Abstract
Injury-induced neuropathic pain remains a serious clinical problem. Recent studies indicate that bone marrow stromal cells (BMSCs) effectively attenuate chronic neuropathic pain in animal models. Here, we examined the therapeutic effect of intrathecal administration of BMSCs isolated from young (1-month-old) rats on pain hypersensitivity induced by tibial nerve injury. Cerebrospinal fluid (CSF) was collected and analyzed to examine the effect of BMSC administration on the expression of 67 soluble factors in CSF. A sustained remission in injury-induced mechanical hyperalgesia was observed in BMSC-treated rats but not in control animals. Engrafted BMSCs were observed in spinal cords and dorsal root ganglia at 5 weeks after cell injection. Injury significantly decreased the levels of six soluble factors in CSF: intercellular adhesion molecule 1 (ICAM-1), interleukin-1β (IL-1β), IL-10, hepatocyte growth factor (HGF), Nope protein, and neurogenic locus notch homolog protein 1 (Notch-1). Intrathecal BMSCs significantly attenuated the injury-induced reduction of ICAM-1, IL-1β, HGF, IL-10, and Nope. This study adds to evidence supporting the use of intrathecal BMSCs in pain control and shows that this effect is accompanied by the reversal of injury-induced reduction of multiple CSF soluble factors. Our findings suggest that these soluble factors may be potential targets for treating chronic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain, especially neuropathic pain, is an important public health challenge. Despite years of research yielding numerous insights into underlying pathogenic mechanisms (Basbaum et al. 2009; von Hehn et al. 2012), there have been few new therapies for chronic neuropathic pain. Implantation of bone marrow stromal cells (BMSCs) has recently emerged as a potential new avenue for long-term therapy to control refractory pain conditions (Chen et al. 2015). BMSCs are populations of skeletal progenitor cells residing in the postnatal BM with high expansion potential, stable genetic phenotype, and immunosuppressive properties. The ability of BMSCs to proliferate in vitro allows generation of a large number of cells from a small initial sample within a relatively short time.
Several types of cell-based therapy have been examined as a potential treatment for a number of painful conditions, with varied methodologies and results. Neural stem cells (NSCs) have been used in multiple studies (Franchi et al. 2012; Xu et al. 2013). The results are promising, but NSCs are more difficult to acquire and culture, and may not be suitable as a translational therapy. BMSCs are especially appealing, as they can be harvested easily with minimal injury to the patient (Pittenger et al. 1999) and administered to the same patient in an autologous fashion, obviating the need for immunosuppressive drugs (Jacobs et al. 2013; Lee et al. 2015; Mao 2010). The data of BMSCs-induced analgesia in animal models have shown important applicative implications (Chen et al. 2015; Hosseini et al. 2015; Shibata et al. 2008; Xu et al. 2013; Yu et al. 2015), although failure to affect behavioral markers of pain has also been reported (Schafer et al. 2014) and some routes of administration are not appropriate for translation, such as intracerebral injection (Siniscalco et al. 2010). Intrathecal injection is an attractive approach, as this is an easily performed procedure that can be repeated if needed for continued relief.
Convergent evidence has demonstrated the roles of cytokines (including chemotactic cytokines or chemokines) in the mechanisms of neuropathic pain (Clark et al. 2013). Cytokines are low molecular weight polypeptides that can be secreted into the cerebrospinal fluid (CSF) during the course of inflammatory and injury responses triggered by various types of damage to the nervous system. Their differential expression has been associated with diverse neuropathic pain conditions (Clark et al. 2013), functioning as either pro- or anti-nociceptive regulators (Meirelles Lda et al. 2009). BMSCs have been shown to exert therapeutic effects through the release of cytokines and trophic factors and the modulation of inflammatory cell function (Liang et al. 2014). Previous studies of intrathecal application of BMSCs have shown efficacy in alleviating pain behavior and have examined a small number of potential factors mediating the analgesic effect (Chen et al. 2015). Therefore, the aim of this study was to better understand the role of soluble factors underlying BMSCs-mediated analgesia by examining the effects of an intrathecal implantation of BMSCs on rat pain behavior induced by peripheral nerve injury and expression levels of 67 different pro- and anti-nociceptive factors in cerebrospinal fluid (CSF).
Results
Characterization of the cultured BMSCs
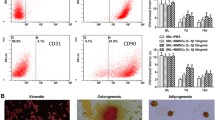
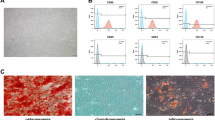
Adherent colonies of BMSCs formed within several days of plating, with the expected fibroblast-like morphology. The identification of expanded BMSCs was confirmed by analysis of BMSCs-related positive and negative marker expression. The results revealed that the majority of the cultured BMSCs 10–12 days after isolation were positive for the BMSCs markers Thy-1, CD105, Stro1, and CD29 and showed little positive staining for the hematopoietic markers CD45 and CD11b (Fig. 1a–i). These markers were used because they are well recognized as criteria for definitive identification of BMSCs (Dominici et al. 2006). In addition, the cells were capable of differentiation into osteocytes and adipocytes when exposed to the appropriate differentiation media (data not shown). Because these characterizations defined the cultured cells as mostly composed of BMSCs, we did not pursue more rigorous quantification, for instance by immunofluorescent flow cytometry. Additional confirmation of Thy-1 and Stro1 expression in BMSCs was obtained through immunoblotting of Thy-1 and Stro1 on BMSC lysates, while both were undetectable in DRG tissue from adult rat (Fig. 1j–l).
Characterization of cultured BMSCs. Typical morphology of rat BMSCs cultures at 3 days in vitro (DIV) (a inset showing enlarged view) and 10 DIV (b, c) after isolation and plating. The culture, composed of cells that are mostly spindle-shaped with thin cell processes, approached confluence around 10 DIV (b, c). Immunofluorescent cytochemistry of cultured BMSCs at 10 DIV after plating. Most of the cultured BMSCs are positive for the markers Thy-1 (d), CD105 (e), Stro1 (f), and CD29 (g). Only few of the cells are positive for the hematopoietic markers CD45 (h) or CD11b (i). Scale bars 200 μm for a and b, 100 μm for the inset of a, c, h, and i, 50 μm for d–g. Immunoblotting of Thy-1 (j) and Stro1 (k) in the top panels and GAPDH (bottom panels) on the lysates of the BMSCs (j lane 1 and 2; k lane 2). The postnatal day 1 mouse brain (P1 mbrain) homogenate (j lane 3 and 4) and cell lysate from MC7 cells (k lane 1) were used as the positive controls of Thy-1 and Stro1, respectively. Note: both Stro1 (k lane 3) and Thy-1 (l) were undetectable in adult rat DRG tissue (rDRG). Arrows point to the expected size bands for Thy-1 (~27 kDa) and GAPDH (~38 kDa) as a loading control
In our previous study, poor engraftment, short-term survival, and tumor-like growth of transplanted BMSCs that had undergone multiple passages were recognized as major limitations in the setting of injection of BMSCs into dorsal root ganglia (DRGs) (Yu et al. 2015), possibly related to cellular aging and loss of immunosuppressive and cytokine productive properties during ex vivo expansion (Bara et al. 2014). These problems are minimized by transplantation of BMSCs within a short period of ex vivo expansion (Chen et al. 2015; Guo et al. 2011). Therefore, BMSCs cultured for 10–12 days after isolation, and without passage were used for injection in the present study.
Effects of intrathecal administration of BMSCs on pain hypersensitivity
We induced neuropathic pain by TNI, which resulted in mechanical hyperalgesia and allodynia in all rats. To test whether BMSCs would alleviate pain behavior development, we injected BMSCs into the CSF by lumbar puncture at the time of TNI surgery. BMSCs expanded ex vivo for 10–12 days after isolation were used for injection (single injection of average 2.5 × 105 cells in 10 μl per rat). In threshold mechanical testing with von Frey fibers, both the TNI + PBS and TNI + BMSCs groups showed a marked decrease in mean withdrawal threshold compared to the non-operated naïve control animals (Fig. 2a, b). This hypersensitivity developed as soon as three days after injury and continued for the duration of the study. A difference in mean withdrawal threshold between the TNI + PBS and TNI + BMSCs groups was observed at the final four-week timepoint, but not at earlier timepoints. Using fully noxious mechanical stimulation by pin, hyperalgesia behavior developed in both the TNI + PBS and TNI + BMSCs groups after injury (Fig. 2c, d), compared to the non-operated naïve controls. The development of hyperalgesia reached its peak by three days after injury and continued for the duration of the study. The hyperalgesia response to pin stimulation, however, was substantially alleviated in animals receiving CSF injection of BMSCs, and this anti-nociceptive effect remained significant at each tested timepoint thereafter, indicating sustained relief of pain hypersensitivity after intrathecal MSC transplantation.
Neuropathic pain remission induced by a single intrathecal injection of BMSCs in TNI rats. Left panels show the time course for the group averages of sensitivity to innocuous punctate mechanical stimulation (a), and hyperalgesia behavior after touching with a pin (c) before and after a single intrathecal injection of BMSCs at the time of TNI. Right panels show averaged area under the curve (AUC) calculated for the time period following TNI for von Frey (b) and Pin (d). Results are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001 for comparison of naïve to TNI + PBS and TNI + PBS to TNI + BMSCs (a, c), and for comparisons of AUC between groups as indicated (b, d). All data are expressed as the mean ± SEM. BL baseline
Destination of transplanted BMSCs
To determine the destination of injected BMSCs, the bilateral L3–L5 DRGs and the corresponding segments of lumbar spinal cord were collected and examined for BMSCs markers. IHC on spinal cord sections, harvested 35 days after TNI combined with CSF injection of either saline or BMSCs, exhibited depleted IB4 staining in the medial portion of the superficial dorsal horn, the site of central termination of tibial nerve (Decosterd and Woolf 2000), indicating successful establishment of TNI (Fig. 3a). In BMSCs-injected rats, Thy-1 and Stro1 positive cells were observed close to the pia mater around the surface of the spinal cord in the L3 to L5 region. Engrafted BMSCs were also observed deeper within the spinal cord white matter and were especially abundant on the dorsal aspect of the spinal cord, although some implantation was also observed on the ventral aspect of the cord, particularly ipsilateral to nerve injury. Distribution was preferential to the side ipsilateral to injury (cf. Fig. 3b, c), but BMSCs were also visible on the contralateral and ventral aspects of the spinal cords (Fig. 3d). No anatomically atypical growths were observed. Thy-1 and Stro1 immunopositive BMSCs were colocalized with Hoechst staining for nuclei, indicating the presence of intact cells, not debris from dead or dying cells. No Stro1/Thy-1 staining was observed in any spinal cords from saline-injected animals (data not shown). The staining patterns of Thy-1 and Stro1 were comparable in all areas of the cord.
Long-term survival of BMSCs in lumbar spinal cord following intrathecal injection in TNI rats. a Depleted IB4 staining (red, arrowheads) in the medial portion of the ipsilateral superficial dorsal horn, the site of central termination of tibial nerve sensory neurons, after TNI. (White dashed line outlines gray matter in a cross section of lumbar spinal cord, and white matter is pseudocolored blue.) Scale bar 200 μm. Representative areas in which the engrafted BMSCs are found by IHC are shown in magnified IHC images in b–d. BMSCs immunopositive for Thy-1 (b, c) or Stro1 (d) immunopositive BMSCs (green) were detected in the lumbar SC at 35 days after injection and engrafted BMSCs are colocalized with Hoechst staining for nuclei (b). b1 and c1 are enlarged images of b and c, respectively. Scale bars 50 μm for b–d. Note that Thy-1-positive (b, b1) and Stro1-labeled (d) BMSCs are colocalized with Hoechst staining for nuclei (color figure online)
Thy-1- and Stro1-labeled BMSCs were also detected in the ipsilateral lumbar DRGs 35 days after cell transplantation (Fig. 4), suggesting long-term survival of BMSCs in DRGs. The Thy-1- and Stro1-positive BMSCs were found clustered on the surface of the DRG and just subcapsular at the proximal pole of the DRG and were also observed engrafting within the DRG among the neuronal somata. DRG sections from animals injected with PBS showed no staining for either Thy-1 or Stro1. Together, our data indicate that, although the number of observed BMSCs may be much less than the number that was initially injected, intrathecal transplantation can lead to engraftment of BMSCs within the lumbar spinal cord and DRGs. An exact count of implanted cells was not performed because their small size and attenuated morphology would make it unreliable to positively distinguish each cell in a section.
Long-term survival of BMSCs in lumbar DRGs following intrathecal injection in TNI rats. A representative image of Thy-1-labeled BMSCs (green) detected by IHC and counter-stained with Hoechst (blue) in an ipsilateral L4 DRG at 35 days after intrathecal BMSCs injection (a). Minimal Thy-1 immunostaining is found in DRG following intrathecal PBS injection (b). Arrows point directions to roots. Right panels c are magnified images showing a cluster of engrafted BMSCs within DRG body (C1), near where the roots join the DRG body (C2), and showing differentiated BMSCs engrafted among the neuronal somata (C3, C4). A representative image of Stro1-labeled BMSCs (red) detected by IHC and counter-stained with Hoechst (blue) in an ipsilateral L4 DRG at 35 days after intrathecal BMSCs injection (d). Right panels (D1, D2) are magnified insets showing a cluster of engrafted BMSCs within DRG body. Arrowheads point to the selected BMSCs that are Hoechst staining for nuclei. Scale bars 100 μm for a, b and d, 50 μm for c, D1 and D2 (color figure online)
Effect of BMSCs transplantation on the profiles of soluble factors in CSF
Next, we analyzed whether the inhibition of neuropathic hyperalgesia by intrathecal administration of BMSCs is associated with alteration of soluble factors in the CSF and whether the injury-associated alterations of cytokine expression could be minimized by intrathecal BMSCs therapy. Antibody array analysis was performed to simultaneously measure the levels of 67 cytokines and other soluble factors in CSF samples taken 35 days after TNI combined with CSF injection of either saline or BMSCs (Fig. 5), as well as non-operated naïve rats (n = 5 rats per group). Comparison of the Naïve and TNI + PBS groups showed that injury significantly decreased the levels of six soluble factors, specifically ICAM-1 (p < 0.0001), IL-1β (p < 0.0001), IL-10 (p < 0.0001), HGF (p < 0.0001), Nope (p < 0.0001), and Notch-1 (p < 0.0001) (Fig. 5). Comparison of the TNI + PBS and TNI + BMSCs groups showed a significant increase and restoration of five of the six soluble factors that were depressed by injury (ICAM-1, p < 0.0001; IL-1β, p < 0.0001; HGF, p < 0.0001; IL-10, p < 0.05; Nope, p < 0.001). No effect of BMSCs implantation was observed for the remaining biomarkers in the panel. Since the aim of this study is to identify the effect of intrathecal BMSCs on chronic neuropathic pain behavior and the associated CSF cytokine profile, we chose the 5-week timepoint for cytokine analysis rather than at an earlier stage of treatment. A full list of soluble factors measured and their levels under each condition is presented in Supplementary Table 1.
Significantly altered soluble factors in CSF. a Bar chart shows 6 of 67 soluble factors, detected by Multiplex ELISA antibody array, that are differentially expressed in CSF. b The values of significantly altered soluble factors in CSF samples. Results are mean ± SEM. ****p < 0.0001 for comparisons of each cytokine between Naïve and TNI + PBS, and # p < 0.05, ### p < 0.001 and #### p < 0.0001 for comparisons of each cytokine between TNI + PBS and TNI + BMSCs; derived from two-way ANOVA followed by post hoc multicity comparisons. NS indicates no significance. Cytokine abbreviations: ICAM-1 intercellular adhesion molecule 1, IL-1β interleukin-1β, IL-10 interleukin-10, HGF hepatocyte growth factor, Nope (Igdcc4) immunoglobulin superfamily DCC subclass member 4, Notch-1 neurogenic locus notch homolog protein 1
Discussion
Our findings add to the mounting evidence that unmodified freshly isolated BMSCs (expanded during a short period of ex vivo culture) are a potentially effective treatment for chronic pain secondary to peripheral nerve injury. The threshold for withdrawing from innocuous touch was not greatly reversed as measured by von Frey testing. An effect of BMSC implantation on withdrawal threshold was observed at only a single timepoint, and this effect was small, but significant. The attenuation of hyperalgesic response to pinprick is an important finding, as this behavior has been associated with conditioned avoidance in nerve-injured rats (Wu et al. 2010), which validates it as a representation of the affective and motivational aspect of pain, unlike von Frey testing which is based upon simple reflex activity. Thus, BMSCs transplantation reduces the aversive aspect of mechanical hyperalgesia in neuropathic pain. In this initial report, we tested the use of BMSC transplantation in a preventive sequence. Although treating established pain is a more common goal and will be pursued in future studies, there are numerous occasions in which nerve injury is expected or possible during surgery, as in nerve transfer procedures and amputations, and even in routine procedures such as intercostal incisions and herniorrhaphy. A treatment with preventive efficacy could reduce the risk of painful neuropathy in these situations.
The initial effect of injury on CSF soluble factors was revealed by comparing levels in naïve animals to those in animals subjected to TNI but with only saline injected into the CSF. This showed that injury depressed the levels of six different soluble factors at 5 weeks after TNI. IL-10 is an anti-inflammatory signaling peptide with analgesic efficacy in neuropathic pain (Ledeboer et al. 2007; Milligan et al. 2006). Therefore, decreased IL-10 after TNI may contribute to hyperalgesia in the neuropathic state. IL-10 has a significant impact on a number of neuroimmune conditions through the regulation of other cytokines (Kwilasz et al. 2015). Adeno-associated virus-mediated overexpression of IL-10 in the intrathecal space has been shown to be effective in treating painful conditions in a variety of contexts, including in large animals (Pleticha et al. 2015). The mitogenic cytokine HGF has been found to have analgesic properties. Specifically, gene transfer of HGF into the muscle of the thigh provides analgesia in neuropathic pain caused by chronic constriction injury of the sciatic nerve (Tsuchihara et al. 2009), and a clinical trial has been initiated exploring gene therapy with HGF for treatment of pain secondary to diabetic neuropathy (Kessler et al. 2015). This trial found significant pain reduction in a plurality of patients without the need for additional medications. There is currently a Phase 3 clinical trial in process for this therapy (https://clinicaltrials.gov/ct2/show/NCT02427464). Thus, reduced expression of HGF may be a causal factor contributing to pain after nerve injury. ICAM-1, an inducible cell surface glycoprotein, is involved with cellular adhesion, including the diapedesis during inflammation, and ICAM-1 is also critical for BMSCs-mediated immunosuppression (Ren et al. 2010). It has been implicated in intrinsic mechanisms of analgesia in peripheral tissues (Machelska et al. 2002), possibly through the chemotactic attraction of endogenous-opioid-containing leukocytes to sites of neuroinflammation (Labuz et al. 2009). A definitive CNS role in pain genesis, however, remains undefined. IL-1β is a proinflammatory cytokine that contributes to pain in inflammatory models and has implication in peripheral nerve injury-induced neuropathic pain mechanisms in rodents (Clark et al. 2013). In humans, patients with a range of painful peripheral neuropathies exhibit enhanced IL-1β levels in their CSF (Backonja et al. 2008). Thus, our observation of depressed CSF IL-1β levels after tibial nerve injury is unexpected. Animals with neuropathic pain have increased IL-1β expression in the damaged nerve tissues, but CSF IL-1β alteration in tibial nerve injury has not been reported in the literature (Clark et al. 2013). IL-1β has been shown to play a role in sensory neuroregeneration (Temporin et al. 2008) and to be necessary for the regeneration of neural pathways in the medium- to long-term after injury (Nadeau et al. 2011). Increased IL-1β secretion could therefore play a role in the restoration of normal signaling pathways that are altered after injury, although further work would be required to determine this. The function of Nope, also known as immunoglobulin superfamily DCC subclass member 4 (Igdcc4), has been minimally explored. There is evidence that it plays a role in CNS development (Salbaum and Kappen 2000), but participation in pain processes has not previously been examined. Initial reports suggest that Notch-1 may play a signaling role in the development of neuropathic pain (Sun et al. 2012; Xie et al. 2015), suggesting that the action of BMSCs on Notch-1 expression is not a factor in producing analgesia in this model. Our array did not examine TGF-β1, but we note that others have recently identified this anti-inflammatory cytokine as a product from intrathecal BMSCs that contributes to analgesia against neuropathic pain (Chen et al. 2015).
Our data do not establish the source of the factors that are increased in the presence of CSF-delivered BMSCs. We infer that the effect is due to the actions of living BMSCs because surviving, intact BMSCs were identified histologically. Furthermore, the anti-hyperalgesic effect of MSC injection was sustained at least 5 weeks, which indicates an ongoing source of these soluble factors. BMSCs and related adipose-derived MSCs have been shown to directly produce a wide variety of cytokines, including IL-1β, IL-10 (Aggarwal and Pittenger 2005; Kucerova et al. 2010), and BMSCs similarly produce HGF (Crisostomo et al. 2008). However, there is growing recognition of the ability of BMSCs to regulate immune function of other cells. For instance, BMSCs modulate immune cells to result in greater production of IL-10 (Aggarwal and Pittenger 2005; Batten et al. 2006; Beyth et al. 2005; Wehner et al. 2009). Similarly, exposure to BMSCs can regulate the expression of ICAM-1 and Notch-1 in other tissues (Abdel Aziz et al. 2013; Del Papa et al. 2013; Wen et al. 2014). Thus, the source of the increased factors we observed in CSF could be either the BMSCs or other cell types influenced by the BMSCs.
Remarkably, the influence of implantation of BMSCs in the subarachnoid space upon CSF cytokine levels was entirely restricted to those soluble factors affected by TNI, and all but Notch-1 showed a reversal of the nerve injury effect in animals treated with BMSCs. It is possible that the anti-hyperalgesic effect of the BMSCs may be due to the recovery of CSF levels of IL-10 and HGF. The CSF was collected at the level of the cisterna magna, while the implanted BMSCs were at lumbar levels, so our findings may underestimate the influence of the BMSCs at the lumbar levels associated with the TNI.
Our injection site for administration of BMSCs into the CSF was in the midline of the lumbar subarachnoid space. However, implantation was preferentially ipsilateral with the nerve injury and at the segmental level of the injured neurons. A homing process has been identified by which BMSCs implantation favors sites of injury or inflammation (Mackenzie and Flake 2001; Xu et al. 2013). This may in part account for the efficacy of a small volume of injected BMSCs despite the substantial comparative volume of CSF and the extensive surface area it contacts. Although implantation was observed on both the dorsal and ventral aspects of the spinal cord, the distribution of analgesic factors in the CSF milieu should allow all cells to impact sensory function. The mechanism of this ipsilateral implantation is unknown, but the initial distribution within the CSF can explain access of the BMSCs to the DRG as the cephalad pole of the DRG is bathed in the CSF in continuity with the injection site. We noted a preferential collection at proximal pole of the DRG, which is surrounded by a layer of CSF in the so-called lateral recess of the subarachnoid space (Himango and Low 1971), which is where intrathecal debris is known to naturally collect (Brierley and Field 1948).
Future work building off the reported studies may include attempts at better understanding the role that individual cytokines and other peptides may play in the development and maintenance of neuropathic pain, both individually and in concert with one another. This could be achieved by using emerging genetic technologies to selectively increase or reduce the levels of peptides in the nervous system. The translational value of CSF injection is due to its relative ease of performance in clinical subjects and wide dispersion of potential segmental targets. Therapeutically, the partial effect of BMSCs observed in this study could be enhanced by genetic modification, in which cells would be engineered to constitutively or conditionally secrete high concentrations of one or more peptides to increase their analgesic effect (Meyerrose et al. 2010; Tran and Damaser 2015).
Methods
Animals
Male Sprague–Dawley rats acquired from Charles River Laboratories (Wilmington, MA, USA) at approximately four weeks old were housed individually on standard sawdust bedding in a room with a constantly maintained temperature (22 ± 0.5 °C) and relative humidity (60 ± 15%) and an alternating 12-h light–dark cycle. Animals were given free access to food (Purina laboratory rodent chow) and filtered municipal tap water throughout the experiments. All procedures were performed with the approval of the Zablocki VA Medical Center and Medical College of Wisconsin Institutional Animal Care and Use Committees, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Bone marrow stromal cell (BMSCs) isolation and culture
Young adult (4 weeks old) Sprague–Dawley rats were anesthetized with isoflurane (2–3% in O2). After decapitation, both femurs were removed and placed in a dish containing sterile Hank’s buffered salt solution (HBSS) on ice. The ends of the femurs were removed and bone marrow extruded into sterile HBSS using an 18-G cannula attached to a 12-ml syringe. Extruded bone marrow was dispersed by trituration and centrifuged at 400g for 10 min, repeated twice. The cells were then resuspended in culture medium consisting of low-glucose Dulbecco’s Modified Eagle Medium supplemented with 10% stem cell-qualified fetal bovine serum and penicillin–streptomycin–neomycin antibiotic mixture (Life Technologies, Carlsbad, CA) and placed in culture. The flasks were washed twice at a 24-h interval to eliminate non-adherent cells. Adherent cells were further cultured in 25-cm2 flasks with medium change at 2–3 day intervals. Prior to cell transplantation, the cultured BMSCs 10–12 days (without any passage after harvest) after isolation were digested using TrypLE (Life Technologies) and centrifuged at 400g for 5 min. The pelleted BMSCs were resuspended in sterile phosphate-buffered saline (PBS). Cell viability was determined by trypan blue exclusion and cell numbers counted by hemocytometer.
Tibial nerve injury (TNI)
Tibial nerve injury was induced as described previously (Hofmann et al. 2003). Briefly, at 6–7 weeks of age, during isoflurane (1.5–3%) anesthesia, an incision was made at the right mid-thigh to expose the trifurcation of the sciatic nerve. The tibial nerve was tightly ligated with 4–0 silk and transected distal to the ligation, leaving the sural and common peroneal nerves intact. The incision was closed in layers with absorbable internal sutures and skin staples.
Intrathecal BMSCs injection
Animals were randomly allocated to one of three groups in a fashion that caused animals from each purchased cohort to be represented in all groups. Naïve rats (n = 5) had no sham operation, no anesthetic, and no intrathecal injection; TNI + PBS rats (n = 8) had nerve injury plus PBS injection into the CSF; and TNI + BMSCs rats (n = 10) had nerve injury plus a single intrathecal injection of BMSCs. Behavior data were collected from all of these animals, while CSF from five animals in each group was used for analysis, and histological samples were obtained from all animals.
At the same time as TNI, an intrathecal injection was performed via lumbar puncture procedure as prior described (Decosterd and Woolf 2000), with some minor modifications. A roll of paper towel was placed beneath the pelvis to arch the lumbar spine and enlarge the intervertebral space. A 30-gauge needle was inserted between the fourth and fifth lumbar vertebrae, guided by the position of the iliac crest. Entry of the needle tip in the intrathecal space was inferred by a change in resistance to advancement of the needle accompanied by an S-shaped flinch of the tail. Either 10 µl of BMSCs (approx. 2.5e + 04 cells/µl suspended in sterile PBS) or 10 µl of sterile PBS was slowly injected over 5 s. The needle was held in place for an additional 5 s to prevent backflow of injectate.
Behavioral analysis
On the day prior to the TNI and CSF injection surgery, and on days 3, 7, 14, 21, 28, and 35 thereafter, rats were placed in Plexiglas enclosures on a raised wire platform and allowed to acclimate to the testing environment. The following behavior tests were then performed. All testing stimuli were applied to the lateral portion of the plantar aspect of the hindpaws, to elicit responses from the non-axotomized sural nerve (Decosterd and Woolf 2000). Testing was performed by a single tester who was blinded to the type of injection each animal received. In the presence of nerve injury, animals may avoid weight bearing, making blinding to the injury status of the animal difficult.
Von Frey (tactile allodynia): Calibrated von Frey filaments of forces (in g) 0.31, 0.51, 0.80, 1.05, 2.75, 4.85, 9.03, 13.92, and 23.44 were applied, beginning with the 2.75-g filament. Filaments were applied with enough force to bend the fiber and held for one second. An up-down sequence was used as described previously (Chaplan et al. 1994). Specifically, if the animal withdrew the foot, we applied in response to the 2.8-g filament, the next weakest was applied, whereas if no response was observed, we applied the next stiffest. This was continued until we observed a reversal (i.e., lack of response following a response to the previous fiber, or vice versa), after which four additional stimulations were performed, and the forces of the filaments were used to calculate the 50% mean withdrawal threshold (MWT) using the previously published equation (Dixon 1980).
Pin (tactile hyperalgesia): a 22-gauge spinal anesthesia needle was applied to the hindpaw at a rate of approximately 1 cm/s, which always elicits a response but does not puncture the skin. This was repeated 10 times with intervals of a minimum of 5 s between applications, in two sets of five stimulations separated by at least 2 min. For each application, this elicits either a simple withdrawal with immediate return of the paw to the floor, or an extended response involving sustained lifting of the paw, sometimes with licking or grooming and possibly shaking. This latter response is termed hyperalgesia behavior (Hogan et al. 2004) and has been shown to be highly correlated with nerve injury. Nerve-injured animals reliably express this behavior, and its expression is limited to the side ipsilateral to injury. Further, the hyperalgesic response to noxious stimulation has specifically been correlated with conditioned avoidance in nerve-injured rats (Wu et al. 2010), indicating that this behavior is associated with and aversive pain-like experience. Data were recorded as the number of hyperalgesic responses per 10 stimulations.
Cerebrospinal fluid (CSF) collection
Cerebrospinal fluid was collected from naïve rats and the animals at the 35th day after TNI with cell or PBS injection surgery, by using a technique described in full elsewhere (Liu and Duff 2008), with minor modifications. Briefly, under isoflurane anesthesia, an incision was made on the back of the neck, and the muscles separated by blunt dissection. The spinal dura at the atlanto-occipital level was exposed, the cisterna magna was entered with a 27-G needle, and an average of 250 µl of CSF was collected. CSF samples collected were frozen and stored at −80 °C until use. Animals were euthanized for tissue harvest after CSF collection.
CSF multiple cytokine analysis
Multiplex ELISA antibody array was performed using rat cytokine array Q67 kit (QAR-CAA-67, RayBiotech, Norcross GA) per manufacture service. Rat cytokine array Q67 kit allows simultaneous quantification of multiple (67) rat cytokines (https://www.raybiotech.com/high-density-quantitative-biomarker-array-services.html#100-rat-biomarkers) in CSF samples, including cytokines, inflammatory factors, growth factors, and receptors (referred to collectively as soluble factors in this report). Each sample was tested individually in quintuplicate, and the measurements were averaged. The actual cytokine concentration was directly quantified according to a standard curve performed in parallel for each cytokine as part of the array.
Immunofluorescence staining
The cultured BMSCs were characterized by immunocytofluorescence staining of BMSC-related marker expression as described previously (Yu et al. 2015). In brief, the cultured cells were fixed with 4% paraformaldehyde (PFA) for 10 min. After blocking with 3% bovine serum albumin (BSA), cells were stained with antibodies against Thy-1 (1:200, SCB), CD105 (1:100, Sigma-Aldrich), Stro1 (1:200, Life Technologies), CD29 (1:200, SCB), CD45 (1:100, SCB), and CD11b (1:100, SCB). All concentrations were in line with the manufacturers’ recommendations for paraffin-embedded histology. The antibodies used were determined by prior work in the field. Positive staining for CD105, Stro1, Thy-1 (also known as CD90), and CD29 in conjunction with the absence of staining for CD45 and CD11b is considered essential qualifiers for cells to be considered “Mesenchymal Stem Cells” (Dominici et al. 2006; Tolar et al. 2010). For immunohistological (IHC) determination of BMSCs engraftment after transplantation, animals tested were terminally anesthetized with isoflurane (3%), bilateral lumbar (L) 3, L4, L5 DRGs and segments of lumbar spinal cords were harvested from the euthanized animal and postfixed by immersion in zinc formalin, embedded in paraffin, and sectioned (Yu et al. 2011). Five-μm-thick sections were de-waxed and treated by heat-induced epitope retrieval in 10 mM citrate buffer, pH 6.0. Sections were stained with IB4 (5 μg/ml, Life Technologies) or immunolabeled with Thy-1 (1:100, SCB) and Stro1 (1:100, Life Technologies) overnight. All antibodies were diluted in 1× PBS supplemented with 0.5% Triton X-100 and 3% BSA. Non-immune immunoglobulin G (IgG) (from the same species as the primary antibodies) was replaced for the first antibody as the negative control used for staining. After 3 washes with PBS, the tissue sections were stained with the appropriate fluorophore-conjugated (Alexa 488 or Alexa 594) secondary antibodies (Jackson ImmunoResearch, West Grove, PA) to reveal immune complex. The sections were imaged on a Nikon TE2000-S fluorescence microscope (El Segundo, CA), equipped with an Optronics QuantiFire digital camera, acquisition software (Ontario, NY), and filters suitable for selectively detecting the green, red and blue fluorescence. All comparative images were acquired with identical settings (e.g., exposure time, gain).
Western blotting
Lysates of cultural cells (BMSCc and MCF7) and tissues (mouse brain at postnatal day 1 and adult rat DRGs) were extracted using 1× RIPA buffer (20 mm Tris–HCl pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, with 0.1% Triton X100 and protease inhibitor cocktail). Protein concentration was determined by using the BCA kit (Pierce, Rockford, IL). Western blotting (20 μg protein) was preceded by SDS-PAGE gel electrophoresis, transferred onto nitrocellulose, and probed with Thy-1 antibody (1:100, Santa Cruz Biotechnology, SCB, Santa Cruz, CA) and Stro1 antibody (1:100, Life Technologies). Immunoreactive proteins were detected by enhanced chemiluminescence (Pierce, Rockford, IL) after incubation with HRP-conjugated second antibodies (1:2000, SCB) and exposed to photographic film. GAPDH (1:2000, Cell Signaling, Danvers, MA) was used as a loading control.
Statistical analysis
CSF cytokine analysis was performed by two-way ANOVA, with the two factors being group membership of the animal (Naïve, TNI + PBS, or TNI + BMSC) and cytokine. Post hoc comparisons were performed for each cytokine between the Naïve group and the TNI + PBS group to identify the effects of injury, and between the TNI + PBS group and the TNI + BMSCs group to identify the effect of BMSCs implantation. These comparisons were tested for significance as planned comparisons with correction for multiple comparisons by the Holm–Sidak method. For between-group analysis of behavior, a two-way ANOVA with repeated measures was performed, with post hoc comparisons between the non-operated Naïve group and the TNI + PBS groups and between the TNI + PBS group and the TNI + BMSCs group. p values were corrected for multiple comparisons by the Holm–Sidak method. Area under the curve (AUC) analysis was performed to capture the influence of treatment upon observed behavior across time, by generating the product of the magnitude of the treatment effect by the duration of the observed effect between successive timepoints at which behavior was evaluated. AUC values were compared between groups by the Kruskal–Wallis test, with post hoc comparisons among all groups with correction for multiple comparisons by Dunn’s test. Analyses were performed using Prism 6.07 (GraphPad Software La Jolla, CA). p values less than 0.05 were considered statistically significant. Data are displayed as mean ± SEM.
References
Abdel Aziz MT et al (2013) Effect of mesenchymal stem cells and a novel curcumin derivative on Notch1 signaling in hepatoma cell line. Biomed Res Int 2013:129629
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Backonja MM et al (2008) Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol 195:157–163
Bara JJ et al (2014) Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: implications for bone and cartilage tissue engineering. Tissue Eng Part A 20:147–159
Basbaum AI et al (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Batten P et al (2006) Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng 12:2263–2273
Beyth S et al (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105:2214–2219
Brierley JB, Field EJ (1948) The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat 82:153–166
Chaplan SR et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Chen G et al (2015) Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Investig 125:3226–3240
Clark AK, Old EA, Malcangio M (2013) Neuropathic pain and cytokines: current perspectives. J Pain Res 6:803–814
Crisostomo PR et al (2008) Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B—but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294:C675–C682
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158
Del Papa B et al (2013) Notch1 modulates mesenchymal stem cells mediated regulatory T-cell induction. Eur J Immunol 43:182–187
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462
Dominici M et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Franchi S et al (2012) Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain 153:850–861
Guo W et al (2011) Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells 29:1294–1303
Himango WA, Low FN (1971) The fine structure of a lateral recess of the subarachnoid space in the rat. Anat Rec 171:1–19
Hofmann HA et al (2003) Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur J Pharmacol 470:17–25
Hogan Q et al (2004) Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 101:476–487
Hosseini M et al (2015) The effect of bone marrow-derived mesenchymal stem cell transplantation on allodynia and hyperalgesia in neuropathic animals: a systematic review with meta-analysis. Biol Blood Marrow Transplant 21:1537–1544
Jacobs SA et al (2013) Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 91:32–39
Kessler JA et al (2015) Double-blind, placebo-controlled study of HGF gene therapy in diabetic neuropathy. Ann Clin Transl Neurol 2:465–478
Kucerova L et al (2010) Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer 9:129
Kwilasz AJ et al (2015) The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 96:55–69
Labuz D et al (2009) Immune cell-derived opioids protect against neuropathic pain in mice. J Clin Invest 119:278–286
Ledeboer A et al (2007) Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21:686–698
Lee MW et al (2015) Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res Ther 6:179
Liang X et al (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23:1045–1059
Liu L, Duff K (2008) A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp JoVE 21:960. doi:10.3791/960
Machelska H et al (2002) Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J Neurosci 22:5588–5596
Mackenzie TC, Flake AW (2001) Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis 27:601–604
Mao F et al (2010) Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res 59:219–225
Meirelles Lda S et al (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20:419–427
Meyerrose T et al (2010) Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev 62:1167–1174
Milligan ED et al (2006) Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol 2:293–308
Nadeau S et al (2011) Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci 31:12533–12542
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Pleticha J et al (2015) High cerebrospinal fluid levels of interleukin-10 attained by AAV in dogs. Gene Ther 22:202–208
Ren G et al (2010) Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 184:2321–2328
Salbaum JM, Kappen C (2000) Cloning and expression of nope, a new mouse gene of the immunoglobulin superfamily related to guidance receptors. Genomics 64:15–23
Schafer S et al (2014) Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury. J Neuroinflamm 11:157
Shibata T et al (2008) Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 57:3099–3107
Siniscalco D et al (2010) Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci CMLS 67:655–669
Sun YY et al (2012) The spinal notch signaling pathway plays a pivotal role in the development of neuropathic pain. Mol Brain 5:23
Temporin K et al (2008) Interleukin-1 beta promotes sensory nerve regeneration after sciatic nerve injury. Neurosci Lett 440:130–133
Tolar J et al (2010) Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28:1446–1455
Tran C, Damaser MS (2015) Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev 82–83:1–11
Tsuchihara T et al (2009) Nonviral retrograde gene transfer of human hepatocyte growth factor improves neuropathic pain-related phenomena in rats. Mol Ther 17:42–50
von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652
Wehner R et al (2009) Mesenchymal stem cells efficiently inhibit the proinflammatory properties of 6-sulfo LacNAc dendritic cells. Haematologica 94:1151–1156
Wen L et al (2014) Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells. PLoS One 9:e101841
Wu HE et al (2010) Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 11:280–286
Xie K et al (2015) Notch signaling activation is critical to the development of neuropathic pain. BMC Anesthesiol 15:41
Xu Q et al (2013) Intrathecal transplantation of neural stem cells appears to alleviate neuropathic pain in rats through release of GDNF. Ann Clin Lab Sci 43:154–162
Yu H et al (2011) Lentiviral gene transfer into the dorsal root ganglion of adult rats. Mol Pain 7:63
Yu H et al (2015) Analgesia for neuropathic pain by dorsal root ganglion transplantation of genetically engineered mesenchymal stem cells: initial results. Mol Pain 11:5
Acknowledgements
This work was supported by grant from the National Institute of Neurological Disorders and Stroke (R01NS079626-01) to QHH. Authors thank Zhen Liu for assistance in histological sample preparation.
Author information
Authors and Affiliations
Contributions
HY and QHH contributed to conception and design of the study; GF, HY, and QHH helped in performing experiments, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; FW and HX performed experiments and data analysis; XB helped with consultant.
Corresponding authors
Ethics declarations
Conflict of interest
The authors indicated no potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
221_2017_5000_MOESM1_ESM.xlsx
Supplementary Table 1. Complete results of antibody array. Mean concentration of each soluble factor in CSF (n = 5 for each group) with SEM. (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Fischer, G., Wang, F., Xiang, H. et al. Inhibition of neuropathic hyperalgesia by intrathecal bone marrow stromal cells is associated with alteration of multiple soluble factors in cerebrospinal fluid. Exp Brain Res 235, 2627–2638 (2017). https://doi.org/10.1007/s00221-017-5000-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5000-x