower 95% confidence intervals. The vertical arrows show the duration of the synchronization peak
Subjects
We recruited 20 healthy, right-handed adult subjects (15 male, 5 female) aged 25.2 ± 7.1 years for single motor unit (SMU) experiments (experiment 1). From 10 of these subjects at least one SMU was isolated during both tasks, making the total number of recovered SMUs 11. Six of the subjects from experiment 1 and nine additional healthy, right-handed subjects (13 male and 2 female, aged 27.4 ± 8.1 years) participated in synchronization experiments on a separate test day (experiment 2). Informed consent was obtained from all individual participants included in the study. The study was approved by the ethics committee for the Capital Region of Denmark (Approval no. H-16021214) and was performed in accordance with the Declaration of Helsinki.
EMG recording and brain stimulations
The subjects were seated at a table with their right arm placed in a supportive armrest (see Fig. 1a) and the height of the chair adjusted, obtaining a 30° abduction and a 45° flexion of the shoulder. The elbow was flexed 50°. The study made use of surface electrodes (Ambu Blue Sensor NF-10-A/12, Ballerup, Denmark) and 0.3 mm needle electrodes (Viasys Healthcare, Medelec REF x53153, Surrey, United Kingdom). Both received 1000x amplification through a pre-amplifier (Digitimer nl844, Welwyn Garden City, United Kingdom) and were filtered (Digitimer Neurolog) with a high pass of 5 Hz and a low pass of 1000 Hz.
During the hotspot search, one surface electrode was placed on the FDI muscle and one on the index finger of the subject’s right hand. For the EMG–EMG coherence studies two surface electrodes were placed on the FDI muscle and two on the abductor pollicis brevis (APB) for simultaneous recording. In both studies, a surface electrode was placed on caput ulnae as a reference point. The needle electrodes were used when isolating the firing of a SMU. The use of needle electrodes instead of wire electrodes made repositioning possible, which was necessary if the initial position had several motor units being recruited at the same time and firing with the same amplitude.
The TMS stimulations were delivered using a 70 mm figure-8 coil (Magstim Rapid2, Whitland, United Kingdom). The coil was oriented 45 degrees from the midline with the handle pointing posterior and lateral, as this orientation produces the lowest MEP thresholds in the FDI and APB (Mills et al. 1992; Souza et al. 2017). To ensure that the stimuli were given at the same location throughout the experiment, the coil and subject were tracked using Brainsight (Rogue Research Inc, Montreal, Canada). Deviations of up to 1 mm from the hotspot were accepted during trials. In search of the hotspot of the FDI muscle, TMS stimulations of M1 were given every 3 s. The hotspot was then determined as the location of the coil where the largest and most consistent MEP’s were observed at a latency of 20–40 ms. The resting threshold intensity was subsequently determined as the TMS intensity resulting in at least 3 MEP’s every 5 stimulations. The subjects were instructed to perform a maximum voluntary contraction (MVC) of the FDI muscle during the execution of precision grip on a force transducer (Dacell ST-AM310). The active threshold was found while performing precision and power grip, respectively, at a force corresponding to 5% of the MVC.
Single motor unit experiments (experiment 1)
When the subject succeeded in isolating a SMU during the performance of one of the two tasks, 200 TMS stimulations were given with an interstimulus interval (ISI) of at least 3 s. The exact ISI was adjusted to the SMU firing rate since the stimulus was triggered by the preceding motor unit action potential (MUAP) within the trigger interval, but in most cases the performance of a single task would last for approximately 10 min. Trials where the SMU stopped firing or other motor units interfered were stopped immediately and the subject was asked to find and isolate the same or a new SMU. When 200 TMS stimulations had been delivered in one task, the second task was performed immediately after. This was done to improve the chances of relocating the same SMU in both tasks. The order in which the tasks were performed was semi-randomised. Stimulations were given at an intensity of 90% of the active motor threshold during the precision grip. The rationale for adopting this intensity was the need for the intensity to be subthreshold in order not to elicit MEPs in inactive motor units, yet still be able to facilitate an earlier discharge of the already active motor unit. This method is explained in greater detail in Petersen et al. (2003). The force of the contraction was not monitored, but was gradually adjusted from almost no contraction to a slight contraction, at which isolation of the firing of a SMU was easiest. SMU’s from FDI were successfully recovered during both the precision grip and power grip in 10 subjects. Two recovered units were retrieved from the same subject, adding to a total of 11 recovered units. The experimenter recognised the motor unit by observing the amplitude, firing frequency and waveform of the unit. Post-stimulus time histograms (PSTH’s) were constructed from the data (see Fig. 1 and data analysis).
Synchronization experiments (experiment 2)
EMG–EMG coherence and synchronization between FDI and APB surface EMG activity was quantified during an isometric precision grip and power grip.
After finding the precision grip MVC (see above), subjects were instructed to perform an isometric contraction at 10% of the MVC, and the corresponding EMG levels were noted. Subjects were then asked to maintain this level of EMG during both tasks guided by visual feedback from an oscilloscope (HP 54602B). The order of the tasks was randomised, and each task was performed for 2 min.
Data analysis
PSTH analysis
The SMU EMG recordings and PSTH constructions were performed using Spike (Spike2 version 7.11c, Cambridge Electronic Design). The firing of the SMU acted as a trigger for the TMS after a pre-set delay to account for the refractory period of the SMU. As the firing frequencies of the SMU in most circumstances were between 8 and 12 Hz, the delay was most often set to 50 ms. For each SMU, two PSTH’s were constructed per task. In one, a TMS stimulus was given (‘stim’), and in the other no stimulus (‘no-stim’) was given. The final PSTH was made in the following analysis, where the counts from the no-stim PSTH were deducted from the stim PSTH at the corresponding latencies. This was done to account for any trigger counts due to background activity in the EMG. After each PSTH, an analysis was made of the average waveform of the trigger-inducing motor unit. The analysis involved a comparison of the two tasks to ensure that the same motor unit had been recovered. The peak of the PSTH was defined as having bins containing a number of counts exceeding the mean background firing plus two standard deviations (SD) (Mao et al. 1984). The bins of the peak were not allowed to be separated by more than 1 ms. The peak of the PSTH was then compared to the PSTH from the same SMU during the opposite task in the following variables; peak onset, maximal peak, peak duration and response probability. Peak onset was defined as the latency of the first bin belonging to the peak. Maximal peak was defined as the latency of the bin with the most counts belonging to the peak. Peak duration was defined as the number of bins belonging to the peak. Response probability was defined as the total number of counts in the bins of the peak divided by the number of stimulations given during the construction of the PSTH.
Synchronization and coherence analysis
The cross-correlation analysis from surface EMG was performed offline, in accordance with the methods described in Gibbs et al. (1997) and Halliday et al. (1995). A trigger level was set manually on each surface EMG recording in order to generate two point processes, which were used for further analysis. The threshold was adjusted so that a minimum of 2000 points was included. Figure 1a depicts the setting of the trigger level in a single subject. A threshold level was used to prioritize motor unit firing times over action potential shape (Halliday and Farmer 2010), but also to minimize the risk of including possible cross-talk between the FDI and APB muscle electrodes. To ensure comparability between the two tasks, the number of spikes included in the analysis of tasks from the same subject was not allowed to differ by 1000 or more points.
The results of analysis of individual records generated estimates of the auto-spectra of the two point processes fxx(λ), fyy(λ), and their cross-spectra fxy(λ). We then estimated three functions that characterize the signals’ correlation structure: coherence, |R xy (λ)|2; phase, ϕ xy (λ); and cumulant density, q xy (u). Coherence estimates are bounded measures of frequency association between the signals and are defined over the range [0, 1]. The time domain cumulant density estimate of synchrony between the signals is not bounded. The phase between the signals is defined over the range [−π, +π]. For the present data, a linear coherence analysis provide a measure of the fraction of the activity in one signal at any given frequency that can be predicted by the activity in the second signal. In this way, coherence estimates quantify the strength and range of frequencies of common oscillations that are shared between two EMGs. The timing relations between the two EMG signals are estimated from the phase. The cumulant density, calculated from the inverse Fourier transform of the cross-spectrum, provides an unbounded time-domain representation of the EMG–EMG correlation structure analogous to the cross-correlogram and thus captures both correlation and timing information between signals (Halliday et al. 1995).
In the synchronization analysis, resulting central peaks of synchrony was identified as significant when differing by 3 SD from baseline. The central synchronization peak width in milliseconds was noted.
Statistics
For all examined variables, the obtained results were compared between the two tasks using a paired student’s t-test. Data are presented as [means ± SD (95% confidence interval)] unless otherwise specified. Statistical significance was given for p values smaller than 0.05. In the cross-correlations analysis, 95% confidence intervals were presented visually in the resulting figures for both the coherence and cumulant analyses.
Results
PSTH
A total of 11 SMUs (from 10 subjects) were recovered and isolated during both the precision grip and the power grip (see example of a PSTH from the same SMU during both tasks in Fig. 1b, c). Data from all SMUs are presented in Fig. 2. There was no significant difference between the average latency [26.6 ± 1.9 (25.3, 27.9) vs. 26.7 ± 2.0 (25.4, 28.0) ms; p = 0.75], or peak duration [2.3 ± 1.1 (1.6, 3.0) vs. 2.3 ± 1.0 (1.6, 3.0) ms; p = 0.75] for the SMUs that were found during both the precision grip and the power grip. The maximal peak occurred at the same time during both tasks in 7 of the 11 recovered SMUs. In two recovered SMUs, the maximal peak was earlier in precision grip and in the remaining two SMUs the maximal peak was earlier in the power grip. Figure 2c presents the response probability measured as the number of TMS stimulations during a trial divided by the number of counts belonging to the PSTH peak. The average response probability was similar for the precision grip [0.18 ± 0.9 (0.11, 0.25)] and the power grip [0.19 ± 0.8 (0.13, 0.25), p = 0.79].
Latency, duration and response probability for the 11 motor units recorded during both precision grip and power grip in response to the TMS stimulus. The latency of the PSTH peak defined as the time from stimulus delivery to the earliest bin belonging to the PSTH peak for precision grip and power grip is shown in a. b The duration of the PSTH peak measured as the number of 1 ms bins belonging to the PSTH peak for precision grip and power grip. The amplitude of the peak calculated as the number of counts belonging to the PSTH peak divided by the number of TMS stimulations given during the trial for precision grip and power grip is shown in c. The grey circles illustrate the group averages for each condition
Coherence–synchronization
Of the 15 subjects participating in experiment 2, only 10 subjects showed central synchronization peaks in both tasks. One subject did not show a central synchronization peak in either task, another subject did not show a peak during the power grip only and the last two subjects showed no peak during the precision grip.
Figure 1d, e shows an example of a cross-correlation analysis while Fig. 1f, g shows an example of two central synchronization peaks from the same subject during the two tasks.
Figure 3 presents the individual results for the 10 subjects where both tasks displayed a central synchronization peak. The average peak widths for precision grip [7.2 ± 3.7 ms (4.5, 9.9)] and power grip [7.9 ± 3.1 ms (4.8, 11.0)] were not significantly different (p = 0.59). Also, no significant difference was observed between precision grip and power grip in the average logarithmic alpha band (− 2.3 ± 0.6 vs. − 2.0 ± 0.9, p = 0.31), beta band (− 1.5 ± 0.5 vs. − 1.4 ± 0.6, p = 0.31) or gamma band coherence (− 1.4 ± 0.6 vs. − 1.3 ± 0.5, p = 0.78), respectively.
Coherence and central synchronization in all subjects. The amount of EMG–EMG coherence between FDI and APB is shown for the alpha (a), beta (b) and gamma band (c) during 2 min isometric precision grip and power grip contraction for each subject. The size (d) and duration (e) of the central synchronization peak is shown for the two tasks. The grey circles illustrate the group averages for each condition. N = 15 in a–d. In e, only observations where both tasks showed significant peaks of synchronization are presented (n = 10)
Discussion
This study has revealed that responses of single finger muscle motor units and the temporal aspects of motor unit synchronization do not differ during precision and power grip in human subjects. Furthermore, no task-dependent changes in neural excitability were found when comparing the two tasks. This study thus fails to confirm any difference in the temporal organization of descending motor commands during the two tasks. Although we cannot exclude that a disynaptic pathway through C3–C4 propriospinal neurons contributes to the activation of the muscles also, our data are fully consistent with the idea that the CM pathway makes an important contribution in both tasks.
CM connections in humans have been studied indirectly through the use of transcranial electrical stimulations (TES) and TMS (de Noordhout et al. 1999; Mazevet et al. 1996; Pauvert et al. 1998). In various muscles, de Noordhout et al. (1999) found that the first voluntarily recruited alpha motor neurons exhibit short rise-times of the compound excitatory post-synaptic potentials (EPSP’s) following TES, which corresponds to the rise-times of CM connections. However, other studies have argued that an important part of the neural drive is mediated through premotoneuronal networks in addition to the CM system (Mazevet et al. 1996; Pauvert et al. 1998). Examining non-monosynaptic premotoneuronal excitation, Marchand-Pauvert et al. (2000) found an even distribution to both low- and high threshold motor neurons, suggesting that the premotoneuronal system is used to activate the muscle in a wide-range and non-selective fashion. This premotoneuronal network is thought to be the C3-C4 propriospinal network (Alstermark et al. 2007). Lately, several studies have also implicated the reticulospinal system as a contributor of neural drive during hand motor function (Baker and Perez 2017; Tazoe and Perez 2017), and hypothesized that the drive from this system could be task dependent, as the extensive collateralization of these connections make them more suited for tasks not requiring independent finger movements, such as the power grip (Federico and Perez 2016). From the constructed PSTH’s of this study, no differences were observed between precision grip and power grip. The short durations of the PSTH peaks are consistent with previous findings of monosynaptic peak durations (de Noordhout et al. 1999). If the active connections during the precision grip are indeed CM connections it would support the hypothesis that CM connections are evolutionary adaptations designed to improve the precise control of fractionated finger movements (Rathelot and Strick 2009). In opposition to this hypothesis however, the findings from this study suggest that there are no differences between precision and power grip, and that the active connections during the power grip are CM connections.
The width of the central synchronization peak was analysed as an addition to the PSTH analyses. It was expected that direct monosynaptic input would produce narrow peaks, whereas the peaks resulting from non-monosynaptic input would be wider (Semmler et al. 2006). Significant central peaks were found in both tasks in 10 of the 15 subjects, which is similar to the synchronization degree of different hand muscles reported in Huesler et al. (1998). Contrary to Huesler et al. (1998) however, no significant differences in the task-related occurrence of a central synchronization peak were found. The reason for this discrepancy is not entirely clear. However, the precise performance of the tasks in Huesler et al. (1998) is not given, and as Perez and Rothwell (2015) have previously reported, small differences in hand posture can affect the cortical activity. No differences between tasks were found in the width of the synchronization peaks, suggesting that there is no difference in the synaptic connections to the FDI and APB muscle during the two tasks.
This is further supported by the lack of difference between precision grip and power grip in the amount of linear coherence. It should be noted, however, that we cannot exclude that performing non-linear coherence rather than linear coherence could have revealed differences in the dynamics of the central drive during the two tasks.
In humans, task dependent changes in neural excitability related to different finger movements have been observed (Datta et al. 1989; Flament et al. 1993; Hasegawa et al. 2001); yet the underlying explanations of the task dependency remain inconclusive. Using TMS, Datta et al. (1989) observed a larger EMG response of the FDI muscle during an isolated finger movement than during a power grip, suggesting higher excitability. Contradictorily, Flament et al. (1993) found a larger EMG response to movements using the whole hand (e.g. power grip, pincer grip etc.) than isolated index finger abductions. On the basis of these conflicting results, Hasegawa et al. (2001) examined the task-dependent excitation of MEP’s in the FDI muscle and found stronger MEP’s during a precision grip than during a power grip. In this study, response probability was used as a measure of the excitability of the active SMU. As Bawa and Lemon (1993) reported, TMS stimulations generate motor unit recruitment comparable to voluntary activation, and as the stimulus intensity was kept identical during both tasks, response probability should reflect the excitability. In agreement with the remaining data of the study, no differences were observed between the two tasks.
It is a possibility that we did not observe differences in the PSTH parameters because the two tasks were not sufficiently different. The PSTH experiments required that the subject voluntarily activated a single motor unit, which imposes demands on the precise control of force in both tasks. However, the synchronization experiments did not require a similar precision in the control, but also failed to demonstrate differences in the activation profile of the muscles.
We also cannot exclude that we may have failed to activate indirect corticospinal pathways due to the low intensity of stimulation used in the study. However, in other studies this intensity has been found to be sufficient to activate C3–C4 PN mediated facilitation in the forearm and proximal muscles of the arm [for review see (Pierrot-Deseilligny 1996)]. Furthermore, the PSTH findings were confirmed by the synchronization analysis, which did not depend on external stimulation.
Other groups have found no evidence for oligosynaptic excitation of the upper limb in either primates (Olivier et al. 2001) or humans (de Noordhout et al. 1999). In this context, it is hard to conclude from stimulation studies whether PN connections simply are difficult to activate cortically or only play a negligible role in the corticospinal performance of the tasks. The results of the coherence-synchronization part of this study seem to support the latter.
To conclude, this study did not find any difference in the temporal organization of descending motor commands during the two tasks, suggesting that the direct corticospinal pathway (the CM system) makes an important contribution in both tasks.
References
Alstermark B, Isa T, Pettersson LG, Sasaki S (2007) The C3–C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol (Oxf) 189:123–140. https://doi.org/10.1111/j.1748-1716.2006.01655.x
Baker SN, Perez MA (2017) Reticulospinal contributions to gross hand function after human spinal cord. Injury J Neurosci 37:9778–9784. https://doi.org/10.1523/JNEUROSCI.3368-16.2017
Bawa P, Lemon RN (1993) Recruitment of motor units in response to transcranial magnetic stimulation in man. J Physiol 471:445–464
Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E (1994) Non-monosynaptic transmission of the cortical command for voluntary movement in man. J Physiol 480(Pt 1):191–202
Datta AK, Stephens JA (1990) Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422:397–419
Datta AK, Harrison LM, Stephens JA (1989) Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol 418:13–23
de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ (1999) Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain 122(Pt 7):1327–1340
Federico P, Perez MA (2016) Distinct corticocortical contributions to human precision and power grip. Cereb Cortex. https://doi.org/10.1093/cercor/bhw291
Flament D, Goldsmith P, Buckley CJ, Lemon RN (1993) Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol 464:361–378
Gibbs J, Harrison LM, Stephens JA (1997) Cross-correlation analysis of motor unit activity recorded from two separate thumb muscles during development in man. J Physiol 499(Pt 1):255–266
Halliday DM, Farmer SF (2010) On the need for rectification of surface. EMG J Neurophysiol 103:3547. https://doi.org/10.1152/jn.00222.2010 (author reply 3548–3549)
Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF (1995) A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor single motor unit discharges electromyograms. Prog Biophys Mol Biol 64:237–278
Hasegawa Y, Kasai T, Tsuji T, Yahagi S (2001) Further insight into the task-dependent excitability of motor evoked potentials in first dorsal interosseous muscle in humans. Exp Brain Res 140:387–396. https://doi.org/10.1007/s002210100842
Huesler EJ, Hepp-Reymond MC, Dietz V (1998) Task dependence of muscle synchronization in human hand muscles. Neuroreport 9:2167–2170
Isa T, Kinoshita M, Nishimura Y (2013) Role of direct vs. indirect pathways from the motor cortex to spinal motoneurons in the control of hand dexterity. Front Neurol 4:191. https://doi.org/10.3389/fneur.2013.00191
Lemon RN (2008) Descending pathways in motor control. Annu Rev Neurosci 31:195–218. https://doi.org/10.1146/annurev.neuro.31.060407.125547
Lemon RN, Griffiths J (2005) Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32:261–279. https://doi.org/10.1002/mus.20333
Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P (2004) Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res 143:263–279. https://doi.org/10.1016/S0079-6123(03)43026-4
Lemon RN, Landau W, Tutssel D, Lawrence DG (2012) Lawrence and Kuypers (1968a, b) revisited: copies of the original filmed material from their classic papers in brain. Brain 135:2290–2295. https://doi.org/10.1093/brain/aws037
Mao CC, Ashby P, Wang M, McCrea D (1984) Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res 56:341–350
Maier MA, Olivier E, Baker SN, Kirkwood PA, Morris T, Lemon RN (1997) Direct and indirect corticospinal control of arm and hand motoneurons in the squirrel monkey (Saimiri sciureus). J Neurophysiol 78:721–733
Marchand-Pauvert V, Mazevet D, Nielsen J, Petersen N, Pierrot-Deseilligny E (2000) Distribution of non-monosynaptic excitation to early and late recruited units in human forearm muscles. Exp Brain Res 134:274–278
Mazevet D, Pierrot-Deseilligny E, Rothwell JC (1996) A propriospinal-like contribution to electromyographic responses evoked in wrist extensor muscles by transcranial stimulation of the motor cortex in man. Exp Brain Res 109:495–499
Mills KR, Boniface SJ, Schubert M (1992) Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol 85:17–21
Muir RB, Lemon RN (1983) Corticospinal neurons with a special role in precision grip. Brain Res 261:312–316
Nakajima T, Tazoe T, Sakamoto M, Endoh T, Shibuya S, Elias LA, Mezzarane RA, Komiyama T, Ohki Y (2017) Reassessment of non-monosynaptic excitation from the motor cortex to motoneurons in single motor units of the human biceps brachii. Front Hum Neurosci 11:19. https://doi.org/10.3389/fnhum.2017.00019
Olivier E, Baker SN, Nakajima K, Brochier T, Lemon RN (2001) Investigation into non-monosynaptic corticospinal excitation of macaque upper limb single motor units. J Neurophysiol 86:1573–1586
Pauvert V, Pierrot-Deseilligny E, Rothwell JC (1998) Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in man. J Physiol 508(Pt 1):301–312
Perez MA, Rothwell JC (2015) Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35:4882–4889. https://doi.org/10.1523/JNEUROSCI.4170-14.2015
Pierrot-Deseilligny E (1996) Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol 48:489–517. https://doi.org/10.1016/0301-0082(96)00002-0
Petersen NT, Pyndt HS, Nielsen JB (2003) Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res 152:1–16. https://doi.org/10.1007/s00221-003-1537-y
Rathelot JA, Strick PL (2009) Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106:918–923. https://doi.org/10.1073/pnas.0808362106
Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y (2004) Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol 92:3142–3147. https://doi.org/10.1152/jn.00342.2004
Semmler JG, Kornatz KW, Meyer FG, Enoka RM (2006) Diminished task-related adjustments of common inputs to hand muscle motor neurons in older adults. Exp Brain Res 172:507–518. https://doi.org/10.1007/s00221-006-0367-0
Souza VH, Vieira TM, Peres ASC, Garcia MAC, Vargas CD, Baffa O (2017) Effect of TMS coil orientation on the spatial distribution of motor evoked potentials in an intrinsic hand muscle. Biomed Tech (Berl). https://doi.org/10.1515/bmt-2016-0240
Tazoe T, Perez MA (2017) Cortical and reticular contributions to human precision and power grip. J Physiol 595:2715–2730. https://doi.org/10.1113/JP273679
Vaughan CW, Kirkwood PA (1997) Evidence from motoneurone synchronization for disynaptic pathways in the control of inspiratory motoneurones in the cat. J Physiol 503(Pt 3):673–689
Acknowledgements
The study was supported by a Grant from the Elsass Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svane, C., Forman, C.R., Nielsen, J.B. et al. Characterization of corticospinal activation of finger motor neurons during precision and power grip in humans. Exp Brain Res 236, 745–753 (2018). https://doi.org/10.1007/s00221-018-5171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5171-0