Abstract
The role of point of application of perturbation in the anticipatory (APAs) and compensatory (CPAs) postural control was studied. Twelve healthy participants stood on a sliding board (that was either locked and as such motionless or unlocked and as such free to move in the anterior–posterior direction). The body perturbations were applied either to the shoulders (by a pendulum impact) or the feet (by the movement of the sliding board). Electromyographic activity (EMG) of the trunk and lower extremity muscles was recorded. Latencies, integrals of EMG and muscle co-contraction (C) and reciprocal (R) activation indices were calculated and analyzed within the intervals typical for the APAs and CPAs. Higher EMG integrals were seen in the APAs phase when perturbation was applied to the shoulders. Reciprocal activation of muscles was seen in the APAs phase in the shoulders perturbation condition, while co-contraction was seen in the feet perturbation condition. Co-contraction was observed within the CPA phase in both experimental conditions. Higher C values were found in the feet perturbation condition in the CPA phase. The results suggest that different motor control strategies are employed by the central nervous system when encounter perturbations of similar magnitude but applied to different parts of the body. The outcome highlights the importance of investigation of the role of the point of application of the perturbation.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Many tasks that humans perform in their daily life involve body perturbations. To control vertical posture when dealing with body perturbations, the central nervous system (CNS) uses two main types of adjustments in the activity of trunk and leg muscles. Anticipatory postural adjustments (APAs) control the position of the center of mass of the body (Toussaint et al. 1997) by activating the trunk and leg muscles prior to a forthcoming body perturbation (Massion 1992), while compensatory postural adjustments (CPAs) serve as a mechanism of restoration of the position of the COM after perturbation has already occurred (Alexandrov et al. 2005).
Previous literature suggests that while both, APAs and CPAs participate in postural control, their contribution depends on many factors. Thus, APAs magnitude depends on the direction and magnitude of the forthcoming perturbation (Aruin and Latash 1995a, 1996), body stability (Aruin et al. 1998) as well as factors affecting perception of stability, such as fear of falling (Adkin et al. 2002). It was also suggested that when body stability is reduced (e.g. standing on an unstable board), the CNS considers APAs as an additional perturbation and suppresses APAs to minimize a probability of losing balance (Aruin et al. 1998). On the other hand, when the body instability is not related to the altered base of support (BOS) as it happens, for example when standing wearing rollerskates, APAs may not be affected in terms of the onset time of muscle activation (Shiratori and Latash 2000). The CPAs magnitude depends on a number of factors including the direction and the magnitude of the perturbation (Henry et al. 2001; Park et al. 2004), dimensions of the BOS (Dimitrova et al. 2004), predictability of the perturbation (Burleigh and Horak 1996) and involvement of a secondary task such as holding an object in the hands (Bateni et al. 2004; Chen et al. 2016). In addition, because APAs and CPAs are interrelated, CPAs are the only mechanism used by CNS to restore balance when APAs are not available; on the other hand, the presence of strong APAs is associated with significantly smaller CPAs (Santos et al. 2010a).
Generally, the CNS employs two main muscle activation patterns for maintaining equilibrium: co-contraction and reciprocal activation (Mochizuki et al. 2004). Co-contraction of muscles increases joint stiffness and provides body stability (Latash et al. 1995; Aruin and Almeida 1997; Massion et al. 1999) while reciprocal activation of muscles is considered as efficiency and energy saving strategy of control of vertical posture (Latash et al. 1995; van der Fits et al. 1998). Moreover, previous literature demonstrated that both the co-contraction and reciprocal activation of muscles could be utilized simultaneously or sequentially to encounter perturbations (Santos et al. 2010a; Lee et al. 2015). Moreover, increased co-contraction of distal muscles and reciprocal activation of trunk muscles was observed while standing on an unstable board (Slijper and Latash 2004).
The majority of studies of standing postural control were conducted using external perturbations induced by movements of the surface on which the subjects stood (Park et al. 2004; Hirata et al. 2010). Studies using such a paradigm revealed that humans use ankle and hip strategies to restore balance after a perturbation (Nashner and Cordo 1981; Horak and Nashner 1986; Hwang et al. 2009). Other paradigms involve studying the effects of a perturbation induced by self-initiated perturbations such as lifting one leg (Toussaint et al. 1997; Hall et al. 2010), a unilateral (Strang and Berg 2007) of bilateral arm-raising maneuver (Aruin and Latash 1995a; Aruin 2003), release of the load from the extended arms (Aruin and Latash 1995b, 1996; Shiratori and Aruin 2007), pushing and pulling with the upper extremities while standing (Elble and Leffler 2000; Lee et al. 2015), or by an external perturbation involving pendulum impact applied to the shoulders (Santos and Aruin 2009; Santos et al. 2010a; Lee et al. 2015). The outcome of these studies provided important information about the organization of postural control. However, the observed variations in the EMG patterns during both APA and CPA phases of postural control reported in different studies could reflect not only the difference in the points of application of a perturbation but also variations in the perturbation magnitude (Aruin and Latash 1996) as well as the effect of other factors.
In the current study, we aim to investigate how the CNS adjusts motor commands, and, therefore, APAs and CPAs, to optimize postural stability in response to perturbations of a similar magnitude applied to different parts of the body. We hypothesized that the CNS uses adaptive strategies when perturbations are applied to either the shoulders or feet levels. We also hypothesized that a condition in which body perturbation is applied to the feet would be associated with decreased APA EMG activity as compared to a condition with perturbation applied to the shoulders. We, furthermore, hypothesized that higher co-contraction and reciprocal activation of muscles would be seen during the balance restoration phase in the condition with perturbations applied to the feet.
Method
Twelve young volunteers (7 males, 5 females, age = 26.42 ± 1.32 years, height = 1.68 ± 0.01 m, mass = 64.80 ± 3.97 kg) participated in the experiment. All participants were free from any neuromuscular disorder that could affect control of vertical posture. The project was approved by the University of Illinois at Chicago Institutional Review Board, and all participants provided written informed consent before taking part in the experimental procedures.
Procedure and instrumentation
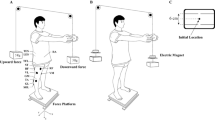
The participants were instructed to stand barefoot on a sliding board with their feet shoulder width apart and in parallel. Foot position was marked on top of the sliding board and reproduced across the trials. The sliding board (lengths 0.50 m, width 0.45 m and height 0.06 m) was made of two layers connected by a linear bearing system. The bearing system includes four pillow blocks, two alloy steel shafts and four shaft supports that allow the top layer to slide with very low friction; the shaft supports prevent the top layer from sliding off the base layer. The top layer made from steel was covered with a 0.005 m sheet of foamy material glued to the top layer to make it comfortable for a participant to stand barefoot on the board. The sliding board had a lock mechanism allowing the top layer to be free to slide in the anterior–posterior direction or remain stationary. The sliding board was positioned on top of a force platform (model OR-5, AMTI, USA). The participants were instructed to maintain a vertical posture while standing on the sliding board after being exposed to perturbations applied to the upper or lower body. First, the participants stood on a stationary sliding board and received a perturbation applied to their shoulders via a pendulum (Lee et al. 2015). Then, the participants stood on a sliding board the top layer of which was free to slide and received the perturbation generated by the movement of the sliding board.
Perturbations to the upper body consisted of unidirectional force applied by the pendulum on the shoulders of the participants. The pendulum was attached to the ceiling with initial angle of 30° and consisted of a height adjustable central rod with the distal end designed as two padded pieces positioned shoulder width apart and projected toward the participant. The central rod was adjusted to each participant’s shoulder height and the padded extension was adjusted to match the shoulder width. 5% of the individual’s body weight was attached to the pendulum next to its distal end. The participants were able to see the pendulum released by the experimenter at all times.
Perturbations to the lower body were delivered by the same experimenter positioned in front of the participants who pulled the rope attached to the top layer of the sliding board. The participants were able to see the pulling process at all times (Fig. 1).
Schematic representation of the experimental setup. a Perturbations are applied to the shoulders by the pendulum impact. Sliding board is locked. b Perturbations are applied to the feet by the movement of the top of the sliding board. 1 accelerometer attached to sternoclavicular joint, 2 accelerometer attached to the sliding board
Two accelerometers were used in the experiment. The first accelerometer (model 208CO3, PCB Piezotronics Inc, USA) attached to the participants’ sternoclavicular joint was used to determine the moment of the pendulum impact (T 0). The second accelerometer (model 208CO3) attached underneath the top layer of the sliding board was used to detect the moment of the sliding board movement (T 0). The perturbation magnitudes determined by acceleration signals were: 2.8 m/s2 when applied to the shoulder level and 2.5–3 m/s2 when applied to the lower body. The pre-determined acceleration was chosen according to a pilot study. The magnitude of the perturbation induced by the movement of the sliding board was monitored in real time using the accelerometer attached to the board; a trial with the acceleration outside of the selected interval was discarded and repeated.
Considering that the perturbations were symmetrical, electrical activity from the following muscles was recorded from the right side of the body: tibialis anterior (TA), medial gastrocnemius (MG), rectus femoris (RF), biceps femoris (BF), rectus abdominis (RA), and erector spinae (ES). Disposable electrodes (Red Dot 3 M, USA) were attached to the muscle bellies (distance between electrodes: 25 mm) after the skin was cleaned with alcohol swipes. The ground electrode was positioned on the lateral malleolus. EMG signals were band-pass filtered (10–500 Hz) and amplified (gain 2000) using the EMG system (Myopac, RUN Technologies, USA).
Five trials were collected in each condition. Each participant was given two practice trials prior the data collection for familiarization with the task. In the current study, the condition of perturbation to the shoulders is called shoulders condition, while the condition of perturbation to the feet is referred as feet condition. The order of experimental conditions was randomized.
Data analysis
The forces, moments of forces, EMG and accelerometer signals were digitalized with a 16-bit A/D card at 1000 Hz using LabView software (National Instruments, USA). MATLAB (MathWorks, USA) was used for data processing. EMG signals were high-pass filtered at 20 Hz, full-wave rectified, and low-pass filtered at 2 Hz (2nd order Butterworth). EMG onset time, timing of pendulum impact and timing of the start of the movement of the sliding board were estimated using the Teager–Kaiser (TKE) onset time detection method (Li et al. 2007).
EMG integrals were calculated for each individual muscle in relation to T 0 in three time intervals: (1) from −150 to +49 ms (anticipatory postural adjustment, ∫APA), (2) from +50 to +249 ms (compensatory postural adjustment, ∫CPA1) and (3) from +250 to +449 ms (compensatory postural adjustment, ∫CPA2). Baseline activities were calculated using a 200 ms time interval (−1000 to −800 ms). The EMG integrals during the APAs and CPAs phase were corrected by subtracting the baseline
Then, ∫APA, ∫CPA1 and ∫CPA2 for each muscle were normalized by the maximum magnitude of the integral in each phase across all experimental trials. As a result, all of the integrals within the three time intervals were converted into −1 to 1. In addition, C and R indices (C refers to co-contraction and R to reciprocal activation of muscles) were obtained for the APA, CPA1 and CPA2 in the three body segments including trunk, thigh and shank. The trunk segment was defined as a combination of RA and ES muscles, the thigh segment was defined as RF and BF muscles, and the shank segment was defined as TA and MG muscles [for more details see (Lee et al. 2015)]
Two C and R based models were used to demonstrate co-contraction and reciprocal activation of muscles serving the shank, thigh and trunk segments: (1) Sum of segments involving shank, thigh and trunk segments (combination model). (2) Muscles serving each body segment (individual model). For the combination model, C and R values were calculated using the sum of trunk, thigh, and shank body segments.
The center of pressure (COP) displacements in the anterior–posterior direction were calculated using equations described in the literature (Winter et al. 1996):
where M x is the moment in the sagittal plane, F z and F y are the vertical and the anterior–posterior components of the ground reaction force and d z is the distance from the origin of the force platform to the surface. When using the equation for COP calculation, we adjusted the d z to 0.098 m; this number reflected the sum of both, the distance from the origin of the force platform to the surface of the force platform (0.038 m) and the height of the sliding board (0.06 m). We calculated anticipatory COP displacements (COP APA) as the COP magnitude at T 0 and compensatory COP displacements (COP CPA) as the peak magnitudes after T 0.
Statistical analysis
Paired t test was used for evaluation of the acceleration of the two experimental perturbations applied at the shoulders and feet level. Repeated measures ANOVA was performed on the EMG onset time and EMG integrals of ∫APA, ∫CPA1 and ∫CPA2 for individual muscles using the experimental condition of the point of application of the perturbation as the within group factor (Shoulders and Feet). All five trials were used in the repeated measures ANOVA. C and R indices were compared for individual segments during each APA, CPA1 and CPA2 periods. If the segment had higher C value than R value, a follow-up repeated measures ANOVA was used to evaluate C rather than R, and vice versa. Post hoc analyses were done using Dunn–Sidak correction for multiple comparison adjustments. Critical value was set at α = 0.05. The effect size is reported using the Omega squared.
Results
Perturbation magnitude
The magnitudes of acceleration in the two experimental conditions were 2.86 ± 0.09 and 2.77 ± 0.06 m/s2 for the shoulders and feet perturbations, respectively. The difference was not significant (t = 1.10, p = 0.29).
EMG onset and integrals
In general, the EMGs were affected by the change in the experimental condition in terms of integrals rather than onset time. EMG onset time is presented in Table 1; the EMG onsets of the postural muscles are observed close to 50 ms before T 0, however, no significant difference was observed between the two experimental conditions.
In the APA phase, while the subjects received perturbations at the shoulders, ∫RF was 0.54 ± 0.05 compared to 0.18 ± 0.03 for experiments involving perturbations applied to the feet [F(1,11) = 33.69, p < 0.01, ω 2 = 0.75]. ∫RA was 0.46 ± 0.05 in the condition of a perturbation applied to the shoulders and 0.08 ± 0.03 in experiments with perturbations applied to the feet [F(1,11) = 38.83, p < 0.01, ω2 = 0.78]. In the CPA1 phase, most of postural muscles showed higher integrals in the feet condition than shoulders condition. It was 0.44 ± 0.06 and 0.68 ± 0.04 for TA in the shoulders and feet condition, respectively, [F(1,11) = 11.85, p < 0.01, ω 2 = 0.52]. However, ∫RF (not significant) and ∫RA were higher in the shoulders condition than feet condition: ∫RA was 0.47 ± 0.06 and 0.27 ± 0.06 for RA in shoulders and feet condition [F(1,11) = 5.095, p < 0.05, ω 2 = 0.32], respectively. In the CPA2 phase all the postural muscles showed higher muscle activities in the feet condition than shoulders condition (Table 2).
Co-contraction and reciprocal activation of muscles
Three segments combination model
Figure 2 shows C and R values calculated during the APA, CPA1 and CPA2 phases for the two experimental conditions. Notice the difference between the magnitude of C and R values. During the APA phase, higher R value than C value was observed in the shoulders perturbation condition (C = 1.26 ± 0.19, R = 1.43 ± 0.16), while higher C value than R value was observed in the feet perturbation condition (C = 0.90 ± 0.14, R = 0.28 ± 0.22). In the CPA1 phase, C value was 1.36 ± 0.17 and R value was 1.03 ± 0.17 in the shoulders condition, while C = 2.62 ± 0.21 and R = 0.33 ± 0.12 were seen in the feet condition. Follow-up repeated measures ANOVA showed that higher C value was found in the feet condition compared to shoulders condition [F(1,11) = 16.59, p < 0.01]. During the CPA2 phase C value was 0.77 ± 0.20 and R value was 0.57 ± 0.16 when the perturbation was applied to the shoulders, while C = 2.99 ± 0.24 and R = 0.96 ± 0.14 were seen in condition with the perturbation applied to the feet. Follow-up repeated measures ANOVA showed that higher C value was found in the feet condition compared to shoulders condition (F1,11) = 35.56, p < 0.01).
C and R values calculated during APA and CPA phases for the combination model as the sum of the shank, thigh and trunk segments for conditions with perturbations applied to the shoulders and to the feet. APA, CPA1 and CPA2 phases are shown on the horizontal axis; Integral values are shown on the vertical axis. Filled circle represents R value and square represents C value
Individual segment model
Figure 3 shows C and R values calculated during the APA, CPA1 and CPA2 phase, segment by segment for the two experimental conditions. In the APA phase, higher R values than C values were observed in all the segments for the shoulders condition, while lower R values than C values were observed in the feet condition. For the shank segment, it was 0.36 ± 0.08 and 0.58 ± 0.07 for C and R values, respectively, in the shoulders condition, and C = 0.36 ± 0.13, R = 0.28 ± 0.11 was observed in the feet condition. For the muscles of the thigh segment, C value was 0.53 ± 0.09 and R value was 0.56 ± 0.14 for in the shoulders condition, and C = 0.36 ± 0.06 and R = −0.01 ± 0.07 were seen in the feet condition. For the muscles of the trunk segment, C value was 0.45 ± 0.12 and R value was 0.46 ± 0.13 in the shoulders condition, and C = 0.38 ± 0.10, R = −0.14 ± 0.06 were recorded in the feet condition. During the CPA1 and CPA2 phases, all of the segments showed higher C value than R value in both experimental conditions.
C and R values calculated during APA and CPA phases for the individual segment model including the shank, thigh and trunk segments for conditions with perturbations applied to the shoulders and to the feet. APA, CPA1 and CPA2 phases are shown on the horizontal axis; Integral values are shown on the vertical axis. Filled circle represents R value and square represents C value
Further repeated measures ANOVA analysis revealed that the point of application of perturbation affects C value for the three individual segments. In the CPA1 phase, C value for the shank segment was 0.50 ± 0.08 for the shoulders condition compared to 0.99 ± 0.13 for the feet condition [F(1,11) = 9.66, p = 0.01]. For the thigh segment, C values were 0.58 ± 0.06 and 0.82 ± 0.08 for the shoulders and feet conditions, respectively, [F(1,11) = 6.28, p = 0.03]. For the trunk segment, C values were 0.61 ± 0.11 for the shoulders condition, and 0.76 ± 0.12 for the feet condition [F(1,11) = 0.58, p = 0.46]. During CPA2 phase, C value for the shank segment was 0.28 ± 0.08 for the shoulders condition compared to 0.98 ± 0.10 for the feet condition [F(1,11) = 25.66, p < 0.01]. For the thigh segment, C values were 0.41 ± 0.06 and 1.00 ± 0.09 for the shoulders and feet conditions, respectively, [F(1,11) = 18.15, p < 0.01]. For the trunk segment, C values were 0.28 ± 0.07 for the shoulders condition, and 0.92 ± 0.09 for the feet condition [F(1,11) = 17.37, p < 0.01].
COP displacement
No condition effect was observed in the COPAPA displacement: it was −0.008 ± 0.004 m for the shoulders condition and −0.005 ± 0.002 m for the feet condition [F(1,11) = 0.38, p = 0.55]. However, significant difference was found in the peak of COP displacement between two experimental conditions. COPCPA was −0.045 ± 0.003 m for the shoulders condition and −0.02 ± 0.003 for the feet condition [F(1,11) = 62.30, p < 0.01].
Discussion
The current study investigated the role of the point of application of perturbation on the generation of the anticipatory and compensatory postural adjustments. Anticipatory and compensatory activities of the trunk and leg muscles were observed in both experimental conditions. As such, our first hypothesis that the CNS adapts to the perturbations applied to either the shoulders or feet was supported. Significant larger muscle activities were observed in the APAs phase when perturbations were applied at the shoulders thus the second hypothesis was supported. Moreover, higher co-contraction of muscles during both CPA1 and CPA2 was seen in the experiments with the perturbation applied to the feet compared to shoulder perturbations. This study outcome supports our third hypothesis that higher C–R indices would be seen during the balance restoration phase in the condition with perturbations applied to the feet. It is important to mention that in both experimental conditions the participants were exposed to perturbations of a similar magnitude. As such, the observed differences in muscle activities are attributed solely to the manipulation of the point of application of the perturbation.
Perturbation applied to the shoulders
It is known that distal to proximal sequence of anticipatory activation of muscles was observed in experiments with self-initiated perturbations (Cordo and Nashner 1982) and pendulum induced external perturbations (Santos et al. 2010b, 2010a). Similar order of muscle activation was seen in the current study when the perturbation was applied to the shoulders and the direction and magnitude of the perturbation was known to the participants. In addition, as reported in Table 1, EMG onsets were seen from −62 to −33 ms; this outcome is in line with prior literature reporting that the CNS is capable of generating APAs prior to either self-initiated (Shiratori and Latash 2001; Kanekar et al. 2008) or externally induced (Aruin et al. 2001; Santos and Aruin 2009) perturbations. Moreover, the CNS might not consider the pendulum-induced perturbation as the balance-challenging task, particularly in our case, otherwise much early APAs (between −250 m and −100 ms) would be observed (Shiratori and Latash 2001; Yiou et al. 2009; Santos et al. 2010b).
During a predictable perturbation to the shoulders, ventral muscles were activated whereas the dorsal muscles were inhibited; as a result, a reciprocal activation of muscles was observed before T 0 in both the combination and individual segments models. Similar pattern of muscles activation was described in experiments involving self-initiated (Aruin and Latash 1995a) and external (Santos et al. 2010a) perturbations. Reciprocal activation of muscles is considered as energy saving and as such more efficient strategy of control of vertical posture (Latash et al. 1995). On the other hand, co-contraction was observed in both the CPA1 and CPA2 phases in the condition when perturbations were applied to shoulders. It appears that by co-activating both ventral and dorsal muscles, the CNS increases the stiffness of the joints which provides better body stability to keep balance (Aruin and Almeida 1997; Lee et al. 2015). The fact that the reciprocal activation and co-contraction were seen when dealing with the shoulder perturbation (that could be considered as not an imminent balance challenging task), suggests that the CNS is capable of using different strategies to control posture. Notably, despite the inhibition of the dorsal muscles in the APAs phase (seen in the EMG integrals), the magnitudes of the integrals of the ventral muscles were relatively similar in the APAs and CPA1 phases. In addition, the magnitudes of EMG integrals were smaller in the CPA2 phase as compared to the APA and CPA1 phases. Such a finding suggests that the CNS was able to activate muscles prior T 0 (APAs) and immediately after perturbation (CPA1). As a result, little compensation was needed during the second compensatory phase (CPA2)(Cordo and Nashner 1982; Santos et al. 2010a).
Perturbation applied to the feet
APAs are associated with the activation or inhibition of trunk and leg muscles prior a forthcoming perturbation. The change in APAs could be quantified with EMG onset/time and EMG magnitude. The APA timing was relatively similar, ranging from −70 to −36 ms prior to T0 in both conditions. The small variation in the timing of the APAs suggests that the CNS was able to adapt its commands taking into consideration changes in the point of application of perturbation. It looks like the CNS generates APAs in a time frame close to the perturbation onset (T 0) depending on the magnitude, rather than the point of the application of perturbation. In addition, previous studies suggested that the CNS would activate muscles similarly in terms of EMG onset time when performing voluntary motor task and standing on a unsteady surface (roller-skates) because of the effective area of the BOS in such a condition is not altered (Shiratori and Latash 2000). On the other hand, the distal to proximal patterns of muscles activation were not seen in the experiments with the perturbation applied to the feet. One possible explanation could be that the condition with perturbations applied to the feet is a more balance-challenging task compared to the perturbation applied to the shoulders.
Previous studies suggested that in case of postural instability (Aruin et al. 1998) or fear of falling (Adkin et al. 2002), the CNS would scale down APAs in conditions involving challenging postural requirements. Similarly, when the perturbation was applied to the feet (thus affecting the ability of the subjects to apply forces to the ground needed to counteract the forthcoming perturbation), the CNS reduced muscle activity to minimize further inter-segmental movements that might additionally destabilize posture (Shiratori and Aruin 2004). Thus, one can suggest that the CNS deliberately utilized such a motor control strategy when a person encounters perturbations the point of application of which is below the COM position.
In the CPAs phase, the CNS utilized co-contraction of ventral and dorsal muscles when perturbations were applied to the feet. In addition, muscles were co-contracted more in that condition than in the shoulders condition. It was suggested in the literature that CPAs rather than APAs would be significantly affected when a perturbation is applied to the feet, especially when it involves a horizontal movement (Memari et al. 2014). It is worth to note that in the CPA1 phase, although the C value is larger in the feet condition than the shoulders condition, a lack of statistical significance between the two indices could be related to the observed higher muscle activities in the RA and BF muscles in the shoulders condition. It could be because muscles are active for a longer period of time when a subject encounters a perturbation applied to the shoulders. On the other hand, we observed a trend of increasing muscle activation, especially in the CPA1 and CPA2 phases in the feet condition. This indicates that when perturbations are applied to the point that is below the COM, the activation of muscles during APAs and CPA1 cannot be successfully minimized, thus a relatively prolonged and large compensation would be needed during the CPA2. Another explanation relates to a possibility that the CNS suppresses the APAs in the more unstable condition (Aruin et al. 1998) of standing on the sliding board and receiving perturbation applied to the feet.
It is worth noting that the COPAPA were not significantly different between two experimental conditions while COPCPA were statistically significant between the two experimental conditions. One possible explanation to this finding could be that the CNS adjusts posture in a manner of controlling COM (Massion 1992) based on multiple feedback gains (Park et al. 2004). In our study, the magnitudes of perturbation were relatively similar when applied to the shoulders and feet. As such, it is quite possible that the CNS did not vary the COPAPA displacement significantly in preparation for the two types of perturbations. However, after the perturbation, higher co-contraction of ventral and dorsal muscles in the feet condition helped to stabilize the body, and as a result, smaller COPCPA displacements were observed.
The study has some limitations. First, while the subjects stood with eyes open and were able to see the sources of perturbation, the information used to trigger anticipatory adjustments in both experimental conditions might be slightly different. Second, the current data set was draw only from young healthy individuals, thus limiting the applicability of the study outcome to older adults and individuals with impairments.
Conclusion
The results allow suggesting that when a point of application of a perturbation is above the COM, the CNS mainly employs activation of muscles during APAs and CPA1. When a point of application of a perturbation is below the COM, the CNS activates muscles during the CPA1 and CPA2. The outcome of the current study highlights the importance of considering the point of application of perturbations in postural control.
References
Adkin AL, Frank JS, Carpenter MG, Peysar GW (2002) Fear of falling modifies anticipatory postural control. Exp Brain Res 143:160–170. doi:10.1007/s00221-001-0974-8
Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S (2005) Feedback equilibrium control during human standing. Biol Cybern 93:309–322. doi:10.1007/s00422-005-0004-1
Aruin AS (2003) The effect of changes in the body configuration on anticipatory postural adjustments. Mot Control 7:264–277
Aruin A, Almeida GL (1997) A coactivation strategy in anticipatory postural adjustments in persons with Down syndrome. Mot Control 1:178–191
Aruin AS, Latash ML (1995a) Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103:323–332
Aruin AS, Latash ML (1995b) The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res 106:291–300
Aruin AS, Latash ML (1996) Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol 101:497–503
Aruin AS, Forrest WR, Latash ML (1998) Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol 109:350–359
Aruin AS, Shiratori T, Latash ML (2001) The role of action in postural preparation for loading and unloading in standing subjects. Exp Brain Res 138:458–466
Bateni H, Zecevic A, McIlroy WE, Maki BE (2004) Resolving conflicts in task demands during balance recovery: does holding an object inhibit compensatory grasping? Exp Brain Res 157:49–58. doi:10.1007/s00221-003-1815-8
Burleigh A, Horak F (1996) Influence of instruction, prediction, and afferent sensory information on the postural organization of step initiation. J Neurophysiol 75:1619–1628
Chen B, Lee YJ, Aruin AS (2016) Control of grip force and vertical posture while holding an object and being perturbed. Exp Brain Res 234:3193–3201. doi:10.1007/s00221-016-4717-2
Cordo PJ, Nashner LM (1982) Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47:287–302
Dimitrova D, Horak FB, Nutt JG (2004) Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol 91:489–501. doi:10.1152/jn.00094.2003
Elble RJ, Leffler K (2000) Pushing and pulling with the upper extremities while standing: the effects of mild Alzheimer dementia and Parkinson’s disease. Mov Disord 15:255–268
Hall LM, Brauer S, Horak F, Hodges PW (2010) Adaptive changes in anticipatory postural adjustments with novel and familiar postural supports. J Neurophysiol 103:968–976. doi:10.1152/jn.00479.2009
Henry SM, Fung J, Horak FB (2001) Effect of stance width on multidirectional postural responses. J Neurophysiol 85:559–570
Hirata RP, Arendt-Nielsen L, Graven-Nielsen T (2010) Experimental calf muscle pain attenuates the postural stability during quiet stance and perturbation. Clin Biomech (Bristol, Avon) 25:931–937. doi:10.1016/j.clinbiomech.2010.06.001
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55:1369–1381
Hwang S, Tae K, Sohn R, Kim J, Son J, Kim Y (2009) The balance recovery mechanisms against unexpected forward perturbation. Ann Biomed Eng 37:1629–1637. doi:10.1007/s10439-009-9717-y
Kanekar N, Santos MJ, Aruin AS (2008) Anticipatory postural control following fatigue of postural and focal muscles. Clin Neurophysiol 119:2304–2313. doi:10.1016/j.clinph.2008.06.015
Latash ML, Aruin AS, Neyman I, Nicholas JJ (1995) Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry 58:326–334
Lee YJ, Chen B, Aruin AS (2015) Older adults utilize less efficient postural control when performing pushing task. J Electromyogr Kinesiol 25:966–972. doi:10.1016/j.jelekin.2015.09.002
Li X, Zhou P, Aruin AS (2007) Teager–Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng 35:1532–1538. doi:10.1007/s10439-007-9320-z
Massion J (1992) Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38:35–56
Massion J, Ioffe M, Schmitz C, Viallet F, Gantcheva R (1999) Acquisition of anticipatory postural adjustments in a bimanual load-lifting task: normal and pathological aspects. Exp Brain Res 128:229–235
Memari S, Le Bozec S, Bouisset S (2014) Particular adaptations to potentially slippery surfaces: the effects of friction on consecutive postural adjustments (CPA). Neurosci Lett 561:24–29. doi:10.1016/j.neulet.2013.12.008
Mochizuki G, Ivanova TD, Garland SJ (2004) Postural muscle activity during bilateral and unilateral arm movements at different speeds. Exp Brain Res 155:352–361. doi:10.1007/s00221-003-1732-x
Nashner LM, Cordo PJ (1981) Relation of automatic postural responses and reaction-time voluntary movements of human leg muscles. Exp Brain Res 43:395–405
Park S, Horak FB, Kuo AD (2004) Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res 154:417–427. doi:10.1007/s00221-003-1674-3
Santos MJ, Aruin AS (2009) Effects of lateral perturbations and changing stance conditions on anticipatory postural adjustment. J Electromyogr Kinesiol 19:532–541. doi:10.1016/j.jelekin.2007.12.002
Santos MJ, Kanekar N, Aruin AS (2010a) The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol 20:388–397. doi:10.1016/j.jelekin.2009.06.006
Santos MJ, Kanekar N, Aruin AS (2010b) The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol 20:398–405. doi:10.1016/j.jelekin.2010.01.002
Shiratori T, Aruin AS (2004) Anticipatory postural adjustments associated with rotational perturbations while standing on fixed and free-rotating supports. Clin Neurophysiol 115:797–806. doi:10.1016/j.clinph.2003.11.015
Shiratori T, Aruin A (2007) Modulation of anticipatory postural adjustments associated with unloading perturbation: effect of characteristics of a motor action. Exp Brain Res 178:206–215. doi:10.1007/s00221-006-0725-y
Shiratori T, Latash M (2000) The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol 111:613–623
Shiratori T, Latash ML (2001) Anticipatory postural adjustments during load catching by standing subjects. Clin Neurophysiol 112:1250–1265
Slijper H, Latash ML (2004) The effects of muscle vibration on anticipatory postural adjustments. Brain Res 1015:57–72. doi:10.1016/j.brainres.2004.04.054
Strang AJ, Berg WP (2007) Fatigue-induced adaptive changes of anticipatory postural adjustments. Exp Brain Res 178:49–61. doi:10.1007/s00221-006-0710-5
Toussaint HM, Commissaris DA, Beek PJ (1997) Anticipatory postural adjustments in the back and leg lift. Med Sci Sports Exerc 29:1216–1224
van der Fits IB, Klip AW, van Eykern LA, Hadders-Algra M (1998) Postural adjustments accompanying fast pointing movements in standing, sitting and lying adults. Exp Brain Res 120:202–216
Winter DA, Prince F, Frank JS, Powell C, Zabjek KF (1996) Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75:2334–2343
Yiou E, Mezaour M, Le Bozec S (2009) Anticipatory postural adjustments and focal performance during bilateral forward-reach task under different stance conditions. Mot Control 13:142–160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Lee, YJ. & Aruin, A.S. Role of point of application of perturbation in control of vertical posture. Exp Brain Res 235, 3449–3457 (2017). https://doi.org/10.1007/s00221-017-5069-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5069-2