Abstract
While concurrent augmented visual feedback of the center of pressure (COP) or center of gravity (COG) can improve quiet standing balance control, it is not known whether such feedback improves reactive balance control. Additionally, it is not known whether feedback of the COP or COG is superior. This study aimed to determine whether (1) concurrent augmented feedback can improve reactive balance control, and (2) feedback of the COP or COG is more effective. Forty-eight healthy older adults (60–75 years old) were randomly allocated to one of three groups: feedback of the COP, feedback of the COG, or no feedback. The task was to maintain standing while experiencing 30 s of continuous pseudo-random perturbations delivered by a moving platform. Participants completed 25 trials with or without feedback (acquisition), immediately followed by 5 trials without feedback (immediate transfer); 5 trials without feedback were completed after a 24-h delay (delayed transfer). The root mean square error (RMSE) of COP–COG, electrodermal level, and co-contraction index were compared between the groups and over time. All three groups reduced RMSE and co-contraction index from the start of the acquisition to the transfer tests, and there were no significant between-group differences in RMSE or co-contraction on the transfer tests. Therefore, all three groups learned the task equally well, and improved balance was achieved with practice via a more efficient control strategy. The two feedback groups reduced electrodermal level with practice, but the no-feedback group did not, suggesting that feedback may help to reduce anxiety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Augmented feedback is additional information about motor skill performance that supplements a participant’s own sensory feedback (Schmidt and Lee 2011). Provision of concurrent augmented feedback during practice of a new motor skill can improve learning of that skill (Sigrist et al. 2013). Concurrent augmented visual feedback has also been applied to re-learning balancing tasks among individuals with impaired balance due to neurologic injury (Shumway-Cook et al. 1988; Winstein et al. 1989; Sackley and Lincoln 1997; Walker et al. 2000; Ioffe et al. 2010; Sayenko et al. 2010; Tsaklis et al. 2012) or aging (Wolf et al. 1997; Rose and Clark 2000; Lajoie 2004; Sihvonen et al. 2004b; Hatzitaki et al. 2009). Typically, this is done by asking the participant to stand on one or two force platforms and providing visual feedback regarding weight distribution between the two limbs (Winstein et al. 1989; Sackley and Lincoln 1997; Hatzitaki et al. 2009), or the location of the center of gravity (COG; Rose and Clark 2000; Walker et al. 2000) or center of pressure (COP; Shumway-Cook et al. 1988; Lajoie 2004; Sihvonen et al. 2004b; Ioffe et al. 2010; Sayenko et al. 2010; Tsaklis et al. 2012). With the goal of improving quiet standing balance control when provided with feedback of the COG or COP, participants are asked to minimize movement of the COG or COP (Shumway-Cook et al. 1988; Lajoie 2004; Sayenko et al. 2010; Tsaklis et al. 2012). This type of concurrent feedback allows the participant to identify that a balance control error has occurred (i.e., that the COG or COP has travelled too far from the ideal location) and to correct this error in real time. Presumably, through repeated practice with augmented feedback, participants eventually learn to identify and correct these errors and retain this skill even after feedback is removed. Indeed, studies suggest that repeated practice with this type of feedback can improve some features of quiet standing balance control, such as stance symmetry (Shumway-Cook et al. 1988) or postural sway (Sayenko et al. 2010; Tsaklis et al. 2012), and can improve functional balance control (Lajoie 2004).
Balance control involves both anticipatory and reactive components (Huxham et al. 2001). Because balance reactions are initiated very quickly (e.g., 100 ms faster than volitionally executed movements; McIlroy and Maki 1996), it is possible that augmented feedback is only effective for improving anticipatory components of balance, and cannot be used in real time to improve reactive components. To our knowledge, only one group has studied concurrent visual feedback during practice of a reactive balance task (Wolf et al. 1997). This study found that older adults who practiced the reactive balance task (responding to external postural perturbations from a moving platform) with concurrent visual feedback of the COP position had greater improvements in some measures of reactive balance control than individuals who practiced an anticipatory balance task without visual feedback (Tai Chi) and a control group who did not do any balance exercise (Wolf et al. 1997). It is not clear whether this improved reactive balance control can be attributed to the feedback or to the reactive balance aspect of the practice. Therefore, the first objective of the current study was to determine whether concurrent augmented feedback can improve reactive balance control in response to external postural perturbations among older adults.
The type of feedback provided is another fundamental question to be addressed. The objective of balance control is to maintain or regain control of the COG relative to the base of support. In quiet standing, this is achieved by activating ankle musculature to change the location of the COP and create moments that cause the body to rotate about the ankle joint (Winter 1995). This so-called ankle strategy can also be used to regain stability following a small-magnitude postural perturbation (Shumway-Cook and Woollacott 2007). Thus, the COP is the ‘controlling’ variable and the COG is the ‘controlled’ variable. We propose that feedback that is more closely related to the goal of balance control (i.e., the COG) is more effective than feedback that relates to how balance is controlled (i.e., the COP). Therefore, the second objective of this study was to determine whether augmented feedback of the COG or COP is better for improving reactive balance control.
We used the difference between COP and COG, which represents ‘error’ in balance control (Winter et al. 1998), as our primary outcome to describe how postural stability improved with practice and learning of the task. We also measured electrodermal skin conductance activity and electromyography (EMG) throughout the sessions. The former was used as a marker of physiologic arousal, with increased arousal potentially suggesting increased physical effort (Mochizuki et al. 2009) and/or increased attention (Maki and McIlroy 1996) dedicated to performing the balance task. Improved performance of the task with increased effort and/or attention would suggest that the task was actually not well learned. EMG was used to study changes in muscle activation with practice of the task and to ensure that improved stability was not achieved via maladaptive neuromuscular strategies (i.e., co-contraction).
Methods
Participants
Forty-eight healthy community-dwelling older adults (60–75 years old) were recruited. Participants were excluded if they had any diagnosed neurologic condition (e.g., stroke or Parkinson’s disease), had any other condition that limited mobility, were unable to understand and follow instructions in English, and/or had corrected or uncorrected Snellen visual acuity worse than 20/40. Participants were allocated in a blocked randomized manner to one of three groups: (1) feedback of the COG (COGf); (2) feedback of the COP (COPf); or (3) no feedback (NoFB). Three additional NoFB participants were recruited to account for missing data for the primary outcome for three participants in the NoFB group (see “Results” section); therefore, there were 15 participants assigned to each of the COGf and COPf groups, and 18 participants assigned to the NoFB group. Participant characteristics are presented in Table 1.
Procedures
Testing occurred within two sessions. The protocol involved: an initial acquisition period; a transfer test immediately after the end of the acquisition period; and a delayed transfer/retention test after a period of approximately 24 h (Table 2). The transfer tests allow us to determine whether any improved performance with practice applies to situations where feedback is not present (Schmidt and Bjork 1992; such as in real-life instances of loss of balance). The delayed transfer test evaluates the potential permanence of improved performance.
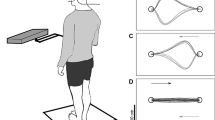
Participants stood barefoot in a standardized position (feet oriented at 14° from the sagittal plane with 17 cm of separation between the heels; McIlroy and Maki 1997) on a force plate (model BP11971197-2000, Advanced Medical Technology, Inc., Watertown, MA, USA) mounted on moving platform (Fig. 1). Participants wore a safety harness attached to an overhead support to prevent a fall to the floor in the event of a failure to recover balance. The harness was worn loosely such that no support was provided unless participants started to fall to the floor (note that no participant actually required support from the safety harness). Participants viewed a computer screen at eye level at a distance of ~75 cm (Fig. 1). Each session began with one 30-s quiet standing trial; the platform did not move for this trial, and participants were instructed to stand as still as possible. For the remainder of each session (25 Acquisition Block trials, and 5 Immediate Transfer trials in Session 1 and 5 Delayed Transfer trials in Session 2; see Table 2), each trial involved 30 s of platform oscillation in a pseudo-random manner in order to evoke balance reactions. The waveforms that described platform movement in each direction were the sum of two sine waves with frequencies ranging from 0.086 to 0.398 Hz and amplitudes ranging from 1.3 to 8 cm. There were 10 such waveforms created with root mean square of position ranging from 1.3 to 5.2 cm and root mean square of acceleration ranging from 4.7 to 9.2 cm/s2 (see Table 3). Each trial involved platform motion in the antero-posterior and medio-lateral directions, with a different waveform describing platform motion in each direction. Therefore, there were 90 possible waveform combinations. This study was concerned with antero-posterior, rather than medio-lateral, balance control as the equation for estimating COG in real time (Eq. 4; see below) is only valid in the antero-posterior direction. The amplitudes of the medio-lateral waveforms were half that of the antero-posterior waveforms as these waveforms were not intended to be as destabilizing as the antero-posterior waveforms. The medio-lateral waveforms were used to ensure that trials with the same antero-posterior waveform would feel different to the participant, and ensure that any improved performance observed would be due to improved reactive balance control rather than consciously learning the characteristics of a specific waveform. The magnitude of the platform movement was small enough that participants were able to maintain stability without moving their feet.
Participant viewing the feedback screen. The participant is standing on a force platform which is used to calculate location of the COP or COG. The empty force plate to the right of the participant was used to correct shear forces due to platform movement in order to estimate COG (see Eq. 4). The location of the COP or COG is represented as a moving line on the screen. The participant is instructed to minimize movement of the COP or COG. He is also wearing a harness attached to an overhead support to prevent a fall to the floor in the event of a failure to recover balance
During platform motion trials, COGf and COPf participants received concurrent visual feedback of the COG or COP on 50 % of practice trials; this practice schedule has been shown to improve learning of novel motor tasks compared to a 100 % feedback practice schedule (Winstein and Schmidt 1990). A yellow line showing the antero-posterior location of either the COG (COGf group) or the COP (COPf group) was displayed on the monitor during every odd-numbered trial (Fig. 2a); a stationary ‘X’ was displayed during every even-numbered trial (Fig. 2b). This type of feedback was chosen based on pilot testing and our earlier study (Lakhani and Mansfield 2015), suggesting that participants can interpret and attend to a two-dimensional (time and amplitude) signal. The display shows the COG or COP location for 1–2 s prior to the current time (Fig. 2a). This historical information on the COG or COP location may be used by participants to modify performance in the current trial. However, because of delays in using visual information to correct balance in real time, it is possible that the feedback could be used to correct performance in future trials. When viewing the COG or COP location, participants in the feedback groups were instructed to minimize movement of the line representing the COG or COP as much as possible and to try to keep the COG or COP between two red target lines (Fig. 2a). The target lines were ±5 cm from the mean antero-posterior COG or COP prior to the start of the trial; we determined from pilot testing that these targets seemed to provide optimal challenge to participants (i.e., not too difficult or too easy to keep COG/COP within the target lines). Participants in the NoFB group viewed the ‘X’ on the monitor for all trials. Participants in the COGf and COPf groups, when viewing the ‘X,’ and participants in the NoFB group were instructed to focus on the ‘X’ and try to stand as still as possible without moving their feet.
Monitors viewed by participants during the trials. a The view during feedback trials. The yellow line represents the mean COP or COG position for 1–2 s before the current time; the position is updated in real time during the trial. The horizontal white line is the mean COG or COP position during the 1 s prior to the start of the trial; the two red lines represent this baseline antero-posterior COG or COP ±5 cm. COGf and COPf participants were instructed to keep the COG or COP within the two red lines and as close to the mean COG or COP position as possible. b The ‘X’ viewed during trials with no feedback (color figure online)
The force plate measured ground reaction forces and moments during standing, which were used to calculate the location of the COP in real time. The antero-posterior location of the COG in real time was estimated using a biomechanical model that assumes that the body acts as an inverted pendulum rotating around the ankles (Winter 1995; Masani et al. 2007):
where M is the participant mass (in kg),
where H is the participant height (in m),
where COG y is the antero-posterior location of the COG, COP y is the antero-posterior location of the COP, g is acceleration due to gravity, F y1 is the antero-posterior shear force recorded from the force plate upon which the participant stood, and F y2 is the antero-posterior shear force recorded by an empty force plate on the motion platform. The shear forces recorded by the force plate that participants stood on are influenced by acceleration of the participants’ COG as well as acceleration of the moving platform; thus, this latter term in Eq. 4 corrected for the component of shear force caused by platform acceleration.
Data collection and processing
Force plate data were sampled at 250 Hz and stored for off-line processing. EMG (Telemyo Direct Transmission System, Noraxon Inc., Scottsdale, Arizona, USA) from the left medial gastrocnemius and tibialis anterior and electrodermal level (EDL; SCA1 Skin Conductance Adaptor, Grass Technologies, West Warwick, Rhode Island, USA) were recorded during all trials. For EMG, patches of skin over the muscle bellies were cleaned using mildly abrasive exfoliating cream and alcohol wipes. Disposable silver/silver chloride electrodes were placed over the belly of each muscle and connected to sensors, which transmitted wirelessly to the data collection computer via a receiver. EMG data were sampled at 1000 Hz. For EDL, the palmar surfaces of the index and middle fingers were cleaned with alcohol wipes. Reusable silver/silver chloride finger electrodes filled with conductive pasted were placed on the fingers. EDL was sampled at 250 Hz. For force plate, EMG, and EDL data, an extra 1 s of data was recorded before the start of each motion trial, and 7 s of data was recorded at the end of each trial; therefore, 38 s of data was collected for each trial.
Data were processed using custom routines implemented in MATLAB (R2014a, The MathWorks, Natick, MA, USA). Force plate data were low-pass filtered at 10 Hz using a fourth-order zero-phase-lag Butterworth filter. The COG was estimated from force plate data using the zero-to-zero point double integration method (King and Zatsiorsky 1997; Zatsiorsky and King 1998). As this method relies on integration of the force plate signal, it cannot be used to calculate COG in real time; thus, Eq. 4 was used to provide a reasonable estimate of COG location for the purpose of providing real-time visual feedback. The accuracy of the zero-to-zero point double integration method relative to the gold standard (three-dimensional kinematics) has been established (Lafond et al. 2004); therefore, this method (rather than Eq. 4) was used to estimate COG for the purpose of data analysis. There is a relatively large error in estimation of the COG at the start and end of the trial as the COG and COP are assumed to be coincident at these points in time, but are likely not; therefore, the first 1 s and last 7 s of data were discarded from each trial. The root mean square (RMS) of COG and COP displacement was calculated as a measure of amplitude of COG and COP motion, respectively, over the trial. The root mean square error (RMSE) of the difference between the COP and COG was calculated using the following equation:
where n is the total number of data points. RMSE is considered an index of effectiveness of overall balance control; since COP–COG represents ‘error’ in balance control (Winter et al. 1998), large RMSE suggests poor balance control. EDL data were low-pass filtered at 3 Hz using a fourth-order zero-phase-lag Butterworth filter. The mean EDL signal over the 1 s at the start of the trial (prior to the platform movement) was calculated as the baseline EDL. The baseline value was then subtracted from the mean EDL over the 30-s duration of each trial. The DC offset was removed from EMG signals, and signals were full-wave rectified, and low-pass filtered at 10 Hz using a fourth-order zero-phase-lag Butterworth filter. EMG signals during the motion trials were normalized to mean EMG during the quiet standing trial. The co-contraction index was then calculated using the method described by Lewek et al. (2004); that is:
that is, for each sample, EMG for the muscle with the lower amplitude is divided by EMG for the muscle with the higher amplitude, and multiplied by the sum of EMG for both muscles combined.
Statistical analysis
Statistical analyses were conducted using SAS (Version 9.2, SAS Institute, Inc., Cary, NC, USA). To better understand the effect of concurrent feedback on the outcome variables, we analyzed differences in RMSE, RMS of COP, RMS of COG, EDL, and co-contraction index between trials with and without feedback during the Acquisition Blocks for COGf and COPf participants with a one-way repeated measures ANOVA (two levels: feedback vs. no feedback). For the main hypothesis, we assumed improved learning would be demonstrated by reduced RMSE (primary outcome), EDL and/or co-contraction index in the Immediate and Delayed Transfer compared to the initial practice trials. Data were analyzed using a two-way repeated measures analysis of variance (ANOVA). The factors in the ANOVA were group (three levels: COGf, COPf, and NoFB) and trial block (seven levels: Acquisition Blocks 1–5, Immediate Transfer, and Delayed Transfer; five trials per block). Dependent variables were RMSE, RMS of COG, RMS of COP, EDL, and co-contraction index. For each ANOVA, group-by-trial block interaction effects were analyzed to determine whether one group changed more than the others between trial blocks for each variable. If significant interaction effects were found, post hoc Tukey–Kramer tests were conducted to determine where the significant pairwise differences lay. In the absence of significant interaction effects, main effects were examined. When there were significant main effects for trial block, we primarily focused on reporting differences between consecutive trial blocks (e.g., differences between Acquisition Block 1 and Acquisition Block 2 or between Acquisition Block 5 and Immediate Transfer), as differences between consecutive trial blocks indicate change over time. Alpha (level of significance) was 0.05 for all analyses.
Results
Missing data
Some data were missing due to equipment failure. Force plate data were missing for three participants (all NoFB group); therefore, analysis of RMSE and RMS of COG and COP is based on 15 participants per group. EDL data were missing for 15 participants (4 COGf, 4 COPf, and 7 NoFB); therefore, analysis of EDL is based on 11 participants per group. EMG data were missing for 12 participants (3 COGf, 3 COPf, and 6 NoFB); therefore, analysis of co-contraction index is based on 12 participants per group.
Differences between feedback and no-feedback trials
During acquisition, individuals who received feedback (either COP or COG) exhibited significantly higher RMSE (feedback trials: 6.3 mm, no-feedback trials: 5.7 mm; F 1,29 = 12.56; p = 0.0014), higher RMS of COP (feedback trials: 16.7 mm, no-feedback trials: 15.9 mm; p < 0.0001), and co-contraction index (feedback trials: 2.1, no-feedback trials: 1.9; F 1,29 = 11.06; p = 0.0029) during feedback trials than no-feedback trials. EDL was also slightly higher for feedback trials (−0.02 μS) compared to no-feedback trials (−0.06 μS); however, this difference was not statistically significant (F 1,29 = 3.80, p = 0.065). There was no significant difference in RMS of COG between feedback (13.4 mm) and no-feedback trials (13.2 mm; p = 0.14).
Learning effects between groups
Figure 3 shows balance reactions, EDL, and EMG activity at the start to the end of the acquisition period for one participant. Figure 4 shows group means for RMSE, RMS of COG and COP, EDL, and co-contraction index across both sessions.
COP and COG position, EDL, and EMG activity throughout the trial. Two trials are plotted for the same participant (COGf): the first (a) and last (b) trials in the acquisition period. Both trials had concurrent feedback and, coincidentally, used the same waveform (see the top graph showing the platform position). On the last trial, compared to the first trial, there is reduced amplitude and variability of COP position, electrodermal activity, and muscle activity. Indeed, reduced co-contraction is evident on the last trial compared to the first trial, with both the tibialis anterior and gastrocnemius being active relatively continuously throughout the first trial, whereas clear ‘on’ and ‘off’ periods are observed on the last trial
Changes in balance control, EDL, and co-contraction throughout the sessions. Changes in root mean square error (RMSE; a), root mean square (RMS) of center of pressure (COP; b), RMS of center of gravity (COG; c), electrodermal level (EDL; d), co-contraction index (e) across all trial blocks are presented. Trial blocks A1–A5 are acquisition blocks 1–5, IT is the Immediate Transfer, and DT is the Delayed Transfer. Values presented are group means with standard error bars, averaged over five trials per trial block. Asterisk indicates significant group effects at individual trial blocks; Dagger symbol indicates significant differences between individual trial blocks (see the text for further details)
Postural control measures (RMSE and RMS of COG and COP)
There was a significant group-by-trial block interaction effect for RMSE (F 12,252 = 1.85; p = 0.041). Post hoc testing revealed that COGf and COPf had significantly higher RMSE than NoFB during Acquisition Block 1 (COGf = 7.8 mm, COPf = 7.5 mm, NoFB = 6.3 mm; p values <0.0007). COGf also had higher RMSE than NoFB during Acquisition Block 3 (COGf = 6.7 mm, NoFB = 5.7 mm; p = 0.0058). There were no significant differences in RMSE between groups during any other trial block (p values >0.16). COGf and COPf significantly reduced RMSE Acquisition Block 1 to Acquisition Block 2 (Acquisition Block 2 values: COGf = 6.2 mm, COPf = 6.0 mm; p values <0.0001) but NoFB did not (Acquisition Block 2: NoFB = 5.6 mm; p = 0.38). There were no other statistically significant differences in RMSE between neighboring trial blocks for any group (p values >0.061). While there were no statistically significant differences in RMSE between neighboring trial blocks for NoFB, from post hoc testing, RMSE was significantly lower for this group during the Acquisition Block 5 (5.2 mm; p = 0.0060) and the Delayed Transfer (5.0 mm; p < 0.0001) compared to Acquisition Block 1.
There was no significant group-by-trial block interaction effect for RMS of COG (F 12,252 = 0.45; p = 0.94) or RMS of COP (F 12,252 = 1.46; p = 0.14), and no significant main effect of group for RMS of COG (F 2,42 = 0.07; p = 0.93) or RMS of COP (F 2,42 = 0.02; p = 0.97). There was a significant main effect of trial block for both RMS of COG (F 6,252 = 15.71; p < 0.0001) and RMS of COP (F 6,252 = 34.70; p < 0.0001). Post hoc testing revealed that RMS of COG was significantly lower in Acquisition Block 2 (13.3 mm) compared to Acquisition Block 1 (14.4 mm; p < 0.0001), and significantly lower in the Delayed Transfer (12.5 mm) compared to the Immediate Transfer (13.4 mm; p = 0.0002). RMS of COP was significantly lower in Acquisition Block 2 (16.3 mm) compared to Acquisition Block 1 (18.2 mm; p < 0.0001), lower in Acquisition Block 4 (16.1 mm) compared to Acquisition Block 3 (16.9 mm; p = 0.022), and lower in the Delayed Transfer (14.9 mm) compared to the Immediate Transfer (16.0; p = 0.0001).
EDL
There was a significant group-by-trial block interaction effect for EDL (F 10,180 = 3.14; p = 0.0004). Post hoc testing revealed that COPf had significantly higher EDL than NoFB during Acquisition Block 1 (COPf = 0.20 μS, NoFB = 0.05 μS; p = 0.034) and that COGf had significantly lower EDL than NoFB during the Immediate Transfer block (COGf = −0.14 μS, NoFB = 0.02 μS; p = 0.020). There were no significant differences between groups during any other trial block (p values >0.052). Both COGf and COPf significantly reduced EDL from Acquisition Block 1 to Acquisition Block 2 (COGf: Acquisition Block 1 = 0.14 μS, Acquisition Block 2 = −0.10 μS; COPf, Acquisition Block 1 = 0.20 μS, Acquisition Block 2 = −0.05 μS; p values <0.0001), NoFB did not (Acquisition Block 1 = 0.05 μS, Acquisition Block 2 = 0.05 μS; p > 0.99). There were no significant differences in EDL between any other neighboring trial blocks for any group (p values >0.93).
Co-contraction index
There was no significant group-by-trial block interaction effect (F 12,198 = 1.68; p = 0.074) or main effect for group for the co-contraction index (F 2,33 = 2.16; p = 0.13); however, there was a significant main effect for trial block (F 6,198 = 10.69; p < 0.0001). Post hoc testing revealed that the co-contraction index was significantly lower for all groups in Acquisition Block 2 compared to Acquisition Block 1 (Acquisition Block 1 = 2.34, Acquisition Block 2 = 1.84; p < 0.0001), although this effect seems to be largely driven by changes in the COGf and COPf groups rather than the NoFB group. There were no other statistically significant differences between neighboring trial blocks (p values >0.92). Of note, from post hoc testing, co-contraction index was significantly lower for all groups during the Immediate Transfer (1.62, p < 0.0001) and Delayed Transfer (1.73, p < 0.0001) compared to Acquisition Block 1.
Discussion
This study aimed to determine whether concurrent augmented feedback can improve learning of a reactive balance task, and whether feedback of the COG or COP position is better for improving reactive balance control. All three groups improved balance control from the start to the end of the acquisition period, and there were no between-group differences in balance control during the transfer tests. Therefore, we can conclude that all three groups learned the task equally well; that is, repeated practice alone is sufficient to improve performance on the task, and augmented feedback is not necessary. These findings conflict with previous work reporting improvement in some features of standing balance control following practice with augmented feedback (Shumway-Cook et al. 1988). Shumway-Cook et al. (1988) reported improved stance symmetry, but not reduced area of COP excursion, following practice of a quiet standing task with concurrent visual feedback of the COP among individuals with stroke compared to a group who practiced balance tasks without feedback. Improved stance symmetry represents a shift in the ‘reference point’ for balance, whereas the area of COP excursion may indicate the magnitude of corrective reactions to maintain equilibrium about this reference point (Zatsiorsky and Duarte 1999). It takes at least 135 ms to execute a corrective movement in response to visual information (Carlton 1981), whereas corrective reactions to low-magnitude postural perturbations (such as those used in the current study) are typically initiated 90–100 ms after the perturbation (Diener et al. 1988). Thus, it is not clear whether there is sufficient time for balance reactions to be influenced by concurrent visual feedback of the location of the COG or COP. However, it is possible that feedback might influence the slower/low-frequency components of postural control. The finding that practice with feedback of the COG and COP resulted in equivalent learning supports the findings of a previous study that included healthy young adults learning a quiet standing task (Lakhani and Mansfield 2015).
RMSE was used as our primary measure of how well participants were able to control balance. The platform movement initially causes motion of the COG, which must be corrected by moving the location of the COP (Winter 1995). High values of RMSE suggest that the COP motion was much greater than that needed to compensate for motion of the COG. In addition to reduced RMSE, RMS of COP and COG also reduced with practice of this balance task. The findings indicate that corrective movements (RMS of COP) reduced with practice, but also that these corrective movements became more effective at maintaining the COG within a stabile region. These combined findings (reduced RMSE and RMS of COP and COG) with practice indicate that participants learned to effectively control balance following instability imposed by the platform motion. In contrast to previous findings (Lakhani and Mansfield 2015), we did not find that RMS of COP was lower in the COPf group than the other groups, or that RMS of COG was lower in the COGf group than the other groups. All groups reduced RMS of COP and COG from the Immediate Transfer to the Delayed Transfer. Since both test sessions occurred on separate consecutive days, it is possible that this reduction was due to consolidation of learning with sleep (Walker and Stickgold 2006). However, there was no significant reduction in RMSE from the Immediate Transfer to the Delayed Transfer. It is possible that this was due to a floor effect in RMSE (mean values 5–6 mm on Immediate Transfer), and that participants did not have much more room for improvement.
The greatest improvements in balance control occurred early in the acquisition period. Participants in this study were healthy older adults with no known problems with balance. Other studies among healthy older adults reported improved performance and learning with practice of standing on a moving platform using similar pseudo-random waveforms to those used in the current study (Van Ooteghem et al. 2009, 2010). However, it is possible that, for participants in the current study, the task was not sufficiently challenging for participants to show improvement throughout the acquisition period. Alternatively, others have reported acquisition and long-term retention of reactive balance tasks among older adults with few practice trials (Bhatt et al. 2012; Pai et al. 2014). These authors have speculated that the threat to postural stability and risk of fall-related injury imposed by external postural perturbations drives this rapid learning (Bhatt et al. 2012); a similar process could underlie the large improvements in postural control early in the acquisition period in the current study.
The two feedback groups were initially less stable than the no-feedback group and, among the two feedback groups, RMSE was significantly higher for trials with feedback than without. Higher RMSE with feedback was due to increased amplitude of COP motion (RMS of COP) without concomitant increase in amplitude of COG motion (RMS of COG) in feedback trials compared to no-feedback trials. Increased RMSE during feedback trials might suggest a strategy to make use of the feedback presented. Alternatively, presentation of feedback creates a ‘dual-task’ situation (Lakhani and Mansfield 2015); that is, a cognitive task (viewing and interpreting the feedback) in addition to the motor task of maintaining stability. Increased RMSE (i.e., less effective balance control) during feedback trials compared to no-feedback trials may be a result of this dual-task situation. EDL was used to measure physiologic arousal during the sessions. High EDL can suggest increased physical or cognitive effort (Maki and McIlroy 1996; Mochizuki et al. 2009), anxiety (Sibley et al. 2010), or readiness to respond (Maki and Whitelaw 1993). EDL was higher for the two feedback groups than the no-feedback group during the initial acquisition block, and there was a trend toward higher EDL for trials with feedback than without feedback. Increased EDL during the initial feedback trials is likely due, in large part, to increased cognitive effort in this ‘dual-task’ situation. It is noteworthy that both feedback groups decreased arousal with practice of the task. Indeed, EDL values were, on average, negative for both feedback groups during the latter acquisition and transfer trials, indicating that arousal was lower during these latter trials than immediately prior to the onset of platform movement (see also Fig. 3b). EDL was lower for both feedback groups than the no-feedback group during these latter portions of the sessions, with the difference between the no-feedback and COGf groups being statistically significant during the Immediate Transfer. One potential explanation for this finding is that feedback that confirms that participants are improving and mastering the task (i.e., feedback showing that they are better able to keep the COG/COP within the target lines with practice) may serve to promote self-efficacy (Wright et al. 2016) and reduce anxiety associated with the platform movement. Thus, improved self-efficacy and balance confidence might explain some of the non-specific improvements in balance control that have previously been observed following balance training with concurrent visual feedback (Sihvonen et al. 2004a, b; Sayenko et al. 2012).
Co-contraction was also higher during trials with feedback compared to trials without feedback, although there were no significant between-group differences in co-contraction index. The increased co-contraction may be due to the additional attentional load imposed by the concurrent feedback. As co-contraction requires less attention than reciprocal control, others have suggested that increased co-contraction of postural muscles might be a strategy to maintain stability when standing and concurrently completing an additional cognitive task (Dault et al. 2001). There was a significant reduction in co-contraction with practice of the task for all groups, suggesting that participants learned a more efficient neuromuscular strategy for maintaining stability in response to the platform movement. This finding supports previous research reporting reduced co-contraction with practice and learning of an upper-extremity task (Osu et al. 2002).
Conclusions
Previous work has found that concurrent augmented feedback during practice can improve learning of novel motor skills. The current study suggests that such feedback is not beneficial for learning a reactive balance task; the groups who received feedback and the group who did not learned the task equally well with reduced co-contraction, which suggests a more efficient control strategy. It is possible that balance reactions are executed too quickly to allow participants to modify their reactions in real time based on information gained from the feedback. However, feedback may have other benefits; for example, the reduced physiologic arousal we observed when practicing with feedback may suggest improved self-efficacy and balance confidence, and may be particularly useful for rehabilitation of patients with reduced balance confidence.
References
Bhatt T, Yang F, Pai Y-C (2012) Learning to resist gait-slip falls: long-term retention in community-dwelling older adults. Arch Phys Med Rehabil 93:557–564
Carlton LG (1981) Processing visual feedback information for movement control. J Exp Psychol 7:1019–1030
Dault MC, Frank JS, Allard F (2001) Influence of a visuo-spatial, verbal and central executive working memory task on postural control. Gait Posture 14:110–116
Diener HC, Horak FB, Nashner LM (1988) Influence of stimulus parameters on human postural responses. J Neurophysiol 59:1888–1905
Hatzitaki V, Amiridis IG, Nikodelis T, Spiliopoulou S (2009) Direction-induced effects of visually guided weight-shifting training on standing balance in the elderly. Gerontology 55:145–152
Huxham FE, Goldie PA, Patla AE (2001) Theoretical considerations in balance assessment. Aust J Physiother 47:89–100
Ioffe ME, Chernikova LA, Umarova RM, Katsuba NA, Kulikov MA (2010) Learning postural tasks in hemiparetic patients with lesions of left versus right hemisphere. Exp Brain Res 201:753–761
King DL, Zatsiorsky VM (1997) Extracting gravity line displacement from stabilographic recordings. Gait Posture 6:27–38
Lafond D, Duarte M, Prince F (2004) Comparison of three methods to estimate the center of mass during balance assessment. J Biomech 37:1421–1426
Lajoie Y (2004) Effect of computerized feedback postural training on posture and attentional demands in older adults. Aging Clin Exp Res 16:363–368
Lakhani B, Mansfield A (2015) Visual feedback of the centre of gravity to optimize standing balance. Gait Posture 41:529–534. doi:10.1016/j.gaitpost.2014.12.003
Lewek MD, Rudolph KS, Snyder-Mackler L (2004) Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthr Cartil 12:745–751
Maki BE, McIlroy WE (1996) Influence of arousal and attention on the control of postural sway. J Vestib Res 6:53–59
Maki BE, Whitelaw RS (1993) Influence of expectation and arousal on center-of-pressure responses to transient postural perturbations. J Vestib Res 3:25–39
Masani K, Vette AH, Kouzaki M, Kanehisa H, Fukunaga T, Popovic MR (2007) Larger center of pressure minus center of gravity in the elderly induces larger body acceleration during quiet standing. Neurosci Lett 422:202–206
McIlroy WE, Maki BE (1996) Influence of destabilization on the temporal characteristics of ‘volitional’ stepping. J Mot Behav 28:28–34
McIlroy WE, Maki BE (1997) Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech 12:66–70
Mochizuki G, Hoque T, Mraz R et al (2009) Challenging the brain: exploring the link between effort and cortical activation. Brain Res 1301:9–19. doi:10.1016/j.brainres.2009.09.005
Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, Kawato M (2002) Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol 88:991–1004
Pai Y-C, Yang F, Bhatt T, Wang E (2014) Learning from laboratory-induced falling: long-term motor retention among older adults. Age 36:1367–1376
Rose DJ, Clark S (2000) Can the control of bodily orientation be significantly improved in a group of older adults with a history of falls? J Am Geriatr Soc 48:275–285
Sackley CM, Lincoln NB (1997) Single blind randomized controlled trial of visual feedback after stroke: effects on stance symmetry and function. Disabil Rehabil 19:536–546
Sayenko DG, Alekhina MI, Masani K, Vette AH, Obata H, Popovic MR, Nakazawa K (2010) Positive effect of balance training with visual feedback on standing balance abilities in people in incomplete spinal cord injury. Spinal Cord 48:886–893
Sayenko DG, Masani K, Vette AH, Alekhina MI, Popovic MR, Nakazawa K (2012) Effects of balance training with visual feedback during mechanically unperturbed standing on postural corrective responses. Gait Posture 35:339–344
Schmidt RA, Bjork RA (1992) New conceptualizations of practice: common principles in three paradigms suggest new concepts for training. Psychol Sci 3:207–217
Schmidt RA, Lee TD (2011) Motor control and learning: a behavioral emphasis. Human Kinetics, Champaign, IL
Shumway-Cook A, Woollacott MH (2007) Motor control: translating research into clinical practice. Lippincott Williams & Wilkins, Philadelphia
Shumway-Cook A, Anson D, Haller S (1988) Postural sway biofeedback: its effect on re-establishing stance stability in hemiplegic patients. Arch Phys Med Rehabil 69:395–400
Sibley KM, Mochizuki G, Frank JS, McIlroy WE (2010) The relationship between physiological arousal and cortical and autonomic responses to postural instability. Exp Brain Res 203:533–540
Sigrist R, Rauter G, Riener R, Wolf P (2013) Augmented visual, auditory, haptic, and multimodal feedback in motor learning: a review. Psychon Bull Rev 20:21–53
Sihvonen S, Sipilä S, Taskinen S, Pertti E (2004a) Fall incidence in frail older women after individualized visual feedback-based balance training. Gerontology 50:411–416
Sihvonen SE, Sipilä S, Era PA (2004b) Changes in postural balance in frail elderly women during a 4-week visual feedback training: a randomized controlled trial. Gerontology 50:87–95
Tsaklis PV, Grooten WJA, Franzén E (2012) Effects of weight-shift training on balance control and weight distribution in chronic stroke: a pilot study. Top Stroke Rehabil 19:23–31
Van Ooteghem K, Frank JS, Horak FB (2009) Practice-related improvements in postural control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp Brain Res 199:185–193
Van Ooteghem K, Frank JS, Allard F, Horak FB (2010) Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res 204:505–514
Walker MP, Stickgold R (2006) Sleep, memory, and plasticity. Annu Rev Psychol 57:139–166
Walker C, Brouwer BJ, Culham EG (2000) Use of visual feedback in retraining balance following acute stroke. Phys Ther 80:886–895
Winstein CJ, Schmidt RA (1990) Reduced frequency of knowledge of results enhances motor skill learning. J Exp Psychol Learn Mem Cogn 16:677–691
Winstein CJ, Gardner ER, McNeal DR, Barto PS, Nicholson DE (1989) Standing balance training: effect on balance and locomotion in hemiparetic adults. Arch Phys Med Rehabil 70:755–762
Winter DA (1995) Human balance and posture control during standing and walking. Gait Posture 3:193–214
Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) Stiffness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Wolf SL, Barnhart HX, Ellison GL, Coogler CE (1997) The effect of Tai Chi Quan and computerized balance training on postural stability in older subjects. Phys Ther 77:371–381
Wright BJ, O’Halloran PD, Stukas AA (2016) Enhancing self-efficacy and performance: an experimental comparison of psychological techniques. Res Q Exerc Sport 87:36–46. doi:10.1080/02701367.2015.1093072
Zatsiorsky VM, Duarte M (1999) Instant equilibrium point and its migration in standing tasks: rambling and trembling components of the stabilogram. Mot Control 3:28–38
Zatsiorsky VM, King DL (1998) An algorithm for determining gravity line location from posturographic recordings. J Biomech 31:161–164
Acknowledgments
This study was supported by the Drummond Foundation. The authors acknowledge the support of the Toronto Rehabilitation Institute; equipment and space have been funded with grants from the Canada Foundation for Innovation, Ontario Innovation Trust, and the Ministry of Research and Innovation. Avril Mansfield is supported by a New Investigator Award from the Canadian Institutes of Health Research (MSH-141983).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00221-017-4899-2.
Rights and permissions
About this article
Cite this article
Mansfield, A., Aqui, A., Fraser, J.E. et al. Can augmented feedback facilitate learning a reactive balance task among older adults?. Exp Brain Res 235, 293–304 (2017). https://doi.org/10.1007/s00221-016-4790-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4790-6