Abstract
Movement complexity is known to increase reaction time (RT). More recently, transcranial magnetic stimulation (TMS) of the motor cortex has revealed that movement complexity can alter corticospinal excitability. However, the impact of a sequential addition of movement components on corticospinal excitability during the preparatory phase of a simple RT task is unknown. Thus, the purpose of this study was to examine how motor evoked potentials (MEPs) in the premotor period were affected by the complexity of a movement in a simple RT paradigm. Participants (n = 12) completed ballistic movements with their dominant arm, in which they directed a robotic handle to one, two or three targets (32 trials per condition). TMS was delivered prior to movement at 0, 70, 80 or 90% of each participant’s mean premotor RT, at the stimulator intensity which yielded a triceps brachii MEP of ~ 10% the maximal M-wave. As expected, premotor RT slowed with increasing task complexity. Although background electromyographic activity (EMG) of the triceps brachii during the preparation phase did not differ among conditions, MEP amplitude increased with movement complexity (i.e., MEPs were greater for the 2- and 3-movement conditions, compared to the 1-movement condition at 80% of premotor RT). We propose the lengthened RTs could be due in part to less suppression of particular motor circuits, while other circuitry is responsible for the increased MEPs. This study demonstrates that, prior to movement, corticospinal excitability increases as a consequence of movement complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When presented with a sensory cue, the time prior to movement onset, or the reaction time (RT) interval, reflects the time required by sensory and motor processes to prepare the desired movement (Salinas et al. 2014; Wong et al. 2015). Notably, these processes, and consequently the RT, can be altered by the complexity of the planned movement. In their seminal experiment, Henry and Rogers (1960) demonstrated that RT to an imperative stimulus increased with the number of movement components. The authors interpreted the lengthened RTs as a complexity effect, related to an increased amount of time required to program and retrieve a motor response from memory. This work provided the foundation for numerous experimental paradigms and theories of the interplay between complexity and movement preparation. While Henry and Rogers (1960) attributed increases in RT simply to the addition of extra movements, other studies have proposed that RT is influenced strongly by more specific elements of the multi-component movement. For example, it has been proposed that the increase in RT is driven by programming related to the sequence (Klapp 1995) and the timing (Maslovat et al. 2014) of the movement components.
There have been several models proposed to describe the sensory and motor processes involved in the preparation for movement (Nazir and Jacobs 1991; Carpenter and Williams 1995; Hanes and Schall 1996). For example, in the “cell assembly model” (Wickens et al. 1994), a group of cortical motor neurons related to performance of the desired action (known as a “cell assembly”) are brought closer to threshold, then held, in preparation of the motor response. Regardless of the specific model, preparatory processes will influence the excitability of the motor system. In humans, this can be quantified using transcranial magnetic stimulation (TMS) of the motor cortex as the motor evoked potential (MEP) recorded in the electromyogram (EMG) of a target muscle allows for the assessment of corticospinal excitability (e.g., Kobayashi and Pascual-Leone 2003; Bestmann and Duque 2016).
Similar to RT, corticospinal excitability appears to increase with movement complexity. Flament and colleagues (1993) demonstrated that MEPs were larger during a variety of static gripping (complex) tasks compared to an isolated finger abduction (simple) movement. Additionally, Abbruzzese and colleagues (1996) demonstrated that MEPs increased with complexity during both real and imagined sequential finger movements. Under multiple movement conditions (observation, imagery and execution), Roosink and Zijdewind (2010) delivered TMS “just before or at the start of the movement of the index finger” and found that MEPs were larger for a complex compared to a simple finger sequence. Notably, an increase in MEP size with movement complexity is not a universal finding. Recently, Kennefick and colleagues (2016) reported that MEPs were smaller in the 75 ms prior to movement onset for a complex three button-press sequence with a timing component, compared to a simple single button-press movement without a timing component.
To date, the relationship between movement complexity and MEP size has not been examined in the premotor portion of the RT interval (prior to the burst of EMG activity that initiates movement) with an experiment that features two key elements of the simple RT paradigm of Henry and Rogers (1960). That is, no experiment has both: (1) increased complexity by adding new components to the same initial movement; and (2) made the required movement pattern known prior to the imperative stimulus so that the entire sequence can be pre-programmed. Such a paradigm would be insightful because the MEP would reveal how task complexity affects corticospinal excitability during movement preparation. Hence, the purpose of the current study was to measure MEP size in the preparation phase of a simple RT task with a step-wise increase in complexity based on the number of movement components. It was hypothesized that both premotor RT and MEP amplitude would increase with complexity; i.e., the number of movement components.
Materials and Methods
Participants
Twelve (eight female, age range 20–38) healthy, self-declared right-handed participants with normal or corrected-to-normal vision, and no history of neurological, sensory, or motor disorders participated in this study. Testing of each participant took place in a single session and required approximately 1.5 h to complete. The study was conducted in accordance with ethical guidelines and was approved by the University of British Columbia’s Clinical Research Ethics Board (CREB approval: H17-00796) and conformed to the guidelines of the Declaration of Helsinki, except for registration in a database.
Experimental setup
Participants sat in front of the KINARM End-Point Lab (BKIN Technologies Ltd., Kingston, Canada) and grasped the handle of the right manipulandum, linked to robotic motors, with their right hand. All arm movements were performed with the participant’s hand hidden from view by the reflective surface of the KINARM. Participants saw a virtual representation of their hand; i.e., a white circle (visual radius: 0.5 cm) that moved in tandem with the handle of the manipulandum. Triceps brachii surface EMG data were recorded via adhesive Ag-AgCl electrodes (10 mm diameter, Cleartrace; ConMed, Utica, NY), with the active lead positioned over the muscle belly and the reference over the distal tendon. Data were recorded using a 16-bit A/D converter (CED Power 1401-3; Cambridge Electronic Design Ltd, Cambridge, UK) and Spike2 software (version 7.10; Cambridge Electronic Design). Signals were sampled at 2000 Hz, amplified (× 100) and bandpass filtered (16–1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design Ltd., Cambridge, UK).
Task details
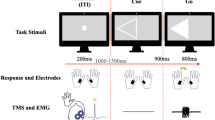
Participants were informed the experiment was a simple RT task consisting of 96 trials of a ballistic arm movement in the horizontal plane that involved one, two or three components (according to the number of targets presented; see Fig. 1). Importantly, the initial movement in each of the three movement conditions was directed to the same first target, requiring activation of the elbow extensors. At the start of each trial, the target(s) appeared on the visual display to inform participants of the required movement pattern for the upcoming task. To initiate the trial, participants had to place their virtual hand within the home position, represented by a red circle (visual radius: 1 cm). Following a random foreperiod (1000–3000 ms), the home position marker turned green (imperative stimulus), signaling the participant to initiate the movement to the first target. For the 1-movement condition, the participant was instructed to move anteriorly (straight ahead) and terminate at the target. For the 2-movement condition, the participant was to hit the first target then perform a change in direction back and to the right and terminate their movement at the second target. The 3-movement condition involved another change in direction after the second target, which required an anterior (straight ahead) movement to reach the final target. Accurate movements were encouraged but it was made clear that it was most important to react as quickly as possible to the imperative stimulus and complete the movement pattern without correcting for missed targets. Accuracy was not measured, and no trials were removed due to missed targets during the ballistic movement.
Visual representation of the behavioural task implemented with the KINARM system. Adapted from Kennefick et al. 2018
Stimulation details
To determine the EMG response to simultaneous activation of the entire triceps brachii motoneuron pool of the responding arm, electrical stimulation was applied to the right brachial plexus to evoke the maximal compound muscle action potential (Mmax). Single stimuli were delivered by a constant-current electrical stimulation (DS7AH; Digitimer Ltd, Welwyn Garden City, UK) at a pulse duration of 200 µs and continuously variable voltage between 100 and 400 V. The cathode and anode (adhesive Ag-AgCl electrodes; Cleartrace) were placed over the supraclavicular fossa and acromion, respectively. Stimuli were delivered as the participant held the robotic handle at the home position and prepared as if to move. Current was increased gradually with successive stimuli until the M-wave reached a plateau (Mmax). Once a plateau was established, an additional two stimuli were delivered at that current to establish a mean Mmax value (25.2 ± 3.8 mV across all participants; average stimulator output was 120 ± 66 mV).
To elicit a MEP from triceps brachii in the responding arm, TMS was applied to the motor cortex using a circular coil (13.5 cm outer diameter) attached to a Magstim 2002 stimulator (Magstim, Dyfed, UK). The coil was held over the vertex of the skull, with the handle pointing straight back (Martin et al. 2008; McNeil et al. 2009; Yacyshyn et al. 2018). Prior to stimulation, the vertex was found in each participant by marking the intersection of the midpoints between the nasion and the inion, and the left and right preauricular points with a dry erase marker. Stimulator output was gradually increased until the elicited MEP amplitude remained consistent at ~ 10% Mmax. Average stimulator output for testing trials was 80 ± 13%.
Experimental procedures
Prior to the 96 trials of the main protocol, participants completed practice blocks of 10 trials for each condition. Practice blocks were done in ascending order (1-, then 2-, then 3-movements) and used to establish the mean premotor RT for each participant. Premotor RT was defined as the time between the imperative stimulus and the point when raw EMG associated with the voluntary movement (i.e., onset of triceps brachii EMG activity) increased from baseline (Kennefick et al. 2014, 2016). The mean value for each condition was calculated after excluding the fastest and slowest trials, leaving eight trials per condition. Trials of the main protocol were identical to those of the practice blocks, with the exception that TMS was presented at four relative time points following the imperative stimulus (0, 70, 80 and 90% of premotor RT for that participant and movement condition). Relative rather than absolute time points were selected because RTs differ among participants (and within participants based on the number of movements) so the delivery of TMS at absolute time points would not measure the same preparatory processes across participants and levels of complexity. The 96 trials of the main protocol were separated into four blocks of 24. Each block included eight trials for each condition (i.e. two trials at each of the four TMS stimulation points for the 1-, 2-, and 3-movement conditions). Representative EMG traces of the triceps brachii at 0% premotor RT for each of the three movement conditions are shown for a single participant in Fig. 2.
Representative EMG traces of the triceps brachii from a single participant in the 1-, 2-, and 3-movement conditions. The shaded box highlights the MEP. In all trials, the TMS pulse was delivered at 0% RT (i.e., at the time of the imperative stimulus), represented by the left edge of the shaded box. Time to voluntary EMG onset (premotor RT) is indicated in each condition by an arrow
Data and statistical analyses
After each trial of the practice blocks, premotor RT (time between the imperative stimulus and EMG onset) was measured manually in Spike2. All other measures were analyzed offline using Signal software (version 6.03, Cambridge Electronic Design). The amplitude of the Mmax and MEPs were measured between the initial deflection from the baseline to the second crossing of the horizontal axis (Martin et al. 2006). Voluntary EMG measures included the root mean square (RMS) of the signal 100 ms prior to the TMS pulse (EMGBACKGROUND). Onset of voluntary EMG was also used to measure the premotor RT of the test trials. Trials with a value for EMGBACKGROUND or MEP amplitude that was < 2 standard deviations from the overall mean for each individual were removed from the analysis. Overall, data from 89 of the 1152 total trials (7.7%) were removed from the analysis, 27 (9.4%) and 28 (9.7%) of which were removed from stimulation points delivered as EMG onset approached (i.e., 80% and 90% of premotor RT, respectively). On a per participant basis, 7.4 ± 5.3 of the 96 total trials were removed. Data were analyzed using repeated-measures (RM) analyses of variance (ANOVA). All analyses were conducted using SPSS version 23 (SPSS Inc., Chicago, IL, USA). MEP amplitudes were first expressed as a percentage of the value at 0% premotor RT (Table 1) and assessed for violations of normality using the Shapiro–Wilk’s test on the studentized residuals. These MEP amplitudes were significantly non-normal (p > 0.05) so were subsequently subjected to a log transform (Stuart-Hamilton 2007). For all RM-ANOVAs, Greenhouse-Geiser Epsilon was used to adjust degrees of freedom for violations of sphericity, when necessary. Unless otherwise stated, all RM-ANOVAs were run as 3 (movement complexity) × 4 (TMS time) comparisons. Dunnett’s pairwise multiple comparison post hoc tests were administered to determine the locus of the differences. Differences with a p < 0.05 were considered significant. Data are presented as mean ± SD in the text and ± SE in the figures.
Results
Premotor reaction time
To determine if the complexity manipulation led to differences in premotor RT, a one-way RM ANOVA was performed on data collected during the practice trials. The analysis (Fig. 3a) revealed a significant main effect of complexity (F(2,22) = 21.1, p < 0.001, ƞp2 = 0.657). The post-hoc analysis indicated that premotor RT was faster for the 1-movement condition compared to both the 2-movement (M = − 33.2 ms, 95% CI [− 55.2, − 11.3], p = 0.004) and 3-movement conditions (M = − 47.8 ms, 95% CI [− 73.3, − 22.3], p = 0.002). Premotor RT did not increase from the 2- to 3-movement condition (M = − 14.6 ms, 95% CI [− 29.5, 0.387], p = 0.057). To ensure the complexity effect on premotor RT remained during the testing protocol, these data were subjected to the same analysis. This also revealed (Fig. 3b) a main effect of complexity (F(2,22) = 24.2, p < 0.001, ƞp2 = 0.687). Similar to the practice trials, post-hoc analysis showed that premotor RT was faster for the 1- than 2-movement condition (M = − 23.5 ms, 95% CI [− 38.4, − 8.50], p = 0.003), and the 1- than 3-movement condition (M = − 31.6 ms, 95% CI [− 46.4, − 16.9], p < 0.001), but not the 2- than 3-movement condition (M = − 8.16 ms, 95% CI [− 17.7, 1.34], p = 0.102). Test premotor RTs were slower than practice premotor RTs; however, this was expected as TMS delays RT when applied close to an expected voluntary response (Pascual-Leone et al. 1992; Ziemann et al. 1997; Leocani et al. 2000).
Boxplot of the mean premotor RT across the 3 complexity levels in both the practice (a) and testing (b) sessions. Box boundaries represent the 25th and 75th percentiles, solid horizontal lines represent medians, the small squares within the box represent means, and error bars represent the farthest outliers within 1.5 times the inter-quartile range from the box boundaries. The asterisk denotes a significantly slower RT compared to the 1-movement condition
MEP amplitude
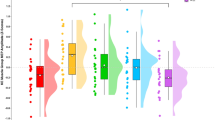
The analysis of log-transformed MEP data revealed a main effect of complexity (F(2,22) = 4.54, p = 0.022) and time (F(3,33) = 34.2, p < 0.001) as well as an interaction between complexity and time (F(6,66) = 2.55, p = 0.028). Therefore, the effect of complexity was assessed at each time point (% premotor RT) and the effect of time was assessed separately for each condition. Post-hoc tests revealed that MEP amplitude was larger for the 3-movement than the 1-movement condition (M = 0.133 mV, 95% CI [0.223, 0.0435], p = 0.008), as well as the 2-movement compared to the 1-movement condition at 80% premotor RT, (M = 0.132 mV, 95% CI [0.216, 0.0471], p = 0.006) (Fig. 4). Furthermore, there was a trend for an increase in MEP amplitude between the 2-movement and 1-movement conditions at 90% premotor RT; however, this comparison did not reach conventional levels of significance. With respect to the time effect, the post-hoc analysis revealed that MEP amplitudes increased from baseline (0% premotor RT) to 90% premotor RT in the 1-movement condition, from baseline to 80% and 90% premotor RT in the 2-movement condition, and finally from baseline to 70%, 80%, and 90% premotor RT in the 3-movement condition. For ease of comparison to the existing literature, normalized MEPs (expressed as a percentage of 0% premotor RT) were also analyzed and are presented in Table 1. The analysis failed to reveal an interaction (p = 0.064) but indicated main effects of complexity (F(2,22) = 3.65, p = 0.043) and time (F(3,33) = 24.6, p < 0.001).
Log transformed MEP amplitudes in each movement condition. MEP amplitudes were greater in both the 2- and 3-movement conditions compared to the 1-movement condition at 80% premotor RT (†). Corticospinal excitability increased from baseline (0% premotor RT) in the 1-movement condition (*) at 90% premotor RT, from baseline in the 2-movement condition (**) at 80% and 90% premotor RT, and from baseline in the 3-movement condition (***) at 70%, 80%, and 90% premotor RT
Background EMG
Background EMG during the premotor period was compared among the three movement conditions in the 100 ms prior to the TMS stimulus (Fig. 5). This analysis revealed there were neither main effects of complexity (F(1.24, 13.6) = 1.67, p = 0.223) or time (F(1.22,13.5) = 3.99, p = 0.060), nor an interaction (F(1.89, 20.8) = 1.66, p = 0.144).
Discussion
The purpose of this study was to examine how step-wise manipulation of response complexity in a simple RT task affects MEP size during the preparation phase of movement production. As anticipated (e.g., Henry and Rogers 1960; Klapp 1995), premotor RT was slower for the 2- and 3-movement conditions compared to the 1-movement condition (Fig. 3), which confirmed the task was made more complex by the inclusion of additional movement components. The major finding was that corticospinal excitability increased with complexity (Fig. 4), without either an effect of complexity or time on the level of background EMG prior to movement (Fig. 5). Movement preparation processes are distinct from initiation processes (Haith et al. 2016), thus highlighting the importance of independently probing how the preparation of movements varying in complexity affects the excitability of the motor pathway (i.e., the amplitude of the MEP).
The size of the MEP is sensitive to cognitive processes such as decision making (Hadar et al. 2016) and motor imagery (Lebon et al. 2018). Therefore, it is reasonable to assume that greater cognitive processing in the preparation of complex movements could contribute to an increase in corticospinal excitability. If this increase in cognitive activity brought more motor neurons to threshold, the level of background EMG in the preparation phase prior to movement should increase with complexity. This would undermine the comparison of MEPs across complexities because MEP size is influenced strongly by voluntary drive (e.g., Taylor et al. 1997), meaning an increase in background EMG could explain an increase in the MEP with complexity. However, this was not the case in the current study as the level of background EMG in the 100 ms prior to TMS was not different among the three movement complexities (Fig. 5). Hence, the increase in MEP size with complexity during the RT interval indicates the involvement of cognitive processes that raise corticospinal excitability, without prematurely discharging additional motor neurons.
Neural activation models have suggested that movement preparation can be considered in terms of an increase of activity in the motor pathway that is held below a certain initiation threshold (Wickens et al. 1994; Hanes and Schall 1996). Premotor RT can, therefore, be thought of as the time required to raise neural activation from a preparatory state to a level beyond threshold. As a simple RT paradigm involves the execution of a singular response known to the participant prior to the imperative stimulus, it is the only type of RT paradigm that allows participants to fully prepare for the upcoming response. Thus, ideal movement preparation involves raising activity levels in the motor pathway as close to threshold as possible (Wickens et al. 1994), which can be inherently difficult due to sensory, cellular and/or motor noise in the pathway (Faisal et al. 2008). Preparatory process can alternatively be considered in terms of the dynamics of preparation. Studies in nonhuman primates (see Churchland et al. 2006) have demonstrated that the reduction in neuronal firing rate variability between target onset and movement onset is indicative of the completeness of the dynamic progress of motor preparation. In this model, shorter RTs are associated with more complete motor preparation, with longer RTs reflecting incomplete preparatory processing. Incomplete processing can be evaluated by disrupting preparation with subthreshold microstimulation. This stimulation lengthens RT, which has been suggested to represent the additional time required to recover the preparatory state (Churchland and Shenoy 2007).
Nevertheless, Harris and Wolpert (1998) have noted that larger motor commands require larger neural activity, thus producing greater noise. Inevitably, a certain amount of noise is present within the pathway, which requires a restriction of the level of activation to ensure random noise does not cause the premature release of a movement (Carlsen et al. 2012). It has been suggested that an inhibitory braking mechanism suppresses the tendency to initiate a movement (Prut and Fetz 1999; Duque et al. 2017); however, recent studies indicate that a general braking mechanism is unlikely. Using a Go/NoGo task in mice, Hasegawa and colleagues (2017) found that preparation for an intended movement was characterized by the selective suppression of certain motor circuits but that “build-up neurons”, or a specific set of neurons in the motor cortex, increase their neuronal activity during the preparation phase of movement. Similarly, Hannah and colleagues (2018) demonstrated in humans that a specific set of excitatory inputs to corticospinal neurons (responsible for late I-waves) are suppressed during motor preparation, while others remain unaffected. Furthermore, Greenhouse and colleagues (2015a, b) used TMS in humans to demonstrate that, during preparation, response selection is facilitated by the inhibition of response representations, including those which are task irrelevant. Such a mechanism would facilitate response preparation by reducing noise within the system. The results of the current study agree with this interpretation, as the simplest movement condition had the fastest premotor RT but lower corticospinal excitability (MEP amplitude) compared to the multi-movement tasks, which had slower premotor RTs.
Direct comparison of the relationship between MEP size and movement complexity observed in the current study to that of previous studies is limited by differences in the task (RT vs. non-RT), the complexity manipulation (additional components vs. discrete movements) and the timing of TMS (before vs. during movement). However, the increase in MEP size with complexity supports the findings of most (e.g., Flament et al. 1993; Abbruzzese et al. 1996) but not all (Kennefick et al. 2016) related studies. The disparity of our data with those of Kennefick and colleagues (2016) is likely caused by key methodological differences. In the current study, the participant was instructed to react as quickly as possible to the imperative stimulus for all movement conditions. There was no constraint on the timing of any movement component and the focus was on movement speed rather than accuracy. In the previous study (Kennefick et al. 2016), there were timing constraints on the duration of each movement (button press) as well as the interval between movements for the complex task. Accuracy was monitored, and trials were discarded if they included a timing error that exceeded the tolerance level. It appears that the inclusion of a timing structure or an emphasis on accuracy influences the interaction between movement complexity and modulation of corticospinal excitability during movement preparation.
The elicited MEPs in the current study are largely reflective of information being transmitted down the corticospinal tract to target muscles (see Di Lazzaro and Ziemann 2013 for a review) during the preparation phase of movement production. While the corticospinal tract is responsible for a broad cortical modulation of motoneuron output (Lemon and Griffiths 2005), subcortical processes have also been shown to be captured in MEPs, potentially via the reticulospinal tract (Fisher et al. 2012). Furthermore, premotor areas have direct and indirect (via motor cortex) connections with spinal motoneurons that are capable of influencing movement (Dum and Strick 2002). Thus, these previous findings demonstrate that corticospinal excitability is not entirely driven by motor cortical processes, and in the context of the current study, the increase of the MEP with complexity is likely to include action selection processes originating from premotor areas.
Conclusion
The current study addressed methodological limitations in other studies to demonstrate that corticospinal excitability prior to movement onset increases with the complexity of the planned task. The increase in MEP amplitude occurred without greater background EMG, which suggests that increased voluntary drive is very unlikely to be a contributing factor. These results suggest that, in line with recent literature, suppression of corticospinal excitability is inversely related to premotor RT.
References
Abbruzzese G, Trompetto C, Schieppati M (1996) The excitability of the human motor cortex increases during execution and mental imagination of sequential but not repetitive finger movements. Exp Brain Res 111:465–472
Bestmann S, Duque J (2016) Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist 22:392–405. https://doi.org/10.1177/1073858415592594
Carlsen AN, Maslovat D, Franks IM (2012) Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol 123:21–33
Carpenter RHS, Williams MLL (1995) Neural computation of log likelihood in control of saccadic eye-movements. Nature 377:59–62. https://doi.org/10.1038/377059a0
Churchland MM, Shenoy KV (2007) Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol 97:348–359. https://doi.org/10.1152/jn.00808.2006
Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV (2006) Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci 26:3697–3712. https://doi.org/10.1523/jneurosci.3762-05.2006
Di Lazzaro V, Ziemann U (2013) The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circ 7:18. https://doi.org/10.3389/fncir.2013.00018
Dum RP, Strick PL (2002) Motor areas in the frontal lobe of the primate. Physiol Behav 77:677–682. https://doi.org/10.1016/S0031-9384(02)00929-0
Duque J, Greenhouse I, Labruna L, Ivry RB (2017) Physiological markers of motor inhibition during human behavior. Trends Neurosci 40:219–236. https://doi.org/10.1016/j.tins.2017.02.006
Faisal AA, Selen LPJ, Wolpert DM (2008) Noise in the nervous system. Nat Rev Neurosci 9:292–303. https://doi.org/10.1038/nrn2258
Fisher KM, Zaaimi B, Baker SN (2012) Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol 590:4045–4060. https://doi.org/10.1113/jphysiol.2011.226209
Flament D, Goldsmith P, Buckley CJ, Lemon RN (1993) Task dependence of responses in 1st dorsal interosseusmuscle to magnetic brain-stimulation in man. J Physiol 464:361–378
Greenhouse I, Saks D, Hoang T, Ivry RB (2015a) Inhibition during response preparation is sensitive to response complexity. J Neurophysiol 113:2792–2800. https://doi.org/10.1152/jn.00999.2014
Greenhouse I, Sias A, Labruna L, Ivry RB (2015b) Nonspecific inhibition of the motor system during response preparation. J Neurosci 35:10675–10684. https://doi.org/10.1523/jneurosci.1436-15.2015
Hadar AA, Rowe P, Di Costa S, Jones A, Yarrow K (2016) Motor-evoked potentials reveal a motor-cortical readout of evidence accumulation for sensorimotor decisions. Psychophysiology 53:1721–1731. https://doi.org/10.1111/psyp.12737
Haith AM, Pakpoor J, Krakauer JW (2016) Independence of movement preparation and movement initiation. J Neurosci 36:3007–3015. https://doi.org/10.1523/jneurosci.3245-15.2016
Hanes DP, Schall JD (1996) Neural control of voluntary movement initiation. Science 274:427–430. https://doi.org/10.1126/science.274.5286.427
Hannah R, Cavanagh SE, Tremblay S, Simeoni S, Rothwell JC (2018) Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J Neurosci 38:1264–1276. https://doi.org/10.1523/jneurosci.2869-17.2017
Harris CM, Wolpert DM (1998) Signal-dependent noise determines motor planning. Nature 394:780–784. https://doi.org/10.1038/29528
Hasegawa M, Majima K, Itokazu T, Maki T, Albrecht UR, Castner N, Izumo M, Sohya K, Sato TK, Kamitani Y, Sato TR (2017) Selective suppression of local circuits during movement preparation in the mouse motor cortex. Cell Rep 18:2676–2686. https://doi.org/10.1016/j.celrep.2017.02.043
Henry FM, Rogers DE (1960) Increased response latency for complicated movements and a memory drum theory of neuromotor reaction. Res Quart 31:448–458
Kennefick M, Maslovat D, Carlsen AN (2014) The time course of corticospinal excitability during a simple reaction time task. PLoS ONE 9:e113563–e113563. https://doi.org/10.1371/journal.pone.0113563
Kennefick M, Maslovat D, Chua R, Carlsen AN (2016) Corticospinal excitability is reduced in a simple reaction time task requiring complex timing. Brain Res 1642:319–326. https://doi.org/10.1016/j.brainres.2016.04.006
Kennefick M, Wright AD, Smirl JD, van Donkelaar P (2018) Anticipatory postural adjustments as a function of response complexity in simple reaction time tasks. Neurosci Lett 684:1–5. https://doi.org/10.1016/j.neulet.2018.06.058
Klapp ST (1995) Motor response programming during simple and choice-reaction time -the role of practice. J Exp Psychol Hum Percept Perform 21:1015–1027. https://doi.org/10.1037//0096-1523.21.5.1015
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2:145–156. https://doi.org/10.1016/S1474-4422(03)00321-1
Lebon F, Ruffino C, Greenhouse I, Labruna L, Ivry RB, Papaxanthis C (2018) The neural specificity of movement preparation during actual and imagined movements. Cereb Cortex. https://doi.org/10.1093/cercor/bhx350
Lemon RN, Griffiths J (2005) Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32:261–279. https://doi.org/10.1002/mus.20333
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123:1161–1173. https://doi.org/10.1093/brain/123.6.1161
Martin PG, Gandevia SC, Taylor JL (2006) Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95:3512–3518. https://doi.org/10.1152/jn.01230.2005
Martin PG, Weerakkody N, Gandevia SC, Taylor JL (2008) Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586:1277–1289. https://doi.org/10.1113/jphysiol.2007.140426
Maslovat D, Klapp ST, Jagacinski RJ, Franks IM (2014) Control of response timing occurs during the simple reaction time interval but on-line for choice reaction time. J Exp Psychol Hum Percept Perform. https://doi.org/10.1037/a0037522
McNeil CJ, Martin PG, Gandevia SC, Taylor JL (2009) The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587:5601–5612. https://doi.org/10.1113/jphysiol.2009.180968
Nazir TA, Jacobs AM (1991) The effects of target discriminability and retinal eccentricity on saccade latencies—an analysis in terms of variable-criterion theory. Psychol Res 53:281–289. https://doi.org/10.1007/bf00920481
Pascual-Leone A, Brasil-Neto JP, Valls-Solé J, Cohen LG, Hallett M (1992) Simple reaction time to focal transcranial magnetic stimulation. Comparison with reaction time to acoustic, visual and somatosensory stimuli. Brain 115(1):109–122
Prut Y, Fetz EE (1999) Primate spinal interneurons show pre-movement instructed delay activity. Nature 401:590–594. https://doi.org/10.1038/44145
Roosink M, Zijdewind I (2010) Corticospinal excitability during observation and imagery of simple and complex hand tasks: implications for motor rehabilitation. Behav Brain Res 213:35–41. https://doi.org/10.1016/j.bbr.2010.04.027
Salinas E, Scerra VE, Hauser CK, Costello MG, Stanford TR (2014) Decoupling speed and accuracy in an urgent decision-making task reveals multiple contributions to their trade-off. Front Neurosci 8:85. https://doi.org/10.3389/fnins.2014.00085
Stuart-Hamilton I (2007) Dictionary of psychological testing, assessment and treatment: second edition. Jessica Kingsley Publishers, London
Taylor JL, Allen GM, Butler JE, Gandevia SC (1997) Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res 117:472–478
Wickens J, Hyland B, Anson G (1994) Cortical cell assemblies: a possible mechanism for motor programs. J Mot Behav 26:66–82. https://doi.org/10.1080/00222895.1994.9941663
Wong AL, Haith AM, Krakauer JW (2015) Motor planning. Neuroscientist 21:385–398. https://doi.org/10.1177/1073858414541484
Yacyshyn AF, Nettleton J, McNeil CJ (2018) The effects of sex and motoneuron pool on central fatigue. Med Sci Sports Exerc 50:1061–1069. https://doi.org/10.1249/mss.0000000000001536
Ziemann U, Tergau F, Netz J, Hömberg V (1997) Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain Res 744:32–40. https://doi.org/10.1016/S0006-8993(96)01062-1
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (DG 04412-2017 and DG 435912-2013) and the Canada Foundation for Innovation/British Columbia Knowledge Development Fund (30979 and 32260).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kennefick, M., Burma, J.S., van Donkelaar, P. et al. Corticospinal excitability is enhanced while preparing for complex movements. Exp Brain Res 237, 829–837 (2019). https://doi.org/10.1007/s00221-018-05464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-05464-0