Abstract
Although studies have investigated tactile and visual processing for perception, sensory processing for ongoing action remains poorly understood. The purpose of this study was to explore modality-specific patterns of cortical activation and functional connectivity in a practiced trajectory tracking task. Participants traced irregular shapes with their index finger using either touch or vision for guidance. In 60 tactile-motor (TM) trials, movement was guided only by tactile feedback of semicircular bumps on a plastic tile. In 60 visuo-motor (VM) trials, movement was guided only by vision of dots on a screen seen through a small window at the finger tip. The order of TM and VM trials was counterbalanced across 16 participants. Electroencephalography (EEG) was used to estimate cortical activation (task-related spectral power) and functional connectivity (task-related magnitude-squared coherence) in the alpha (8–12 Hz) and beta (13–30 Hz) bands during the last 12 movement trials in each sensorimotor task. TM vs. VM tasks exhibited a larger drop in global beta cortical activation, and greater alpha coherence between central, parietal, and occipital regions. VM vs. TM tasks were characterized by low global alpha coherence. Trace time and cortical activation of the last 12 VM trials were reduced in the group performing the VM task after the TM task compared to those performing the VM task first. Beta connectivity initiated by the first task was maintained on the subsequent second task, regardless of the task order. Identification of modality- and order-specific EEG characteristics provides insight into the sensory control of movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans successfully complete a wide range of manual tasks in everyday life with relative ease. This is made possible by a complex combination of internal forward models that control a preprogrammed voluntary plan which is continuously updated by sensory stimuli in a feedback loop during the task (Desmurget and Grafton 2000). These sensory stimuli are either exteroceptive, sensing the state of the external environment, or proprioceptive, sensing information from within the body. Two exteroceptive sensory modalities of primary importance to movement are vision and cutaneous tactile sensation (Scott et al. 2015). Vision provides a high-speed representation of environmental features (Liu et al. 2009). Tactile sensation provides information to select the appropriate magnitude of hand forces once an object is held in the hand and maintains regularity and speed during reach-to-grasp movements (Gentilucci et al. 1997). Visual and tactile inputs reach their primary cortical targets in separate parallel neural pathways, which can be modulated by both internally generated (endogenous), and externally cued (exogenous) attention (Corbetta and Shulman 2002; Hsiao et al. 1993). Endogenous attention is typically biased toward vision over touch when sensory feedback is multimodal (Klein 1977); however, it can be focused on only vision or touch when only one type of task-relevant sensory feedback is available.
A distinction is commonly made between sensory processing for an action goal (e.g., voluntary movements) and sensory processing for a perceptual goal (e.g., object knowledge and conscious decision making). The processing of visual information originates in the primary visual cortex, and either culminates in the posterior parietal cortex to inform action (dorsal visual stream), or in the infero-temporal cortex for the end goal of visual perception (ventral visual stream) (Goodale and Milner 1992). An analogous action–perception distinction has also been described in the processing of tactile information (Dijkerman and De Haan 2007). Tactile processing begins in the primary somatosensory cortex, continues into the secondary somatosensory cortex, and either culminates in the posterior parietal cortex to process tactile information for action, or the insula to inform tactile perception (Dijkerman and De Haan 2007; Mishkin 1979; Reed et al. 2005).
In the context of sensory processing for perception, it has been shown that neural regions that are activated by both visual and tactile stimuli, including the postcentral sulcus, intraparietal sulcus, and occipital–temporal regions of cortex (Amedi et al. 2002). This suggests that, in addition to modality-specific primary sensory areas of cortex, there are multimodal areas involved in perception-centered sensory processing. However, regarding sensory processing for an ongoing action, far less empirical evidence exists to describe the similarities and differences between neural correlates of vision and touch during an ongoing sensory-guided movement.
The purpose of this study was to explore patterns of cortical activation and functional connectivity that are specific to processing tactile or visual feedback in a practiced trajectory tracking task, which requires ongoing sensory feedback to inform movement. We used electroencephalography (EEG) to noninvasively assess neural correlates of movement along a trajectory that was either indicated by tactile cues in the absence of vision, or visual cues in the absence of object contact. By requiring subjects to trace the same trajectories with feedback from touch and vision separately, we were able to minimize confounding due to motor output and the visual bias of endogenous attention and focus on EEG parameters associated with the processing of each sensory modality for movement.
Materials and methods
Design and participants
This was an observational cross-sectional study that included 16 healthy, right-handed young adults (eight females and eight males) with a mean age of 24.0 ± 4.9 (SD) years. All participants had normal or corrected-to-normal vision. The participants were assessed in one visit lasting 2 h.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee at the Catholic University of America and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data acquisition
Continuous EEG signals were recorded (Curry 7.0.7, Compumedics Neuroscan, Charlotte, NC) from 28 Ag/AgCl electrodes, embedded in a Lycra stretch cap (QuikCap, Compumedics Neuroscan, Charlotte, NC) that was placed on the scalp according to the International 10–20 system (Klem et al. 1999). There were 12 lateral pairs (FP1/2, F3/4, F7/8, FC3/4, FT7/8, T7/8, C3/4, CP3/4, TP7/8, P3/4, P7/8, O1/O2) and four midline EEG electrodes (Fz, Cz, Pz, Oz). In addition, one electrode was placed on each earlobe. The signals were referenced to the left earlobe electrode during data collection and later re-referenced to linked-earlobes offline and before analysis. Electrode impedance was kept below 20 kΩ. Before recording data (band-pass filter = DC-400 Hz, sampling rate = 1 kHz), the participants were shown EEG signals in real time to train them on minimizing eye blinks, and other movements of the face, torso, and neck.
Task procedure
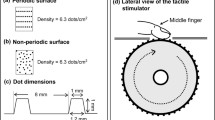
The participants traced six irregular two-dimensional shapes with their right index fingertip in each of two feedback conditions: (1) fingertip tactile feedback during movement behind a curtain to preclude vision of the hand (tactile-motor, TM task); and (2) unobstructed vision of the hand and experimental apparatus during movement without object contact to minimize fingertip tactile feedback (visuo-motor, VM task). The trajectory associated with each shape, composed of a unique combination of straight lines and partial circles, was continuous and did not cross itself along its path (Fig. 1a). All six shapes had the same starting point, but had different end points (mean path length = 45.7 ± 3.6 cm). During both tasks, the participants were seated comfortably with the shapes presented on a 30° incline relative to table height, such that the tracing occurred at the height of the mid-torso. The shapes were presented between the midline of the participant’s body and their right shoulder (Fig. 1b, d). The participants performed the traces without wrist support.
During both VM and TM tasks, the participants traced six irregular shapes that consisted of a combination of non-intersecting lines and partial circles (a). They had the same starting point (solid large dots), but had unique paths (dotted lines) and end points (hollow large dots). During the TM task (b, d), vision was blocked and tracing was guided by tactile feedback only. During the VM task (c, e), tracing was guided by vision only. Only the dots directly under the polarized window attached to the tip of the index finger were visible
In the TM task, movement was guided only by tactile feedback of hemispheric bumps along the tracing path (dia = 0.26 cm, height = 0.13 cm, spacing = 0.76 cm) (Fig. 1b, d). The spacing was designed to be roughly half the width of the pad of an adult index fingertip, such that the participants were always in contact with approximately two bumps, while they traced the shapes. The start and end points of the shape were circular wells (dia = 1.5 cm, depth = 0.4 cm). The tactile shapes were 3-D printed (uPrint SE, Stratasys Ltd., Eden Prairie, MN) using acrylonitrile butadiene styrene. During this task, participants kept their eyes open and focused on a curtain approximately 30 cm in front of their body that blocked their ability to see the shapes.
In the VM task, movement was guided by vision of black dots along the tracing path (dia = 0.26 cm, spacing = 0.76 cm) presented against a white background on a liquid–crystal display (LCD) screen (1908 FPC, Dell, Round Rock, TX). To reduce visibility of the tracing path to the area around the index fingertip (similar to the spatial focus of tactile feedback on the fingertip in the TM task) the LCD screen’s outer polarizing filter was removed, rendering any displayed image invisible. Then, a small circular window of polarized film was attached to a rubber thimble that was placed on the participant’s index fingertip. Thus, participants were only able to see approximately two black dots of the tracing path in the limited circular field of view of the film at a time (Fig. 1c, e). The start and end dots during the VM task had the same diameter as the wells in the tactile shapes (1.5 cm). During the VM task, the participants were instructed to hover their finger above the screen while tracing, thereby minimizing tactile feedback during this task. Prior to data collection, each task was explained and demonstrated by an investigator.
The participants began each trial by placing their index finger at the starting point. At the sound of a beep (f = 400 Hz, duration = 0.3 s), they started tracing the shape at a speed that allowed them to accurately trace the entire shape. Tracing accuracy was visually checked by the experimenter. The time of the start beep was recorded using MATLAB (V8.3, MathWorks, Natick, MA) and the Psychophysics Toolbox (V3.0.11, http://psychtoolbox.org). The end of each trial was recorded with a key press by the experimenter when the participant reached the end point of each shape.
Previous work has indicated that measurements of EEG power and coherence during novel tasks are sensitive to practice, suggesting that sufficient practice trials are required in order to study the neurophysiological process of interest, rather than task learning (Smith et al. 1999). Consequently, in our study of sensory processing for movement, participants performed 60 consecutive trials of each task type (TM and VM) to achieve stable performance. The 60 trials of each type were completed in five blocks, each consisting of the six shapes presented twice in a pseudorandom order (the same pseudorandom order was used across participants) for a total of 12 shapes per block. The order of TM and VM tasks was counterbalanced, with eight participants performing the TM task first, followed by the VM task (TM–VM), and the remaining eight participants performing the VM task first, followed by the TM task (VM–TM). There were four females and four males in both groups, and there was no significant difference in age (TM–VM 24.4 ± 5.3 years, VM–TM 23.5 ± 4.7, t(30) = 0.50, p = 0.14) between the groups. To avoid fatigue, each traced shape was preceded and followed by a break of approximately 5 s, and each block was preceded and followed by a 2–3 min break. Baseline EEG signals were also recorded during five 1-min rest periods. The first rest was collected prior to the first block, and the remaining rest periods were evenly spaced throughout the experiment.
Signal processing
All data processing was done using MATLAB and the EEGLAB toolbox (Delorme and Makeig 2004). Raw EEG signals were band-pass filtered (1–40 Hz) with a finite impulse response filter. Continuous EEG signals were visually inspected, and trials with gross artifacts were eliminated. For each participant, the TM and VM trials were then epoched from 0.5 s after the beep to the end of the shortest VM or TM trace across all 120 shapes. For each participant, the rest trials were divided into epochs of the same length, meaning that each TM, VM, and rest epoch had the same length per participant. The mean epoch time across participants was 6.9 ± 2.0 s, resulting in approximately 82 s of data per block. After visual inspection, 96.9% (96.4 and 97.4% for TM and VM tasks, respectively) of epochs were suitable for further analysis. Independent component analysis (ICA) was then used to identify non-neural artifacts. Multiple Artifact Rejection Algorithm (MARA) (Winkler et al. 2011) was used to select non-neural artifacts for rejection based on spatial, spectral, and temporal features of the EEG signals. After this decomposition, approximately 56% of the components were considered to be from a neural source and these components were transformed back to the channel space for further analysis. To spatially filter the data and minimize volume conduction features of the data, EEG data were converted to current source density (CSD) estimates of surface potentials (Kayser and Tenke 2006). CSD estimates were computed by subtracting the signal in the neighboring EEG leads weighted by their inverse distance from the EEG signal measured at a given location.
To study cortical activity, power spectra were computed for each lead during each task and rest epoch using the fast Fourier transform with a 10% Tukey window. Mean spectral power was then computed over each task or rest block in the alpha (8–12 Hz) and beta (13–30 Hz) frequency ranges. The mean spectral power was computed over the entire block to increase the signal-to-noise ratio by averaging across all the data within an entire block. Magnitude-squared coherence was estimated between each lead for each task and rest epoch using Welch’s averaged periodogram method with non-overlapping Hann windows (1024 data points). Mean coherence estimates were then computed over all trials within one task or rest block in the alpha and beta frequency ranges. Only mean coherence values that were above the threshold for significance with 95% confidence were retained for subsequent analysis. This threshold was computed as 1 − (0.05)1/(L−1), where L is the number of cumulative windows in a rest or task block (Rosenberg et al. 1989).
Outcome measures
The four primary outcome measures were mean alpha task-related spectral power (TRP), mean beta TRP, mean alpha task-related coherence (TRC), and mean beta TRC. Given that cortical processing is associated with changes in both the alpha and beta frequencies (Andres et al. 1999), data were analyzed separately in both frequency bands. TRP was quantified (Eq. 1) for each EEG lead as the ratio of spectral power in each task (PowTask) to the closest preceding rest trial (PowRest) (Bulea et al. 2014). As defined, a negative value of TRP, or a decrease in EEG spectral power in the task compared to the rest period, corresponds to cortical activation (Andres et al. 1999). TRC was quantified (Eq. 2) as the ratio of magnitude-squared coherence between pairs of leads in each task (CohTask) and the closest preceding rest trial (CohRest). Since coherence reflects an interaction between different brain areas or a functional neural connection involved in a particular task (Buschman and Miller 2007), a task-related increase would reflect more functional connectivity in the task compared to rest.
As a secondary outcome measure, mean trace time was computed across trials in each block to describe the tracing behavior.
Statistical analysis
Primary analyses
Full-factorial repeated measures analysis of variance (ANOVA) was used to model each of the four primary outcome measures: alpha TRP, beta TRP, alpha TRC, and beta TRC. In these models, EEG data from the last block of the VM and TM tasks (i.e., the last 12 shapes which represented stable tracing performance) were analyzed. Since four outcome measures were analyzed, we used a Bonferroni correction to adjust p values in the four models by multiplying them by a factor of four (p adj = 4 × p value). TRP and TRC calculations included a log-transformation (Eqs. 1, 2) to satisfy the assumptions necessary to use ANOVA modeling. Greenhouse–Geisser corrections were used to compensate for violations of sphericity. All post hoc pairwise comparisons for ANOVA models were done using the Bonferroni correction for multiple comparisons. Statistical analysis was done using SPSS 22 (IBM SPSS Statistics, Armonk, NY).
Cortical activation was assessed through the alpha and beta TRP models, which included a within-participant factor of modality (TM, VM) and a between-participant factor of subject group (TM–VM or VM–TM). To visualize the spatial distribution of cortical activation, topographic plots of alpha and beta TRP were created. Cortical connectivity was assessed through the alpha and beta TRC models. In these analyses, the dependent variable was mean TRC across all pairs, the within-participant factor was modality, and the between-participant factor was subject group. To visualize cortical connectivity, each of the 28 leads was classified into one of 10 bilateral brain regions (frontal, temporal, central, parietal, and occipital). Electrode pairs between pairs of regions were then grouped. The mean TRC between regional groups was assessed, reducing the 378 electrode pairs to a more tractable 45 regional connections (excluding pairs consisting of the same electrode or the same region). Link plots of regional coherence values representing at least a 10.5% change in coherence from rest (TRC ≥ 0.1, and TRC ≤ −0.1) were created to visualize the spatial patterns of functional connectivity. After visually assessing multiple plots with a range of threshold values, we choose ±0.1 to prevent oversaturation (a lower threshold that retains a large number of connections) or undersaturation (a higher threshold that catches only a few of the strongest connections) of the link plots as described by Rubinov and Sporns (Rubinov and Sporns 2010). This threshold retained 33% of the strongest significant (95% confidence) TRC links.
Secondary analyses
Mean trace time was computed for all blocks to describe how participants performed the trajectory tracking task. Since the primary EEG analysis focused only on block 5, independent samples t tests were used to assess the difference between modalities (TM and VM) on trace time of the first and second task, and the difference between ordering of tasks (first and second) on TM and VM performance in block 5.
Results
Tracing behavior
All participants completed the 120 trials in approximately 1 h. Although we observed that some participants made visible tracing errors in the first block of each modality, we did not observe visible errors by the final block of that modality. Across blocks, trace time appeared to decrease in each modality with practice (Fig. 2a, b). Block 5 trace time was similar between modalities in the first task [t(14) = 0.30, p = 0.77], and greater for the TM compared to the VM condition in the second task [t(14) = 2.8, p = 0.016; Fig. 2c]. Block 5 trace time was lower for the second vs. first VM task [t(14) = 2.31, p = 0.036], but similar between the first and the second TM tasks [t(14) = −0.21, p = 0.84; Fig. 2c].
Tracing behavior. Trace times of the TM and VM tasks across blocks are shown for the first (a) and second task (b). Data from only block five (c) indicate that there was no modality difference at the end of the first task, but there was a modality difference at the end of the second task. The difference in trace time for the first vs. second task was significant for the VM task, but not significant for the TM task (c). Each marker/error bar is the mean/standard error over eight participants. *p < 0.05
Cortical activation: task-related power (TRP)
In the alpha band, there was a significant two-way interaction between modality and order on TRP [F(1.0, 446.0) = 53.89, p adj < 0.004]. Post hoc testing indicated that five out of six pairwise comparisons were significant, as shown by the asterisks on Fig. 3a. The comparisons revealed greater alpha TRP loss (more cortical activation) in TM vs. VM in the TM–VM group (p < 0.006) but less alpha TRP loss in the TM vs. VM task in the VM–TM group (p = 0.018). There was more alpha TRP loss in the VM task when it was done first compared to second (p < 0.006), but there was no order difference in the TM tasks (p = 0.054). The VM vs. TM tasks had more TRP loss in the first task (p < 0.006, Fig. 3c), while TM vs. VM tasks had more TRP loss in the second task (p < 0.006, Fig. 3e).
Cortical activation: task-related power. In both frequency bands, there were significant interactions between modality and order in the mean TRP over all 28 leads in the alpha (a) and beta (b) bands. *p < 0.05, and error bars represent one standard error. Topographic scalp maps of the task-related power (TRP) in the alpha and beta bands during both the first task (c, d) and the second task (e, f). Cortical activation (TRP loss) is associated with negative values (cooler colors), and TRP gain is associated with positive values (hotter colors) (color figure online)
In the beta band, there was also a significant two-way interaction between modality and order [F(1.0, 446.0) = 12.67, p adj < 0.004]. Pairwise comparisons (Fig. 3b) indicated more beta TRP loss in the TM vs. VM task in both the TM–VM (p < 0.006) and VM–TM groups (p = 0.036). There was a gain in beta TRP when the VM task was performed second compared to a loss of beta TRP when the VM task was performed first (p < 0.006), while there was no difference in beta TRP for the TM task based on when it was performed (p = 2.68). In the first task, there was no difference in beta TRP between the modalities (p = 0.462, Fig. 3d), while there was more TM vs. VM beta TRP loss in the second task (p < 0.006, Fig. 3f).
Over both tasks and frequency bands, the greatest TRP loss (greatest cortical activation) was around the left sensorimotor cortical area (Fig. 3c–f). The right sensorimotor cortical area also had TRP loss to some extent. In block 5 of the first task, when the trace time was well matched between participant groups, the bilateral parietal regions (right greater than left) appeared to have more activation during the VM vs. TM tasks (Fig. 3c, d). Figure 3c, e together indicate that the VM–TM group had and maintained a more widespread drop in alpha TRP throughout the first and second tasks than the TM–VM group, which maintained a more focused activation pattern around the left sensorimotor area independent of task modality. The amplitude of TRP was lower in the beta frequencies (Fig. 3d, f) than the alpha frequencies (Fig. 3c, e).
Cortical connectivity: task-related coherence (TRC)
In the alpha band, there was a significant two-way interaction between modality and order on overall TRC between all regions (F(1.0, 4222.0) = 16.53, p adj < 0.004). The four significant post hoc pairwise comparisons (out of six) are indicated by the asterisks in Fig. 4a. There was less alpha TRC in the VM vs. TM task in the TM–VM group (p < 0.006), but there was no modality difference in the VM–TM group (p = 0.23). Alpha TRC in the TM task was minimal and similar whether it was completed first or second (p = 0.51); however, alpha TRC in the VM task was lower when it was completed second vs. first (p < 0.006). There was a larger drop in alpha TRC in the VM vs. TM tasks in both the first task (p = 0.006), as indicated by the larger number of green central, parietal, temporal, and occipital links in Fig. 4c, and the second task (p < 0.006), as indicated by the large number of green links across all regions in Fig. 4e. In the first task, when trace time at block 5 was similar between modalities, links denoting an increase in alpha TRC in the TM task mostly involved the central, parietal, and occipital regions, while the links denoting an increase in TRC in the VM task involved the frontal regions (Fig. 4c). In the first and second tasks in the alpha band, the VM condition retained more links after thresholding than the TM condition (VM first task and VM second task = 19 links each, TM first task = 9 links, TM second task = 15 links, Fig. 4c, e). Of the numerous VM links, the majority (VM first task = 79%, VM second task = 95%) were green, indicating a decrease in TRC whether the VM task was performed first or second (Fig. 4c, e).
Cortical connectivity: task-related coherence. There was a significant interaction between modality and order on mean TRC over all brain regions in the alpha (a) and beta (b) bands. *p < 0.01, and error bars represent one standard error. Link plots of regional coherence values in the alpha and beta bands are shown for the first (c, d) and second (e, f) tasks. The five bilateral brain regions used to assess regional coherence (shown in c) were left and right frontal (LF and RF), temporal (LT and RT), central (LC and RC), parietal (LP and RP), and occipital (LO and RO). Thin magenta lines represent positive TRC between 0.1 and 0.2, and thick magenta lines represent TRC greater than 0.2. Similarly, green lines represent negative TRC values (color figure online)
In the beta band, there was also a significant two-way interaction between modality and order on overall TRC between all regions (F(1.0, 4222.0) = 12.02, p adj = 0.004). The five significant post hoc pairwise comparisons (out of six) are indicated by the asterisks in Fig. 4b. There was less beta TRC in the VM vs. TM tasks in the TM–VM group (p < 0.006), and no difference between the two modalities in the VM–TM group (p = 0.68). There was greater beta TRC in the VM–TM group than the TM–VM group for both tasks (TM: p < 0.006; VM: p < 0.006). During the first task, the VM vs. TM tasks had more beta TRC (p < 0.006), with increased TRC among numerous links in the VM tasks involving all five bilateral regions, and fewer beta TRC links that were positive in the TM task (Fig. 4d). During the second task, there was greater beta TRC in the TM vs. VM task (p < 0.006), with an increase in TRC that involved all regions, while the VM tasks had elevated TRC in far fewer links (Fig. 4f). In the first and second tasks in the beta band, the number of links after thresholding was greater in the VM–TM group compared to the TM–VM group (VM first task and TM second task = 21 links each, TM first task and VM second task = 8 links each, Fig. 4d, f). Of these links in the VM–TM group, the majority (VM first task = 95%, TM second task = 86%) were magenta, indicating an increase in TRC whether the modality was TM or VM (Fig. 4d, f). In both frequency bands, the greatest drop in overall TRC was associated with the VM task when it was performed second (i.e., the TM–VM group).
Discussion
We explored patterns of cortical activation and functional connectivity that are specific to tactile or visual processing during trajectory tracking. Task-related power and task-related coherence were assessed in the alpha band, associated with the bottom-up processing involved in the binding of sensory and motor information (Andres et al. 1999; Chen et al. 2003; Rilk et al. 2011), and the beta band, associated with top-down processing involving cognitive activity of perception and motor planning (Andres et al. 1999; Buschman and Miller 2007; Campus et al. 2012). In agreement with previous research (Andres et al. 1999), both tasks in our study were associated with cortical activation around the left, and to a lesser extent, the right sensorimotor cortical area (Fig. 3c–f).
Tactile processing for movement
At the end of the first task, alpha cortical activation was more spatially specific to the sensorimotor areas in the TM tasks compared to the VM tasks, in which activation was more spatially distributed (Fig. 3c). This was expected since this area includes the primary somatosensory cortex, which is the cortical target for tactile stimulation of the hand. Similarly, previous work has also shown focal activation in these areas during active touch (Stoeckel et al. 2003). Activation around the sensorimotor areas may also be related to a focus of endogenous attention on the tactile sensory modality, which is associated with an increase in firing rate of neurons in the primary somatosensory cortex and secondary somatosensory cortex (Hsiao et al. 1993), and synchronization of neuronal spiking within the secondary somatosensory in humans and non-human primates (Gomez-Ramirez et al. 2014; Steinmetz et al. 2000).
The TM vs. VM tasks were associated with greater alpha TRC in the first and second tasks, involving the central, parietal, and occipital regions (Fig. 4c). The alpha band is associated with the binding of sensory and motor information in these regions, and coherence is thought to aid inter-regional communication. Therefore, greater coherence during the TM task may indicate a greater need for connectivity between the sensory and motor cortices for sensorimotor integration compared to the VM task. The observation that alpha TRC was similar in the first and second TM tasks suggests that this pattern of connectivity is related to the type of feedback being processed rather than any other task order-related or cognitive process.
Visual processing for movement
By the end of the first task, the VM vs. TM tasks had more widespread activation across the cortex in the alpha band (Fig. 3c). This is similar to previous findings of bilateral global activation of the central, parietal, and occipital areas during tracking of an irregularly fluctuating visual target with pinch grip modulation (Rilk et al. 2011). Like previous research reporting parietal activation during the processing of visual information (Wojciulik and Kanwisher 1999), we observed bilateral, right greater than left, activation of the parietal regions during the VM vs. TM tasks in the alpha band during the first task (Fig. 4c). Other studies have also highlighted the involvement of the right temporo-parietal area, a part of the ventral attention network, in motor tasks requiring visual attention (Shulman et al. 2010). Moreover, unilateral right parietal damage is associated with a loss of motion perception in both the left and right fields of view (Battelli et al. 2001), suggesting right-sided dominance in the processing of visual stimuli. This is in agreement with greater right vs. left parietal activation during the first task in the alpha band of the VM task in our study.
Contrary to the TM task, there was a decrease in global alpha TRC in the VM task. This drop in task-related coherence may represent the relatively automatic nature of the highly practiced VM task. As Fig. 2a indicates, trace time for the VM task adapted and plateaued early in task practice compared to the TM task. Previous studies of expert visuo-motor task practice indicate decreased EEG coherence following learning (Busk and Galbraith 1975), suggesting a more efficient cortical organization in the VM vs. TM tasks with practice.
Effects of task practice order
The counterbalanced design of this study allowed us to investigate the potential for motor practice guided by one sensory modality to support future tracing with the other modality. We found that initial TM practice led to faster VM tracing in the TM–VM group by the end of the series of 60 trials, but initial VM practice did not lead to faster TM tracing in the VM–TM group (Fig. 2c). This discrepancy may indicate that adaptation to the shorter and potentially easier VM task led to a motor plan specialized for visual input, while TM task practice, which was longer and possibly more difficult, led to a more general motor plan that was able to successfully accommodate a switch in sensory input for the task. In support of this idea, previous research on the transfer of a learned visuo-spatial movement sequence to new spatial locations has indicated that more difficult practice sequences are stored in an abstract form that facilitates a more efficient transfer on subsequent visuo-spatial movements, compared to practice on an easier task (Braden et al. 2008). Alternatively, it is possible that differences in movement variability between the two modalities affected learning rate, since previous research has shown that greater movement exploration facilitates motor learning and that the nervous system dynamics regulates it to improve motor learning (Wu et al. 2014). Unfortunately, since we did not collect kinematic data during this study, the role of movement variability on the learning rate for the TM and VM tasks in this study remains unknown, but a potential avenue of study in the future.
Another potential factor contributing to the order effect is that participants may have been consciously or unconsciously engaging visual processing areas or creating a visuo-motor image during the TM task by imagining the shape of the path being traced (Jeannerod 1995). This mental rehearsal may have been driven by the participants trying to relate the TM task to visual guidance, which is dominant in daily activities, and subsequently recalling that image during the VM task. By contrast, although tactile imagery exists (Uhl et al. 1994), it is less utilized in healthy sighted individuals than motor imagery, and the VM task practice likely did not activate neural regions for processing tactile information or creating a tactile-motor image. However, it is unclear whether the shorter trace time and low cortical activation seen in the VM task when executed after the TM task was due to initial TM practice or some other factor (e.g., non-specific motor practice). Future studies will test whether repeated exposure to sensorimotor tasks with either feedback modality alone can also lead to adaptive changes in EEG parameters that correlate with behavioral changes.
In both frequency bands, there was an absence of or slight decrease in cortical activation compared to rest during the VM tasks of the TM–VM group, compared to increased cortical activation in the other conditions (Fig. 3a, b). With respect to order and cortical connectivity, the least amount of overall TRC was associated with the VM tasks of the TM–VM group in both frequency bands (Fig. 4a, b). These results suggest there may be a cortical activation or connectivity pattern associated with initial tactile-motor practice that predisposes an individual to perform well on future visuo-motor tracking tasks. This raises the intriguing possibility that coaches and therapists involved with physical education and rehabilitation may recommend a specific order of task practice to yield improvements in motor control.
With regard to functional cortical connectivity, the VM–TM group had more global beta TRC than the TM–VM group (Fig. 4d, f). Since the beta band is associated with top-down processing in the frontal, parietal, and central regions that convey knowledge derived from previous experience rather than sensory stimulation (Buschman and Miller 2007), beta coherence in the second task in this study likely represents the participants' recent sensorimotor experience in the first task rather than responses evoked by the current sensory stimuli. On the contrary, there was a similarity in alpha TRC between the subject groups on each sensory modality (Fig. 4c, e), which likely represents the association of the alpha band with current sensory processing.
Limitations and future work
Like posterior parietal cortex, the cerebellum is also involved in the acquisition and storage of internal forward models serving sensorimotor control of the hand (Wolpert et al. 1998). In our study, although we recorded EEG signals that reflect the activity of postsynaptic potentials near the surface of the cerebral cortex, this cortical activity is believed to be the result of a disruption of a phase-coherent regulatory process at rest between subcortical structures such as the thalamus and basal ganglia and the cerebral cortex (Miller et al. 2007). Since the cerebellum influences movement and has separate pathways to the basal ganglia and cerebral cortex (Bostan et al. 2010), changes in EEG signals in this study likely indicate the involvement of integrated functional networks involving the thalamus, basal ganglia, brainstem, and cerebellum, in addition to the cortex.
A direct comparison between a visually guided task to another sensory-guided task is inherently difficult since visual guidance is dominant in daily activities (Blum et al. 2007). To control for this, we reduced the visual field of view to the same as the tactile field of view, but perceptual equivalence may be different from spatial equivalence. With the same field of view between the modalities, the tracing performance was similar between modalities at the end of the first task (Fig. 2b), suggesting there was no difference in task difficulty after practice.
Tracing accuracy was not directly measured, but we visually observed TM–VM and VM–TM participants transitioning from making a few corrective movements during the first block at the start of a new modality to almost none during the last block. Given that the electrophysiological signals were assessed during the last block, and each block of data consisted of over 1.2 min of data (12 shapes with a mean trace of 6.9 s), we do not believe that our results are driven by differences in tracing accuracy due to rare short-duration corrective movements. The experimental protocol was designed with novel shapes that required participants to rely on sensory feedback to guide movement, with the intention that improved performance would reflect improved sensorimotor processing, not learning of the shapes. When asked after the experiment, participants indicated they did not explicitly learn the shapes, but became more efficient in their use of sensory guidance. Although motor output was designed to be similar across both tasks, we noticed slight variations in finger postures that could have mildly effected EEG outcomes. During the TM task, participants intuitively orientated their index finger so the angle between their finger and shape (angle of attack) was small and they could feel the bumps with the maximum area of the finger pad (Fig. 1d). In contrast, participants used a greater angle of attack during the VM task to see the maximum area through the polarized window without moving their head (Fig. 1e).
Conclusion
Tactile and visual guidance of trajectory tracking movements were each characterized by unique EEG features. TM trials were associated with more spatially focused cortical activation patterns and high alpha coherence between central, parietal, and occipital regions. VM trials were associated with more global cortical activation patterns and exhibited low global alpha coherence. While alpha coherence patterns were dominated by the sensory modality used for movement, beta connectivity was more influenced by task practice history, with the pattern initiated by the first task maintained during the second task. The presence of practice order effects suggests that deliberate task selection (i.e., practicing tactile-guided tasks before attempting similar visually guided tasks) may be a means to create particular cortical states with positive downstream behavioral effects. This may be of relevance to neurorehabilitation of hand function. Neurophysiological and behavioral characterization of modality-specific and order-specific features of trajectory tracking movements provides insight into the sensory control of ongoing movement.
References
Amedi A, Jacobson G, Hendler T, Malach R, Zohary E (2002) Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex 12:1202–1212
Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C (1999) Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122:855–870
Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Hénaff M-A, Michèl F, Barton JJ (2001) Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron 32:985–995
Blum J, Lutz K, Jäncke L (2007) Coherence and phase locking of intracerebral activation during visuo-and audio-motor learning of continuous tracking movements. Exp Brain Res 182:59–69
Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci 107:8452–8456
Braden HW, Panzer S, Shea CH (2008) The effects of sequence difficulty and practice on proportional and nonproportional transfer. Q J Exp Psychol 61:1321–1339
Bulea TC, Prasad S, Kilicarslan A, Contreras-Vidal JL (2014) Sitting and standing intention can be decoded from scalp EEG recorded prior to movement execution. Front Neurosci 8:376. doi:10.3389/fnins.2014.00376
Buschman TJ, Miller EK (2007) Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices science 315:1860–1862
Busk J, Galbraith G (1975) EEG correlates of visual-motor practice in man. Electroencephalogr Clin Neurophysiol 38:415–422
Campus C et al (2012) Tactile exploration of virtual objects for blind and sighted people: the role of beta 1 EEG band in sensory substitution and supramodal mental mapping. J Neurophysiol 107:2713–2729
Chen Y, Ding M, Kelso SJ (2003) Task-related power and coherence changes in neuromagnetic activity during visuomotor coordination. Exp Brain Res 148:105–116
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21
Desmurget M, Grafton S (2000) Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 4:423–431
Dijkerman HC, De Haan EH (2007) Somatosensory processes subserving perception and action. Behav Brain Sci 30:189–201
Gentilucci M, Toni I, Daprati E, Gangitano M (1997) Tactile input of the hand and the control of reaching to grasp movements. Exp Brain Res 114:130–137
Gomez-Ramirez M, Trzcinski NK, Mihalas S, Niebur E, Hsiao SS (2014) Temporal correlation mechanisms and their role in feature selection: a single-unit study in primate somatosensory cortex. PLoS Biol 12:e1002004
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Hsiao SS, O’shaughnessy D, Johnson KO (1993) Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 70:444–447
Jeannerod M (1995) Mental imagery in the motor context. Neuropsychologia 33:1419–1432
Kayser J, Tenke CE (2006) Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol 117:348–368
Klein RM (1977) Attention and visual dominance: a chronometric analysis. J Exp Psychol Hum Percept Perform 3:365
Klem GH, Lüders H, Jasper H, Elger C (1999) The ten-twenty electrode system of the International Federation. Int Fed Clin Neurophysiol Electroencephalogr Clin Neurophysiol Suppl 52:3
Liu H, Agam Y, Madsen JR, Kreiman G (2009) Timing, timing, timing: fast decoding of object information from intracranial field potentials in human visual cortex. Neuron 62:281–290
Miller KJ et al (2007) Spectral changes in cortical surface potentials during motor movement. J Neurosci 27:2424–2432
Mishkin M (1979) Analogous neural models for tactual and visual learning. Neuropsychologia 17:139–151
Reed CL, Klatzky RL, Halgren E (2005) What vs. where in touch: an fMRI study. Neuroimage 25:718–726
Rilk AJ, Soekadar SR, Sauseng P, Plewnia C (2011) Alpha coherence predicts accuracy during a visuomotor tracking task. Neuropsychologia 49:3704–3709
Rosenberg J, Amjad A, Breeze P, Brillinger D, Halliday D (1989) The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53:1–31
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069
Scott SH, Cluff T, Lowrey CR, Takei T (2015) Feedback control during voluntary motor actions. Curr Opin Neurobiol 33:85–94
Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M (2010) Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30:3640–3651
Smith ME, McEvoy LK, Gevins A (1999) Neurophysiological indices of strategy development and skill acquisition. Cogn Brain Res 7:389–404
Steinmetz PN, Roy A, Fitzgerald P, Hsiao S, Johnson K, Niebur E (2000) Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404:187–190
Stoeckel MC, Weder B, Binkofski F, Buccino G, Shah NJ, Seitz RJ (2003) A fronto-parietal circuit for tactile object discrimination: an event-related fMRI study. Neuroimage 19:1103–1114
Uhl F, Kretschmer T, Lindinger G, Goldenberg G, Lang W, Oder W, Deecke L (1994) Tactile mental imagery in sighted persons and in patients suffering from peripheral blindness early in life Electroencephalography and clinical. Neurophysiology 91:249–255
Winkler I, Haufe S, Tangermann M (2011) Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav Brain Funct 7:30
Wojciulik E, Kanwisher N (1999) The generality of parietal involvement in visual attention. Neuron 23:747–764
Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2:338–347
Wu HG, Miyamoto YR, Castro LNG, Ölveczky BP, Smith MA (2014) Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci 17:312–321
Acknowledgements
We wish to thank Ruby Huynh, Gabriela da Silva, and Maira Dos Santos Rodrigues for their help with data collection. This study was made possible with the financial support of the Catholic University of America School of Engineering’s Burns Fellowship and Millennium Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, P.J., Kukke, S.N. Neurophysiological features of tactile versus visual guidance of ongoing movement. Exp Brain Res 235, 2615–2625 (2017). https://doi.org/10.1007/s00221-017-4999-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-4999-z