Abstract
Effects of low-threshold afferents from the flexor digitorum superficialis (FDS) to the extensor carpi radialis (ECR) motoneurons were examined using a post-stimulus time–histogram (PSTH) and electromyogram-averaging (EMG-A) methods in eight healthy human subjects. In the PSTH study in five of the eight subjects, electrical conditioning stimuli (ES) to the median nerve branch innervating FDS with the intensity below the motor threshold induced excitatory effects (facilitation) in 39 out of 92 ECR motor units. In 11 ECR motor units, the central synaptic delay of the facilitation was −0.1 ± 0.3 ms longer than that of the homonymous facilitation of ECR. Mechanical conditioning stimuli (MS) to FDS with the intensity below the threshold of the tendon(T)-wave-induced facilitation in 51 out of 51 ECR motor units. With the EMG-A method, early and significant peaks were produced by ES and MS in all the eight subjects. The difference between latencies of the peaks by ES and MS was almost equivalent to that of the Hoffmann- and T-waves of FDS by ES and MS. The peak was diminished by tonic vibration stimuli to FDS. These findings suggest that a facilitation from FDS to ECR exists in humans and group Ia afferents mediate the facilitation through a monosynaptic path.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal reflex arcs mediated by low-threshold afferent fibers among muscles in the human upper limb have been investigated extensively (Baldissera et al. 1983; Day et al. 1984; Cavallari and Katz 1989; Katz et al. 1991; Cavallari et al. 1992; Creange et al. 1992; Aymard et al. 1995; Rossi et al. 1995; Naito et al. 1996, 1998; Marchand-Pauvert et al. 2000; Ogawa et al. 2005; Suzuki et al. 2005, 2012; Wargon et al. 2006; Lourenço et al. 2007; Miyasaka et al. 2007; Nakano et al. 2014). The low-threshold afferents explored in these studies include group I fibers from muscle spindles (Ia) and Golgi tendon organs (Ib). These reflex arcs should modulate excitabilities of motoneurons to coordinate smooth voluntary movements (Rothwell 1994; Pierrot-Deseilligny and Burke 2012). A facilitatory reflex arc (facilitation) should function for co-contraction of the muscles and an inhibitory one (inhibition) for alternating contraction among the muscles (Naito et al. 1998, 2012; Naito 2003, 2004a, b; Fujii et al. 2007). The reflex arcs have been studied using a Hoffmann(H) reflex (Baldissera et al. 1983; Day et al. 1984; Creange et al. 1992; Aymard et al. 1995; Wargon et al. 2006; Lourenço et al. 2007), electromyogram-averaging (EMG-A) method (Ogawa et al. 2005; Suzuki et al. 2005, 2012), and post-stimulus time-histogram (PSTH) method (Cavallari and Katz 1989; Katz et al. 1991; Cavallari et al. 1992; Creange et al. 1992; Aymard et al. 1995; Naito et al. 1996, 1998; Marchand-Pauvert et al. 2000; Wargon et al. 2006; Lourenço et al. 2007; Miyasaka et al. 2007; Nakano et al. 2014). The H-reflex and EMG-A method are employed to evaluate effects of the reflex arcs on excitabilities of the motoneuron pool and enable us to examine effects of tonic vibration stimuli (TVS) on the transmission of group Ia afferents (Ogawa et al. 2005; Suzuki et al. 2012). The PSTH method is employed to evaluate the effects on each individual motoneuron and enable us to estimate the central synaptic delay of reflex arcs (Katz et al. 1991; Naito et al. 1996, 1998; Marchand-Pauvert et al. 2000; Miyasaka et al. 2007; Nakano et al. 2014).
Movements of hand grasping are frequently used as activities of daily living. Our recent electromyographic (EMG) study has confirmed that co-contraction of the extensor carpi radialis longus and brevis muscles (ECR), flexor digitorum superficialis muscle (FDS), thenar muscles (TM), and hypothenar muscles (HTM) is observed during the movements (Fig. 1). This observation seems to indicate a possibility that facilitations among the muscles support their co-activation during the movements. Previous studies using the PSTH and EMG-A methods showed that the ECR motoneurons received facilitation from the intrinsic hand muscles including TM and HTM (Marchand-Pauvert et al. 2000; Ogawa et al. 2005; Suzuki et al. 2005, 2012). Group Ia afferents should mediate the facilitation through a monosynaptic path in the spinal cord. The facilitation would function for supporting the hand during manipulatory movements. However, no author has presented an analysis of reflex arcs from FDS to the ECR motoneurons. In the present study, we showed a facilitation of the ECR motoneurons by low-threshold afferents from FDS using the PSTH and EMG-A methods.
Electromyographic (EMG) activities of the extensor carpi radialis (ECR), thenar muscles (TM), hypothenar muscles (HTM) and flexor digitorum superficialis (FDS) during hand grasping movements using surface electrodes. The bottom thick lines indicate the period of the movements. Co-contraction of the muscles is observed during the movements. Vertical and horizontal calibration bars represent 1.0 mV and 1.0 s
Subjects and methods
Subjects
The experiments were performed in eight healthy human subjects (six males and two females; aged 20–30 years), all of whom gave their informed consent to the experimental procedures, which were approved by the Ethics Committee of Yamagata University School of Medicine, Yamagata, Japan. The PSTH study was carried out in five (five males, 20–30 years) of the eight and the EMG-A study was in the eight subjects. During the experiments, the subject seated comfortably in an armchair. The examined right arm lay on an armrest with the shoulder slightly flexed (about 20°) and the elbow semi-flexed (about 45°). The PSTH and EMG-A studies of each subject were performed on the separate days.
Conditioning stimulation
In both the PSTH and EMG-A studies, electrical (ES) and mechanical stimulation (MS) was used as conditioning stimulation (Ogawa et al. 2005; Suzuki et al. 2005, 2012; Nakano et al. 2014). For ES, electrical rectangular pulses (1 ms duration) were delivered using an electrical stimulator (SEN-7203, Nihon Koden, Tokyo, Japan) and an isolator (SS-104J, Nihon Koden). The median nerve branch innervating FDS (FDS nerve) was stimulated with bipolar surface electrodes (0.8 cm diameter, 1.5 cm interelectrode distance) placed on the FDS muscle belly 9–15 cm distal to the medial epicondyle of the humerus. The stimulus intensity immediately below the threshold of the motor (M) wave (1.0 × MT) of FDS was used. To confirm that we stimulated the nerve branch to FDS instead of the muscle tissue itself, we selected the stimulating position carefully so that a small-step wise increase in stimulus intensity from 1.0 × MT resulted in a rapid increase in amplitude of the M-response of FDS. It was checked that no contraction of any other muscles was induced with the intensity of more than 2.5 × MT. To test the effect of the cutaneous afferents, ES with the same intensity was applied to the skin with the electrodes placed 1.0 cm medial (ulnar) to the stimulation site. For MS, a quick tap (within 5 ms) was delivered to the distal tendon of FDS 2.5–4.0 cm proximal to the proximal wrist crease between the distal tendons of the palmaris longus muscle and flexor carpi ulnaris muscle with a mechanical stimulator (DPS-280SP, Dia Medical System, Kunitachi, Japan). The tip of the actuator was covered by hard rubber (0.5 × 1.0 cm). The stimulus intensity below the threshold of a tendon(T) wave was used (depth of 0.8–2.5 mm). It was carefully checked that the homonymous facilitation of ECR was never provoked by MS with PSTH or EMG-A methods. We took care that the tip of the actuator did not deviate from the stimulation site throughout the experiment. M- and T-waves were recorded with Ag/AgCl surface electrodes (0.8 cm diameter) placed on the FDS muscle belly 11–17 cm distal to the medial epicondyle of the humerus.
The PSTH study
The PSTH method used in this study was previously described by Fournier et al. (1986). We used a personal computer with a PSTH analysis program developed by us (MTS0014, Gigatex, Osaki, Japan). Each PSTH (bin width, 0.1–0.2 ms) of the discharge of a voluntarily activated ECR motor unit was constructed for the period following the conditioning stimuli to FDS. Stimuli were triggered with a delay after voluntary activation of the motor unit. A histogram of the firing probability was constructed in a control situation without stimulation. The control and stimulus situations were alternated randomly using the same number of triggers within a sequence. To examine the stimulus effect, the number of triggers in each bin in the control situation was subtracted from that obtained after stimulation. A χ 2-test was performed for determining whether the firing probabilities after the stimulation differed from those obtained in the control situation within different time-interval windows. The period of facilitation was defined as consecutive bins by judging from a fluctuation of cumulative sum curve (Ellaway 1978). Moreover, a χ 2-test was also performed for the period from onset of the facilitation up to 1.0 ms.
Firings of an ECR motor unit were recorded with a pair of needle electrodes (Seirin acupuncture needle, 0.16 mm diameter, Seirin Kasei, Shizuoka, Japan) inserted into the muscle belly (Naito et al. 1996, 1998; Miyasaka et al. 2007; Nakano et al. 2014). The subject was requested to perform static wrist extension in the prone position of the forearm for recruiting the ECR motor unit firings. To isolate single motor unit, the subject performed a very weak (below 3 % of maximal voluntary power) but steady contraction. The contraction was achieved easily using the auditory and visual feedback of the EMG potential. Because the needle electrodes were very thin and flexible, the subject felt slight or no pain during the contraction.
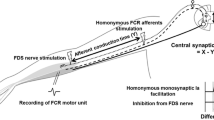
In motor units receiving facilitation, to estimate the central synaptic delay of the facilitatory process, latencies between the facilitations in PSTHs were compared following stimuli to the respective FDS nerve and homonymous ECR nerve in an ECR motor unit (Katz et al. 1991; Naito et al. 1996, 1998; Marchand-Pauvert et al. 2000; Miyasaka et al. 2007; Nakano et al. 2014). The homonymous ECR nerve (radial nerve) was stimulated at the proximal part of the medial intermuscular septum of the arm (axilla) with the above-mentioned surface electrodes. Since the efferent conduction time of the effect by the FDS nerve stimulation and the homonymous facilitation of ECR were identical, the difference between the two latencies (A) reflects the afferent conduction time between the two stimulation sites (B) and the central synaptic delay (C). B was determined by the distance between the two stimulation sites and the afferent conduction velocity of the median nerve in each subject. The velocity of each subject was determined by the difference between latencies of the homonymous facilitation evoked by the median nerve stimulation at the FDS nerve and axilla in an FDS motor unit using the PSTH method and the distance between the two stimulation sites. The subject was requested to perform static finger flexion in the supine position of the forearm for recruiting the FDS motor unit firings. The central synaptic delay (C) of the facilitation was obtained through subtraction of B from A (C = A − B).
The EMG-A study
The EMG-A technique used in this study has been described in previous reports (Capaday et al. 1990; Meunier et al. 1996; Miller et al. 1995; Petersen et al. 1998, 1999; Suzuki et al. 2005, 2012; Ogawa et al. 2005). We used a personal computer with an EMG-averaging program developed by us (TeraAve MTS00140, Gigatex). The EMG signals were rectified and averaged at least more than 100 sweeps by this program. Under conditions of delivering the conditioning stimuli to FDS, the subject performed static wrist extension. The effects of ES and MS were examined during the different sessions of the experiment. Either ES or MS was delivered at random intervals of 0.8–1.2 s during tonic voluntary contraction of ECR. The mean amplitude of a peak after the stimuli was compared with that of 10–60 ms before the stimuli (100 % control).
EMG signals were recorded with pairs of Ag/AgCl disk surface electrodes (0.8 cm diameter, NT-211U, Nihon Kohden). The electrodes were secured to the skin over the corresponding muscle belly of ECR. A ground electrode (wet gauze) put around the forearm was used as the reference. EMG signals were amplified (×2000), bandpass filtered (15–1000 Hz), and sampled at 0.2 ms. To obtain EMG activities of ECR, the subject performed isometric contraction with the level of 5 % of the maximum voluntary contraction (%Max). The level was determined by ratio to the amplitude of rectified and integrated EMG activities recorded during maximum voluntary contraction. During experiments, the level of 5 %Max was displayed on an oscilloscope (VC-6723, Hitachi, Tokyo, Japan).
To examine effects of TVS on the facilitation, TVS (100 Hz) was delivered using a portable vibrator (MD-011; Daito Electric Machine, Higashi-Osaka, Japan) for about 5 min (Ogawa et al. 2005; Suzuki et al. 2012). A steel screw bar (5 mm in diameter, 5 cm in length) was installed with the vibrator using a plastic (Ezeform Sprinting Material, Sakai Medical, Tokyo, Japan). A bell-shaped head made of the plastic was fitted with the tip of the stick. The head placed at the FDS muscle belly 18–25 cm distal to the medial epicondyle of the humerus by the experimenter’s hand. While giving the TVS with an intensity that a tonic vibration reflex was not produced, ES and MS were delivered. The stimulus intensity was adjusted by changing pressure of the head against the muscle belly for each subject. Changes of the rectified and averaged EMG activity after ES and MS with TVS were compared with those without TVS. The EMG activity was recorded before and after TVS.
To determine whether the fluctuation after the conditioning stimuli differed from those obtained in the control (μV), a paired t test was performed for each individual subject. To examine effects of TVS, one-way ANOVA with Games-Howell post hoc tests was used. The level of statistical significance was established at a p < 0.05 (Suzuki et al. 2012).
Results
The PSTH study
A total of 92 ECR motor units were studied with ES in the five subjects. In 39 of the 92 motor units (42 %), an early and significant (p < 0.05 for 20 motor units; p < 0.01 for 17 motor units; p < 0.001 for 2 motor units) peak (facilitation) was induced in every subject (Fig. 2). The latency and duration of the facilitation ranged between 20.8 and 27.0 ms (24.0 ± 1.5 ms, mean ± SD) and 0.6 and 3.2 ms (1.8 ± 0.7 ms), respectively. When a χ 2-test was performed for the period from the onset of the facilitation up to 1.0 ms, there was also significant difference (p < 0.05 for 27 motor units, p < 0.01 for 12 motor units). In the remaining 53 motor units, no significant facilitatory and inhibitory effects were provoked by ES. Such facilitation was never provoked by ES to the skin overlaying the FDS nerve.
Changes in firing probabilities of ECR motor unit evoked by an electrical conditioning stimulation (ES) to the homonymous and heteronymous group I afferents. a, b Time histograms obtained in control situation without stimuli (a) and after stimuli to FDS nerve (b). Number of triggers, 710 in a and b. c Each column represents the differences between the two histograms, the value in a is subtracted from that in b. d Cumulative sums of c. e Time histograms were obtained in the difference between the control situation without stimuli and after stimuli to the radial nerve at the axilla. Number of triggers, 240 in e. f Cumulative sums of e (f). Bin width was 0.2 ms in a–c and e. Vertical dotted lines indicate the latencies of the facilitation (24.6 ms, p < 0.01 in d; 19.4 ms, p < 0.001 in f). Abbreviations in this as well as Fig. 1
The central synaptic delay was estimated in 11 ECR motor units receiving facilitation in four subjects (Table 1; Fig. 2). The central synaptic delay of the facilitation was ranged between −0.6 and 0.5 ms (−0.1 ± 0.3 ms), which was similar as the delay of the homonymous facilitation of FDS.
A total of 51 ECR motor units were studied with MS in the five subjects. In all the 51 motor units (100 %), an early and significant (p < 0.001 for all the 51 motor units) peak (facilitation) was induced (Fig. 3). The latency and duration of the facilitation ranged between 27.6 and 36.0 ms (30.6 ± 1.9 ms), and 5.0 and 13.4 ms (8.6 ± 2.2 ms), respectively.
Changes in firing probabilities of an ECR motor unit evoked by mechanical conditioning stimulation (MS) to the FDS. a Time histograms obtained the value in control situation is subtracted from that after mechanical conditioning stimulation to FDS. Number of triggers, 435. b Cumulative sums of a. Bin width was 0.2 ms. Vertical dotted line indicates the latency of facilitation (30.8 ms, p < 0.001). Abbreviations in this as well as Fig. 1
The EMG-A study
Effects of ES and MS were studied in the eight subjects. ES produced an early and significant peak in rectified and averaged EMG of ECR in all the subjects (p < 0.05 for four subjects; p < 0.01 for four subjects) (Table 2; Fig. 4a). The amplitude, latency, and duration of the peaks were 110–135 % (120 ± 9 %), 18.2–23.0 ms (20.2 ± 1.7 ms), and 4.5–8.6 ms (6.7 ± 1.4 ms), respectively. Such effects were never provoked by ES to the skin overlaying the FDS nerve. MS also produced an early and significant peak in all the subjects (p < 0.001 for all the eight subjects) (Table 2; Fig. 4b). The amplitude, latency, and duration of the peaks were 172–230 % (200 ± 21 %), 23.4–28.8 ms (25.7 ± 1.9 ms), and 14.6–20.2 ms (17.1 ± 1.9 ms), respectively. The difference between latencies of the peaks by ES and MS was almost equivalent to that of the H- and T-waves of FDS in each subject (Table 3; Fig. 4).
Results of electromyogram-averaging study in a subject. a, b Early and significant peaks of rectified and averaged EMG activity of ECR produced by ES (a, latency, 19.0 ms; peak amplitude, 110 %; p < 0.01) and MS (b, latency, 24.6 ms; peak amplitude, 206 %; p < 0.001). The sweep is 1300 in a, and 400 in b. c, d Hoffmann(H) (c, latency, 21.4 ms) and tendon(T) waves (d, latency, 27.0 ms) of FDS produced by ES and MS. Abbreviations in this as well as Figs. 1, 2 and 3
In five subjects, the peak was diminished by TVS to FDS (Fig. 5). The peak recovered 20–30 min after TVS (ES: F 4, 175 = 69.9, p < 0.0001; MS: F 4,455 = 45.6, p < 0.0001).
Effects of tonic vibration stimulation (TVS) to FDS on facilitatory effects of ECR by ES (a–d) and MS to FDS in a subject (e–h). The peaks produced by electrical (a: mean amplitude, 109 %, p < 0.05) and mechanical stimulation (d: mean amplitude, 144 %, p < 0.001) are diminished by TVS to FDS (b, f). After TVS, the peaks disappeared or decreased (c: mean amplitude, 96 %, g: mean amplitude, 132 %). The peaks recovered 20 min after TVS (d: mean amplitude, 107 %, p < 0.05, h: mean amplitude, 144 %, p < 0.001). The sweep is 300 in a–h. A vertical calibration bar represents 10 μV. Changes in the mean amplitude of peaks before and after TVS (i: ES, j: MS). There is no significant difference between before (a, e) and after TVS (d, h; 20 min after TVS). Abbreviations in this as well as Figs. 1, 2 and 3
Discussion
In the present PSTH study, ES with intensity just below 1.0 × MT produced facilitation in 39 of 92 (42 %) ECR motor unit in every subject. Such facilitation was never provoked by ES to the skin. The remaining motor units received no effects. Since the stimulus intensity activates both group Ia and Ib afferents, it cannot be denied that group Ib afferents were responsible for the facilitation. On the other hand, MS using a quick tap with the intensity below the threshold of T-wave produced the facilitation in all (100 %) motor unit. Since a quick tap easily activates muscle spindles of the homonymous muscle (Lundberg and Winsbury 1960; Burke et al. 1983; Rothwell 1994), the facilitation would be produced by an activation of group Ia afferents rather than group Ib afferents from FDS. In the present study, the central synaptic delay of the facilitation was estimated to be −0.1 ± 0.3 ms longer than that of the homonymous facilitation of ECR. Incidentally, the afferent conduction velocity of the median nerve was almost equivalent to that of the radial nerve in each subject. This finding suggests that the facilitation is provoked through a monosynaptic path as well as the homonymous facilitation. Since the duration of the facilitation produced by ES ranged between 0.6 and 3.2 ms, it may be claimed that activities of non-monosynaptic path could be responsible for this facilitation. However, because even the very early part of facilitation (up to 1.0 ms from its onset) was significant and the duration of peaks in PSTH will be longer than the rise time of EPSPs when EPSPs are not large (Fetz and Gustafsson 1983), it is most probable that the observed facilitation was induced through a monosynaptic path. Several other mechanisms may be raised as possible candidates for producing facilitation: disynaptic Ib facilitation, oligosynaptic facilitation by cutaneous afferents, or long latency facilitation through a propriospinal path. Contributions of these mechanisms, however, are very unlikely. The central synaptic delay was too short to pass for Ib afferents with slower conduction velocity than Ia fibers or for the propriospinal system with a long pathway in the spinal cord. Further, the facilitation was never provoked by ES to the skin.
In the present EMG-A study, ES and MS produced facilitation in every subject. The latency of the facilitation in the PSTH study seems to be limited within the latency and duration of the peak in the EMG-A study in each subject [As for the EMG-A method, an argument might be raised that the extensor carpi ulnaris muscle (ECU) was activated as well as ECR during wrist extension and the facilitation observed could have been on ECU motoneurons. However, since ECU acted as wrist adductor with the prone forearm (Sagae et al. 2010), the facilitation will most likely be ascribed to that of ECR motoneurons]. The difference between latencies of the facilitation by ES and MS was almost equivalent to that of H- and T-waves of FDS. The facilitation was diminished for 20–30 min after TVS. Since TVS suppresses transmission of the homonymous group Ia afferents and the effect lasts even after TVS, group Ia afferents from FDS should be responsible for the facilitation (Katz et al. 1977; Van Boxtel 1986; Rothwell 1994; Pierrot-Deseilligny and Burke 2012; Suzuki et al. 2012). Considering the results of the present PSTH and EMG-A studies, it is suggested that ECR motoneurons receive a facilitation from FDS. Group Ia afferents should mediate the facilitation through a monosynaptic path in the spinal cord. In the present PSTH and EMG-A studies, we examined only during weak contraction. We thus investigated facilitatory effects on the low-threshold ECR motor units. The effects on the high-threshold ECR motor units are still unclear.
In the present PSTH study, MS easily produced the facilitation. The duration of the facilitation by MS was seen to be longer than that by ES. In the present EMG-A study, the peak by MS was larger in amplitude and longer in duration than that by ES. These findings suggest that MS provokes larger and longer excitatory post synaptic potentials (EPSPs) of the ECR motoneuron pool than ES. Since ES with the intensity immediately below MT activates low-threshold afferents that have lower threshold than α motor fibers, it was not efficient enough to activate all of group Ia afferents from FDS. Furthermore, it is known that a single ES provokes one firing of afferents, although a single MS provokes repetitive and desynchronized firings of group I afferents (especially group Ia afferents) (Birnbaum and Ashby 1982; Burke et al. 1983; Pierrot-Deseilligny and Burke 2012). Probably, the repetitive and desynchronized firings by MS should cause large and long-lasting EPSPs in the motoneuron pool. Because MS may have diffused to other muscle spindles, the possibility cannot be excluded that activation of heteronymous group Ia afferents participated in the facilitation. However, since the difference between latencies of the facilitation by ES and MS was almost equivalent to that of H- and T-waves of FDS, it is considered that, at least, the initial component of the facilitation by MS was induced by group Ia afferents of FDS through a monosynaptic path.
Fritz et al. (1989) and Caicoya et al. (1999) demonstrated monosynaptic facilitations mediated by group Ia afferents among muscles in the cat forelimb. Based on observations of EMGs (English 1978a, b; Illert 1996; Prochazka and Gorassini 1998a, b), they discussed the functional significance of the facilitations for locomotion behaviors of the forelimb. The present study showed a monosynaptic facilitation mediated by group Ia afferents from FDS to ECR motoneurons in the human upper limb. Since co-contraction of TM, HTM, FDS, and ECR was observed during hand grasping movement (Fig. 1), the facilitation would function for maintenance of wrist position during manipulatory movements as well as that from the intrinsic hand muscles (Marchand-Pauvert et al. 2000; Ogawa et al. 2005; Suzuki et al. 2005, 2012). Since wrist extension movements result in an increase in grasping power with tenodesis action (Kapandji 1982; Johanson et al. 1990), it should be very convenient for holding an object in the hand. Further studies are required to elucidate the other reflex arcs (e.g. reverse directions) among the muscles.
References
Aymard C, Chia L, Katz R, Lafitte C, Penicaud A (1995) Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurones? J Physiol (Lond) 487:221–235. doi:10.1113/jphysiol.1995.sp020873
Baldissera F, Campadelli P, Cavallari P (1983) Inhibition of H-reflex in wrist flexors by group I afferents in the radial nerve. Electromyogr Clin Neurophysiol 23:193–197
Birnbaum A, Ashby P (1982) Postsynaptic potentials in individual soleus motoneurons in man produced by Achilles tendon taps and electrical stimulation of tibial nerve. Electroencephalogr Clin Neurophysiol 54:469–471. doi:10.1016/0013-4694(82)90211-5
Burke D, Gandevia SC, McKeon B (1983) The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol (Lond) 339:535–552. doi:10.1113/jphysiol.1983.sp014732
Caicoya AG, Illert M, Jänike R (1999) Monosynaptic Ia pathways at the cat shoulder. J Physiol (Lond) 518:825–841. doi:10.1111/j.1469-7793.1999.0825p.x
Capaday C, Cody FW, Stein RB (1990) Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. J Neurophysiol 64:607–616
Cavallari P, Katz R (1989) Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res 78:465–478. doi:10.1007/BF00230235
Cavallari P, Katz R, Penicaud A (1992) Pattern of projections of group I afferents from elbow muscles to motoneurones supplying wrist muscles in man. Exp Brain Res 91:311–319. doi:10.1007/BF00231664
Creange A, Faist M, Katz R, Penicaud A (1992) Distribution of heteronymous Ia facilitation and recurrent inhibition in the human deltoid motor nucleus. Exp Brain Res 90:620–624. doi:10.1007/BF00230946
Day BL, Marsden CD, Obeso JA, Rorhwell JC (1984) Reciprocal inhibition between the muscles of the human forearm. J Physiol (Lond) 349:519–534. doi:10.1113/jphysiol.1984.sp015171
Ellaway PH (1978) Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45:302–304. doi:10.1016/0013-4694(78)90017-2
English AW (1978a) An electromyographic analysis of forelimb muscles during overground stepping in the cat. J Exp Biol 76:105–122
English AW (1978b) Functional analysis of the shoulder girdle of cats during locomotion. J Morphol 156:279–292. doi:10.1002/jmor.1051560209
Fetz EE, Gustafsson B (1983) Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol (Lond) 341:387–410. doi:10.1113/jphysiol.1983.sp014812
Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M (1986) Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol (Lond) 377:143–169. doi:10.1113/jphysiol.1986.sp016179
Fritz N, Illert M, De La Motte S, Reeh P, Saggau P (1989) Pattern of monosynaptic Ia connections in the cat forelimb. J Physiol (Lond) 419:321–351. doi:10.1113/jphysiol.1989.sp017875
Fujii H, Kobayashi S, Sato T, Shinozaki K, Naito A (2007) Co-contraction of the pronator teres and extensor carpi radialis during wrist extension movements in humans. J Electromyogr Kinesiol 17:80–89. doi:10.1016/j.jelekin.2005.11.01
Illert M (1996) Monosynaptic Ia pathways and motor behavior of the cat distal forelimb. Acta Neurobiol Exp 56:423–433
Johanson ME, Skinner SR, Lamoreux LW, St Helen R, Moran SA, Ashley RK (1990) Phasic relationships of the extrinsic muscles of the nomal hand. J Hand Surg Am 15:587–594
Kapandji LA (1982) The physiology of the joints, 2nd edn, vol 1. Churchill Livingstone, Edinburgh
Katz R, Morin C, Pierrot-Deseilligny E, Hibino R (1977) Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry 40:575–580. doi:10.1136/jnnp.40.6.575
Katz R, Penicaud A, Rossi A (1991) Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol (Lond) 437:269–286. doi:10.1113/jphysiol.1991.sp018595
Lourenço G, Iglesias C, Marchand-Pauvert V (2007) Effects produced in human arm and forearm motoneurones after electrical stimulation of ulnar and median nerves at wrist level. Exp Brain Res 178:267–284. doi:10.1007/s00221-006-0729-7
Lundberg A, Winsbury G (1960) Selective adequate activation of large afferents from muscle spindles and Golgi organs. Acta Physiol Scand 49:155–164. doi:10.1111/j.1748-1716.1960.tb01939.x
Marchand-Pauvert V, Nicolas G, Pierrot-Deseilligny E (2000) Monosynaptic Ia projections from intrinsic hand muscles to forearm motoneurones in humans. J Physiol 525:241–252. doi:10.1111/j.1469-7793.2000.t01-1-00241.x
Meunier S, Mogyoros I, Kiernan MC, Burke D (1996) Effects of femoral nerve stimulation on the electromyogram and reflex excitability of tibialis anterior and soleus. Muscle Nerve 19:1110–1115. doi:10.1002/(SICI)1097-4598(199609)19:9<1110:AID-MUS5>3.0.CO;2-2
Miller TA, Mogyoros I, Burke D (1995) Homonymous and heteronymous monosynaptic reflexes in biceps brachii. Muscle Nerve 18:585–592. doi:10.1002/mus.880180604
Miyasaka T, Naito A, Shindo M, Kobayashi S, Hayashi M, Shinozaki K, Chishima M (2007) Modulation of Brachioradialis motoenuron excitabilities by group I fibers of the median nerve in humans. Tohoku J Exp Med 212:115–131. doi:10.1620/tjem.212.115
Naito A (2003) Spinal mechanisms for motor control: neural connections among muscles in the human upper limb. Yamagata Med J 21:155–169 (in Japanese with English abstract)
Naito A (2004a) A method for studying spinal reflex arcs among muscles in the human upper limb: a post-stimulus time-histogram technique. Acta Anat Nippon 79:47–55. doi:10.1111/j.1447-073x.2004.00072.x
Naito A (2004b) Electrophysiological studies of muscles in the human upper limb: the biceps brachii. Anat Sci Int 79:11–20. doi:10.1111/j.1447-073x.2004.00064.x
Naito A, Shindo M, Miyasaka T, Sun Y-J, Morita H (1996) Inhibitory projection from brachioradialis to biceps brachii in human. Exp Brain Res 111:483–486. doi:10.1007/BF00228739
Naito A, Shindo M, Miyasaka T, Sun Y-J, Momoi H, Chishima M (1998) Inhibitory projections from pronator teres to biceps brachii motoneurones in humans. Exp Brain Res 121:99–102. doi:10.1007/s002210050441
Naito A, Fujii H, Sato T, Suzuki K, Nakano H (2012) Functional significance of facilitation between the pronator teres and extensor carpi radialis in humans: studies with electromyography and electrical neuromuscular stimulation. In: Schwartz M (ed) EMG methods for evaluating muscle and nerve function. InTech, Rijeka, pp 259–278. doi:10.5772/26382
Nakano H, Miyasaka T, Ogino T, Naito A (2014) Facilitation between extensor carpi radialis and pronator teres in humans: a study using a post-stimulus time histogram method. Somatosens Motor Res 31:214–220. doi:10.3109/08990220.2014.928615
Ogawa K, Suzuki K, Fujii H, Sato T, Nakano H, Sagae M, Miyasaka T, Naito A (2005) Facilitation from thenar muscles to the extensor carpi radialis in humans: a study using an electromyogram-averaging method with mechanical conditioning stimulation. Yamagata Med J 23:107–115 (in Japanese with English abstract and figure legends)
Petersen N, Morita H, Nielsen J (1998) Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods 84:1–8. doi:10.1016/S0165-0270(98)00044-2
Petersen N, Morita H, Nielsen J (1999) Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol (Lond) 520:605–619. doi:10.1111/j.1469-7793.1999.00605.x
Pierrot-Deseilligny E, Burke D (2012) The circuitry of the human spinal cord. Cambridge University Press, Cambridge
Prochazka A, Garassini M (1998a) Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J Physiol (Lond) 507:277–291. doi:10.1111/j.1469-7793.1998.277bu.x
Prochazka A, Garassini M (1998b) Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol (Lond) 507:293–304. doi:10.1111/j.1469-7793.1998.293bu.x
Rossi A, Decchi B, Zalaffi A, Mazzocchio R (1995) Group Ia non-reciprocal inhibition from wrist extensor to flexor motoneurones in humans. Neurosci Lett 191:205–207. doi:10.1016/0304-3940(95)11583-I
Rothwell J (1994) Control of human voluntary movement, 2nd edn. Chapman & Hall, London
Sagae M, Suzuki K, Fujita T, Sotokawa T, Nakano H, Naganuma M, Narita A, Sato T, Fujii H, Ogino T, Naito A (2010) Strict actions of the human wrist extensors: a study with an electrical neuromuscular stimulation method. J Electromyogr Kinesiol 20:1178–1185. doi:10.1016/j.jelekin.2010.06.003
Suzuki K, Nakano H, Sato T, Fujii H, Ogawa K, Watanabe H, Naito A (2005) Facilitation from the median nerve innervating hand muscles to the extensor carpi radialis shown in humans. Yamagata Med J 23:59–68 (in Japanese with English abstract and figure legends)
Suzuki K, Ogawa K, Sato T, Nakano H, Fujii H, Shindo M, Naito A (2012) Facilitation from hand muscles innervated by the ulnar nerve to the extensor carpi radialis motoneurone pool in humans. A study with an electromyogram-averaging technique. J Clin Neurophysiol 29:472–476. doi:10.1097/WNP.0b013e31826bdd48
Van Boxtel A (1986) Differential effects of low-frequency depression, vibration-induced inhibition, and posttetanic potentiation on H-reflexes and tendon jerks in the human soleus muscle. J Neurophysiol 55:551–568
Wargon I, Lamy JC, Baret M, Ghanim Z, Aymard C, Penicaud A, Katz R (2006) The disynaptic group I inhibition between wrist flexor and extensor muscles revisited in humans. Exp Brain Res 168:203–217. doi:10.1007/s00221-005-0088-9
Acknowledgments
We thank students of Yamagata University School of Medicine. This work was partly supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (24590230) and Grant from Yamagata Health Support Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nito, M., Hashizume, W., Miyasaka, T. et al. Facilitation from flexor digitorum superficialis to extensor carpi radialis in humans. Exp Brain Res 234, 2235–2244 (2016). https://doi.org/10.1007/s00221-016-4629-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4629-1