Abstract
Gaze pursuit is the coordinated movement of the eyes and head that allows humans and other foveate animals to track moving objects. The control of smooth pursuit eye movements when the head is restrained is relatively well understood, but how the eyes coordinate with concurrent head movements when the head is free remains unresolved. In this study, we describe behavioral tasks that dissociate head and gaze velocity during head-free pursuit in monkeys. Existing models of gaze pursuit propose that both eye and head movements are driven only by the perceived velocity of the visual target and are therefore unable to account for these data. We show that in addition to target velocity, the positions of the eyes in the orbits and the retinal position of the target are important factors for predicting head movement during pursuit. When the eyes are already near their limits, further pursuit in that direction will be accompanied by more head movement than when the eyes are centered in the orbits, even when target velocity is the same. The step-ramp paradigm, often used in pursuit tasks, produces larger or smaller head movements, depending on the direction of the position step, while gaze pursuit velocity is insensitive to this manipulation. Using these tasks, we can reliably evoke head movements with peak velocities much faster than the target’s velocity. Under these circumstances, the compensatory eye movements, which are often called counterproductive since they rotate the eyes in the opposite direction, are essential to maintaining accurate gaze velocity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex organisms have multiple motor systems that can work independently, but often require precise coordination to accomplish everyday tasks. Humans can independently move their eyes and head, but accurate vision requires coordinated movements of these two independent systems. While control of eye movements in isolation has been studied extensively, there has been significant progress on the coordination of the eyes and head during gaze shifts [Review: (Freedman 2008)] and some effort to understand their coordination during pursuit of moving targets (Ackerley and Barnes 2011; Barnes 1993; Dubrovsky and Cullen 2002; Lanman et al. 1978).
Whereas the visual field extends nearly 90° to the left and right of the midsagittal plane, the maximum excursion of a saccade is limited to around ±40° by the neuromechanical properties of the oculomotor plant and orbits. Head rotation and saccadic eye movements can be made concurrently and coordinated to produce gaze shifts that extend the range of visual targets that can be foveated to include the entire visual field (Freedman and Sparks 1997). It is possible to predict the head contribution to a gaze shift with reasonable accuracy given the amplitude and direction of the target displacement and the initial positions of the eyes in the orbits. Head contribution to the gaze shift increases when the eyes are initially rotated in the orbits in the same direction as the gaze shift, compared with gaze shifts of the same amplitude beginning with the eyes centered or deviated in the direction opposite to the direction of the impending gaze shift.
When target velocity is sufficiently low, visual tracking using a combination of head movements and smooth pursuit eye movements is often referred to as gaze pursuit. Although a few studies have investigated gaze pursuit behavior and neural circuitry, less is known about this system than gaze shifts to stationary targets. See Chapter 7 of Leigh and Zee (2006) for a review of gaze pursuit. Head movements do not reduce the latency or improve accuracy of pursuit when target motion is unpredictable (Ackerley and Barnes 2011; Dubrovsky and Cullen 2002; Lanman et al. 1978). When comparing pursuit movements made in response to predictable stimuli such as sinusoidal motion, velocity traces of gaze pursuit are not readily distinguishable from head-restrained smooth pursuit eye movements, except that when the head is free to move, targets can be pursued over larger amplitudes. Despite little change in the pursuit behavior quantifiable in gaze position and velocity, eye movements are very different when the head is free to move. Some head movement accompanies nearly all pursuit when the head is free, so the eyes must move less to compensate. Often, the eyes remain stationary in the orbits for significant portions of pursuit as the head moves to match the velocity of the target.
Because head movements are ubiquitous, models of gaze pursuit, such as described in Lanman et al. (1978), have proposed that a head controller generates a velocity command during pursuit using only the perceived velocity of the target, essentially extending the smooth pursuit models developed from head-restrained data to move the head using similar mechanisms, though with different controller parameters to account for differences between the eye and head plant. For example, Ackerley and Barnes (2011) extend the head-restrained model of Barnes and Collins (2008) by sending the same target velocity-related signal to both eye and head controllers during pursuit. Other models, such as the one described by Belton and McCrea (2000), differ from these models in their treatment of the vestibulo-ocular reflex (VOR), but still derive the head command from the velocity of the target. For simplicity, we will refer to this as the velocity-only hypothesis. It should be noted that models incorporating this hypothesis have been very accurate in predicting eye movements during head-restrained pursuit, specifically because of the impact of the saccadic system, which compensates for the position of a target. A mechanism analogous to the saccadic system has not been identified for controlling head movements, so the velocity-only hypothesis may not be effective for describing gaze pursuit when the head is not restrained.
An alternative to the velocity-only hypothesis proposes that head movements during pursuit are programmed based on both the velocity of the target and the position of the target relative to the head. Although smooth pursuit eye movements are sensitive mainly to the velocity of the target (Rashbass 1961), the behavior of tracking a moving target includes the coordinated execution of both smooth pursuit and saccades, which are sensitive to the position of the target. Both types of eye movements affect the positions of the eyes in the orbits and must be accounted for when considering the maximum amplitude of future movements. Head movements must therefore be responsive to both the velocity and position of the target in order to extend this limit under all circumstances. This is particularly important in naturalistic settings, when the trajectory and amplitude of movements are not predictable. Head movements must be integrated with saccades and smooth pursuit eye movements to achieve the behavioral goal of tracking a moving target in the real world.
In this study, we test the position–velocity hypothesis by manipulating the position of the target relative to the head without altering the velocity of the target. To determine the position of the target relative to the head, information about the visual world must be transformed from retinocentric coordinates. Two pieces of information are required to perform this calculation—the positions of the eyes in the orbits and the position of the target on the retina. We use two approaches to control each of these independently. The position–velocity hypothesis predicts that both manipulations will influence head movement made during pursuit, while the velocity-only hypothesis predicts head movement will not be altered as long as target velocity is consistent.
Methods
Two rhesus monkeys were used in this experiment. A scleral search coil was implanted in one eye of each monkey (Judge et al. 1980), and a small, stainless-steel post was attached to the skull. After recovery, monkeys were trained to perform two tasks (described below). Because these monkeys also participated in neurophysiological studies, after training, a second surgery was performed to implant a recording chamber over a trephine craniotomy. All surgical and experimental procedures were approved by the University of Rochester Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Animals.
During experiments, monkeys were placed in a custom-designed monkey chair that restrained the body but allowed free movement of the head. Before each day of data collection, a small, lightweight cam-lock device was attached to the head. A coil of Teflon-coated wire attached to the device allowed the position of the head to be determined using the same method as the implanted search coil. Three red (650 nm) lasers were also attached to the head-mounted device. The center laser aimed straightforward, aligned with the midsagittal plane, while the other two were aimed 18° away from center in the horizontal plane. The monkey chair was placed in the center of a cube containing three pairs of magnetic field coils (CNC Engineering, Seattle, WA, USA). Signals from the gaze and head coils were sampled at 1 kHz and filtered using a five-pole, low-pass Bessel filter with a cutoff frequency of 3 kHz. A second low-pass filter with a time constant of 0.3 ms was applied to the signal before digitizing. The current in the coils was linearly related to the horizontal rotational position of the coils in the field within 2° over 360°.
Visual targets were presented on the inner surface of a 1.5-m hemisphere (0.5 in acrylic; Capital Plastics, Beltsville, MD USA) using two additional red lasers attached to independent, two-axis-motorized gimbals (RGV 100 rotation stages; Newport, Irvine, CA, USA) which can present targets with <0.01° accuracy. An infrared camera positioned behind the monkey allowed the experimenters to verify that visual targets were displayed accurately and that the monkey was positioned correctly in the chair at all times.
Data analysis was performed using custom software developed in MATLAB (Mathworks). Eye and head movements were identified using a velocity and acceleration threshold that enabled more accurate detection than a velocity criterion alone. Additional plotting and statistical analysis was performed in R (R Core Team) using the ggplot2 package (Wickham 2009).
We use two behavioral paradigms to independently test the hypothesis that the position of the target relative to the head influences head movement during pursuit. In Task 1, monkeys rotate their heads so that the head is pointing some distance away from the target, and rotate their eyes in the opposite direction to maintain gaze fixation on the target before it begins to move. In Task 2, the eyes and head are both initially aligned with the target, but the target is displaced horizontally, in either direction, immediately before it begins to move.
Task 1: initial eye position
At the beginning of each trial, a red laser spot was presented on the inner surface of the presentation hemisphere, directly in front of the monkey, and monkeys were required to direct their gaze to the target. In addition, we imposed a head-position requirement. One of the three head-mounted lasers was randomly chosen to be illuminated, and monkeys were trained to align this laser with the visual target. We track the position of the head using the head-mounted gaze coil described above and required monkeys to move their head within one of three computer-defined windows corresponding with whichever of the three head-mounted lasers was chosen. The head-mounted lasers were only to indicate to the monkeys where they should align their heads, and the position of the head-mounted lasers was calibrated to maintain consistency.
With the head rotated eccentrically relatively to the target, monkeys rotate their eyes in the opposite direction to maintain accurate gaze fixation, resulting in three initial eye positions, producing the three experimental conditions. We call these conditions: centered initial eye position (IEP), forward IEP and backward IEP. It is important to note that the description of “forward” or “backward” refers to the positions of the eyes relative to the eventual direction of target motion, and that the alignment of the head does not give the monkey any cues as to which way the target will move (Fig. 1).
Diagram of target presentation and initial eye position for Task 1. This diagram shows the relative positions of the target presentation screen, a 1.5-m hemisphere, and the monkey seated at the center. We define 0° horizontal to be straightforward, aligned with the monkey’s midsagittal plane. We can present targets 90° in either direction. The top panel shows the eyes-centered condition, where the eyes, gaze and head are all aligned with the same point on the screen. The bottom panel shows the monkey maintaining the eyes rightward in the orbits. Depending on which direction the target moves, this corresponds with either the forward IEP condition for rightward movements or the backward IEP condition for leftward movements in Task 1
Once the monkey’s gaze and head were detected to be within the predetermined computer-defined windows, fixation was required for a brief, random period after which the laser spot began moving either left or right along the horizontal meridian of the hemisphere and accelerated to a randomly chosen speed (10–100°/s). Monkeys needed to maintain gaze within a computer-defined window around the target for the duration of the movement in order to receive a reward. The size of the window was dependent on the velocity of the target and was large enough that the trials continued as long as monkeys made an active effort to pursue the target. After continuing at a constant velocity, covering a distance of 40°–70°, a new velocity and trajectory were chosen that often caused the target to move obliquely, off the horizontal midline. The new velocity was randomly selected from the same range (10–100°/s) and could be the same as the initial velocity. The endpoint of the second trajectory was chosen randomly from a range of positions up to 85° in either direction horizontally and 40° vertically. This meant that the target could sometimes continue moving the same direction, but often changed direction and speed, preventing monkeys from estimating the endpoint of the first trajectory, even after thousands of repetitions, since the endpoint was unrelated to the direction or velocity of the initial trajectory, and required monkeys to pursue visual targets using only information available from the current trial. After both trajectories were complete, the target laser was turned off and monkeys were given an audible success signal and a juice reward. We observed that when the target reversed direction and began moving toward the center, monkeys often made a large, rapid head movement, uncorrelated with the velocity of the target that simply brought the head back to the central position. We therefore included only the first trajectory in our analysis, when target motion was least predictable.
Task 2: retinal position error
For the second test, we introduced a step in horizontal position before the target began to accelerate. At the beginning of each trial, a red laser spot was displayed directly in front of the monkeys. Monkeys were required to align their gaze and head within a computer-defined window around the visual target. Head alignment was obtained by illuminating the central head-mounted laser, and requiring monkeys to align this laser with the stationary target laser. Once the monkeys moved their gaze and heads within the required windows, the visual target was turned off and a second target was displayed to either the left or right of the initial fixation target (the step). The new target immediately began to accelerate to a randomly chosen speed (10–100°/s) (the ramp). We also randomly interleaved trials without a step.
This gave us three conditions to compare: a backward step, where target steps in the opposite direction of the ramp motion, a forward step, and a ramp comparable to those used in Task 1 without the second trajectory. The amplitude of the step was chosen based on the latency of the first catch-up gaze shift, and the velocity of the target, so that when the target was stepped backward, the initial catch-up saccade would often be suppressed. This is an implementation of the typical step-ramp paradigm used for both head-fixed and head-free experiments. The amplitude of the forward step was chosen to be identical, but in the opposite direction, and was expected to produce larger amplitude gaze shifts, due to the increase in retinal position error it produces.
Unlike the first task, there was no second trajectory. Monkeys were rewarded after successfully following the ramp until the target disappeared. To prevent them from learning that the endpoints of pursuit were predictable, we continued to require completion of both tasks, sometimes in the same day or interleaved. Additionally, these monkeys had been previously trained to make gaze shifts to static targets. Approximately one-third of trials on each day of data collection required making a gaze shift rather than pursuit. This meant that once the monkey had fixated on the initial target and aligned the head as required by the head-mounted laser, it had no way to predict which direction the target would move, whether it would be required to pursue the target or make a gaze shift, or whether the target would change directions at some point during pursuit. Task 2 was developed after monkeys had learned for perform Task 1. Only one task was presented during each day of data collection, but monkeys continued to perform both tasks. We did not employ a balanced presentation, so tasks are evaluated against separate control conditions described above.
Results
Task 1: controlling initial eye position
We recorded 37,476 trials (14,790 from monkey S; 22,686 from U) in which monkeys successfully completed all requirements described in the methods for Task 1. At the start of each trial, monkeys aligned their heads in one of three orientations relative to the fixation target, resulting in three different initial positions of the eyes in the orbits. First, we will describe the behavior when the eyes begin centered in the orbits, which is likely to be the assumed normal behavior in experiments that do not control initial eye position. Despite variability and idiosyncratic behavior between monkeys, as well as slight differences between movements to the left and right, we can discuss the relative timing of the gaze, eye and head movements that we observed.
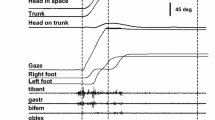
In Fig. 2, we present the positions and velocities of the eyes, head and gaze as a monkey pursues a visual target that accelerates to and maintains a speed of 40°/s. This figure and the values described here describe the behavior of monkey S. Data from monkey U are similar and can be found in Table 1. In the trials presented here, the eyes are centered in the orbits at the beginning of the trial. This means that the head and gaze are both pointed directly at the target, which appears at 0° horizontally in our coordinate system (see Fig. 1). We align a head-mounted laser with the midsagittal plane of the monkeys and consider the head to be aligned with the target when the head-mounted laser overlaps with the target laser. We show 100 ms of fixation before the target (red line) begins to move. Approximately 100 ms later, the eyes (purple lines) and gaze (black lines) begin to accelerate. The horizontal purple bar on the inset panel represents plus or minus one standard deviation from the mean pursuit latency from this monkey. The acceleration of smooth pursuit is interrupted by a gaze shift, visible as a rapid acceleration and deceleration of the eyes. This corrects for the position error that accumulates during the latency period before smooth pursuit begins. The amount of position error that accumulates increases with faster target velocities meaning the amplitude of this gaze shift is dependent on the velocity of the target. The horizontal black bar indicates plus or minus one standard deviation from the mean time of the first gaze shift during pursuit.
Gaze pursuit with eyes centered (centered IEP condition—Task 1). Example of monkey’s behavioral responses (gaze—black, head—blue, eye—purple) to visual targets moving at 30°/s (red) to the right when the eyes begin centered in the orbits. Horizontal bars indicate plus or minus one standard deviation of the latency of the different components of the movement (see Table 1)
About the same time as the gaze shift is initiated, the head (blue) also begins to move in the direction of target motion. The horizontal blue bar in Fig. 2 (inset) extends one standard deviation from the mean time of head movement onset. In the examples shown here, the head continues to accelerate, exceeding the velocity of the target. The variability of the peak head velocity is quite large and will be described in more detail in Fig. 3. During this head movement, gaze pursuit velocity is relatively stable, proportional to the velocity of the target, though occasionally interrupted by gaze shifts. To facilitate this, the eyes decelerate, and begin to rotate in the orbits in the opposite direction of target motion at the appropriate speed to maintain a constant gaze pursuit velocity.
Head velocity during pursuit with eyes centered (Task 1). This figure demonstrates the relationship between peak head velocity and target velocity. For each trial (n = 7412 from monkey U), the peak velocity of the head during the first 500 ms of pursuit is plotted against the velocity of the target on that trial. Negative velocities indicate leftward motion. Unity line (peak head velocity = target velocity) is shown (black dashed). Best fit linear regressions are shown for each direction independently (gray dashed)
In Fig. 3, we examine the relationship between peak head velocity and the velocity of the target. We find significant correlations in each direction, with R 2 > 0.6, indicating that the target’s velocity was influencing head movement, but we also see a large range off peak head velocities for each of the target velocities we tested. Although the majority of successful trials include peak head velocities greater than the target’s, we also see many instances where this is not the case, indicated by points on the opposite side of the y = x line. Linear fit models are plotted (red, dashed) and show a slope slightly greater than one with a positive y-intercept for rightward movements and a negative slope for leftward movements. This indicates that we should expect to find monkeys move their heads faster than the target, with a greater difference between peak head velocity and target velocity at higher target velocities. We also see a slightly larger slope for the fit for leftward versus rightward movements. The data shown in this figure are from monkey U. Data from monkey S are similar and reported in Table 1.
Examples of the effects of eccentric eye position on the movements are shown in Figs. 4 and 5. In the forward initial eye position (IEP) condition, the eyes begin forward in the orbits relative to the direction of target motion. In general, gaze pursuit was initiated by movement of the eyes with approximately the same latency as when the eyes were centered in the orbits, while the first gaze shift was initiated with mean latencies between 10 and 35 ms longer than when the eyes were centered. The head began moving slightly earlier (5–22 ms on average) and reached significantly higher peak velocities, which we describe in detail later. Figure 4 shows the positions and velocities of the eyes, head and gaze as a monkey pursues a visual target moving at 40°/s. Notice in the top panel that although the gaze (black) is aligned with the target (red), the head (blue) is aimed to the left of the target. The eyes (purple) are rightward in the orbits to allow gaze to be on target. This is the forward IEP condition because the eyes are to the right in the orbits when the target begins moving to the right. When the target begins to move, the head makes a large rightward movement, often overtaking the position of the target, which is similar to what was observed with the eyes centered, but the head movements are faster and larger in amplitude due to the starting position.
Gaze pursuit with the eyes forward (forward IEP condition—Task 1). Example of monkey’s behavioral responses (gaze—black, head—blue, eye—purple) to visual targets (red) moving at 30°/s to the right when the eyes begin forward in the orbits. Horizontal bars indicate plus or minus one standard deviation of the latency of the different components of the movement (see Table 1)
Gaze pursuit with the eyes backward (backward IEP condition—Task 1). Example of monkey’s behavioral responses (gaze—black, head—blue, eye—purple) to visual targets (red) moving at 30°/s to the right when the eyes begin forward in the orbits. Horizontal bars indicate plus or minus one standard deviation of the latency of the different components of the movement (see Table 1)
In the backward IEP condition, the eyes are deviated in the opposite direction of target motion. Gaze pursuit latency remained the same, while latency of the first catch-up gaze shift as slightly shorter. Head movements were slower compared to the other conditions. This effect was most noticeable at velocities 40°/s and above, where head movements were significantly slower than the target. Figure 5 shows the behavior of one monkey made in response to targets moving at 40°/s with the eyes leftward in the orbits at the start of the trial. The layout of this figure is similar to the previous two figures. The most apparent difference is that the velocity of the head does not exceed the target’s velocity. As the target moves, it moves closer to the where the head is pointed, and at 40°/s would be aligned with the head in 500 ms or less. In all of the example trials shown in Fig. 5, the head begins moving to the right, away from the position of the target, but in the direction of target motion. This finding is consistent across all velocities in both monkeys.
Table 1 summarizes the mean and standard deviation of the latency of gaze pursuit, head movement and the first gaze shift. The mean latency of pursuit is very consistent across the changes in initial eye position. There are small, but inconsistent changes in the timing of the first gaze shift. The most consistent effect appears to be that the head begins moving later during the backward IEP condition. We show the distribution of head latencies as box plots in Fig. 6. Each of the three conditions is shown for leftward and rightward movements. We also show results from monkey S and U separately. The central line of the box plot indicates the median value. The box extends to cover the 25–75th percentile. Whiskers extend for 1.5 times the length of the box, and values outside of this range are plotted individually. Notice that the median head latency is similar across conditions and monkeys, but there are significantly more outliers during the backward IEP condition. The long latencies on some trials indicate that on many trials during the backward IEP condition, pursuit continues without any noticeable head movement, even though the head is free to move.
We compare the relationship between target velocity and peak head velocity for the three conditions at each target velocity we presented (from 20 to 100°/s in 5° increments) using box plots (Fig. 7), constructed as in the previous figure. We see very little overlap of this interval between conditions for all of the velocities tested. This figure makes clear that although we can find overlap and outliers in individual trials, as the peak velocity of the head is quite variable, the overall behavior matches the intuition gained from the example trials described above. In black we represent the same data shown in Fig. 3, the centered IEP condition. The forward IEP condition, in medium gray (see Fig. 4), shows consistently higher peak head velocities, while the backward IEP condition, shown in light gray (see Fig. 5), shows consistently slower peak head velocities.
Comparison of peak head velocity during pursuit with different initial eye positions (Task 1). Box plots indicating the median (center) and 25–75th percentile (box) for the three IEP conditions at each of the rightward target velocities tested (20°–100°/s in 5 increments). Whiskers extend 1.5 times the length of the box, and individual trials not within this interval are plotted individually
In Fig. 8, we show the best linear fits for each of these conditions to quantify the effect of IEP on the relationship between target velocity and peak head velocity. These lines were fit based on the same raw data represented in Fig. 7 for rightward movements, with best fits for leftward movements also shown here. This figure also allows us to compare the behavior of our two monkeys. In terms of peak head speed, monkey S (solid lines) consistently makes faster head movements to the right, while of the two monkeys, monkey U (dashed lines) makes faster head movements to the left. Despite these differences, both monkeys show the same effects of IEP. With the eyes centered or forward in the orbits, both monkeys consistently move the head faster than the velocity of the target, represented by the unity line, y = x (dashed, gray), while with the eyes backward (red) in the orbits, head movements may be faster or slower than the target’s velocity. The largest effect of initial eye position is seen in the intercepts of these regressions. The slopes of the fits for the center and forward IEP conditions are comparable, with the magnitude of the slope slightly larger in the forward condition, especially for leftward movements. For the backward IEP condition, the slopes of the regressions are consistently less steep, but still with significant correlations.
Comparison of linear fit relationships between target and peak head velocities for pursuit with different initial eye positions and monkeys (Task 1). This figure compares the linear fit regression models for the relationship between target velocity and peak head velocity. Leftward and rightward movements are fit independently. Line color indicates the IEP condition (light gray—backward IEP, black—centered IEP, dark gray—forward IEP). Fits from monkey S are shown as solid lines, and fits from monkey U are shown in dashed lines. The unity line (target velocity = peak head velocity) is labeled y = x (thin dashed)
Task 2: controlling retinal position error
Monkeys successfully performed 37,480 trials (18,929 from monkey S; 18,551 from U) of Task 2 that introduced a step in position prior to target motion (step-ramp). Example trials are shown in Fig. 9. The left panel shows monkey S’s response to a target that begins centered at 0°, steps to the left approximately 10° and begins moving to the right at 60°/s. Notice the absence of any gaze shifts during the first 400 ms of these trials. The examples shown in the right panel are identical except that the target initially steps to the right before accelerating to 60°/s. In these trials, there is a large amplitude gaze shift to the right at the beginning of each trial. In general, stepping the target in the opposite direction of its eventual ramp motion (backward step) reduced the incidence and amplitude of the first catch-up gaze shift that is stereotypically observed during the initial portion of pursuit. We observed more than 80 % of responses with no gaze shifts in the first 400 ms of pursuit after a backward step. For the remainder, gaze shifts were usually small (<5°) and in both directions. When the target was stepped in the direction of target motion (forward step), the amplitude of the initial catch-up gaze shift was larger and always in the direction of target motion. The pursuit portion of the movement accelerated to approximately match target velocity regardless of the step and its effect on the initial gaze shift, in accordance with previous studies using the step-ramp paradigm.
Gaze pursuit of forward and backward step-ramp stimuli (Task 2). Example of monkey’s behavioral responses (gaze—black, head—blue, eye—purple) to visual targets (red) moving at 60°/s to the right when the target initially steps backward (left panel) or forward (right panel) just prior to target motion
Table 2 summarizes our findings regarding the latency of pursuit, gaze shifts and head movement, as well as the amplitude of the first gaze shift (if one was made) under the different step-ramp trial types used. The top panel of Fig. 10 shows the distribution of head latencies found on individual trials using box plots. We compare the results from the two monkeys as well as comparing leftward versus rightward movements. Although the median latency is similar across conditions, during trials with a backward step, we see many examples of head latencies of 400 ms or more. For constructing Table 2, we omit latencies longer than 400 ms, considering these to be trials without head movement. Including such outliers would bias the mean calculation to suggest that the latency of a typical head movement was longer, when a better explanation is that head movements are often not made as part of pursuit of slow-moving targets after a backward step change. Because the head is not restrained in our experiments, it is likely that some head movement will be detected during a trial, which can last 3000 ms or longer.
Head latency and gaze shift amplitude during step-ramp trials (Task 2). The latency of head movements and the amplitude of the first gaze shift during step-ramp trials for monkeys S (black) and U (gray). Top: The effect of step direction on head latency during leftward (left panel) and rightward (right panel) pursuit. Bottom: The effect of step direction on the amplitude of the first gaze shift during pursuit. Negative amplitude represents gaze shifts in the opposite direction of target motion
Head velocity varied significantly with step direction, with monkeys consistently making much faster head movements on trials with a forward step and producing slower head movements on trials with a backward step. This is apparent when comparing the head movements (blue) for the two conditions shown in Fig. 9. In example trials with a backward step, the head continues to accelerate for the duration of the movement, reaching a peak velocity slightly greater than the target by the end of the period shown. In contrast, those with a forward step show head movements that immediately accelerate to peak velocities several times the target’s velocity and to decelerate within the same period. In Fig. 11, we assess whether this effect persists at all of the velocities that we tested. We see an effect consistent with the example trials for movements in response to target velocities above 50°/s. For the slower target velocities, there was not a large separation of peak head velocities between conditions. It is important to consider that the amplitude of the step was chosen to be dependent on the velocity of the target, and thus, we were introducing greater changes in position during faster movements, which is where we also see the greatest differences between the trial types presented here.
Comparison of peak head velocities during pursuit of step-ramp stimuli of different step sizes (Task 2). Box plots indicating the median (center) and 25–75th percentile (box) for the three step-ramp conditions at each of the rightward target velocities tested (20°–100°/s in 5 increments. Also 10°/s movements from monkey S). Whiskers extend 1.5 times the length of the box, and individual trials not within this interval are plotted individually. Black—no step, ramp only. Dark gray—forward step. Light gray—backward step
Average head latency was 40–70 ms longer on trials with a backward step, and about the same or slightly longer, depending on monkey, for forward steps. We also calculated best-fit lines for the relationship between target velocity and peak head velocity for the three conditions, which are plotted in Fig. 12. The regressions are nearly identical between monkeys for rightward movements, with clear separation at higher target velocities. For leftward movements, there is more disparity between monkeys, but the influence of step direction on peak head velocity is still apparent. The regressions of backward step trials produce a smaller slope, which means that head movements may be slower than the target when it moves quickly (>70°/s). The correlations are often poor between head velocity and target velocity (as low as R 2 = 0.088), on trials with backward steps. Along with the lower slope, this indicates head movements are less influenced by the velocity of the target during these trials. We fit a larger slope for trials with a forward step, likely reflecting the combined influence of faster target velocities and larger position steps.
Comparison of linear fit relationships between target and peak head velocities for pursuit of step-ramp stimuli with different step sizes and monkeys (Task 2). Linear fit regression model for the relationship between target velocity and peak head velocity. Line color indicates the step condition (black—no step, ramp only. Dark gray—forward step. Light gray—backward step). Fits from monkey S are shown as solid lines, and fits from monkey U are shown in dashed lines. The unity line (target velocity = peak head velocity) is labeled y = x (thin dashed). Table 2. Latencies and amplitudes of movements during step-ramp trials (Task 2)
Discussion
These experiments were designed to assess the influence of target position on head movement, independent of target velocity. There are two sources of information used to determine the position of a target relative to the head: the location of the target on the retina and the location of the eyes in the orbits. Our findings demonstrate that each of these factors has significant influence on head movements made during head-free gaze pursuit and we must reject the hypothesis that these head movements are driven using only information about the target’s velocity.
In previous studies of head-free gaze pursuit, gaze and head movements have been tightly coupled, differing in latency but both moving at speeds proportional to the velocity of the target (Ackerley and Barnes 2011). Our experiments demonstrate two simple methods for dissociating gaze and head velocities during pursuit, even when the target’s movement is not predictable, and further demonstrate two additional sources of information used to generate head movements during pursuit. Introducing a position step prior to target motion was originally shown to selectively affect the saccadic system without influencing smooth pursuit (Rashbass 1961), and this paradigm has since been used repeatedly in head-free gaze pursuit for the purposes of suppressing the initial catch-up saccade without affecting non-saccadic gaze pursuit acceleration. Our results from Task 2 demonstrate that this position step also influences head movement. A step in the opposite direction of target motion produces slower, smaller head movements, while a step in the direction of target motion produces larger, faster head movements. Any experiments of head-free gaze pursuit employing a step-ramp paradigm must consider how these position steps are influencing the observed head movements. Since we chose the amplitude of the position step based on the velocity of the ramp portion of the movement (which is the standard method for using step-ramp stimuli to suppress saccades), we were introducing greater differences in the position of the target relative to the head at faster ramp velocities. Consistent with the hypothesis that head movements are sensitive to this information, we found greater disparity in the peak head velocity at faster target velocities when larger position steps were presented (Figs. 11, 12).
We also demonstrate that gaze and head movement during pursuit can be disassociated by controlling the initial positions of the eyes in the orbits, as in Task 1. The importance of considering the initial positions when assessing the contributions of the eyes and head to gaze shifts has been clearly established (Freedman and Sparks 1997). Our results indicate that the initial positions of the eyes and head are also vital to accurately predicting head and eye movement during gaze pursuit (see Figs. 4, 6, 7). It is possible that differences observed between experiments or between monkeys could result from experimental paradigms that do not control the initial positions of the eyes in the orbit and allow monkeys to make idiosyncratic choices on each trial. Wellenius and Cullen (2000) have previously reported the influence of monkeys’ choices of initial eye and head positions on head-free gaze pursuit latency, but concluded that the differences in gaze pursuit initiation were due to the elastic properties of the orbit and they did not provide any analysis of the accompanying head movements. Our present experiments show that when the eyes are deviated in the direction of target motion, larger head movements will accompany pursuit than when the eyes are centered or deviated in the opposite direction. This is consistent with the hypothesis that head movements during pursuit have peak head velocities that are increased or decreased by an amount proportional to the initial positions of the eyes in the orbits, one of the measures of the target’s position relative to the head. In all cases, gaze pursuit velocity continues to follow the velocity of the target, which is chosen independently from the initial positions of the eyes in the orbits, allowing gaze and head movements to be dissociated.
One unanticipated finding was that head movements were often faster than target velocity, even when the eyes were initially centered in the orbits. While the existence of head movements faster than the target has appeared in the literature [Dubrovsky and Cullen 2002, their Fig. 3b, Ackerley and Barnes 2011, their Fig. 5c], there has not been any discussion of these movements. One explanation is that during the latency period between the time that the target begins to move and the time when the head begins to move, the position of the target relative to the head is changing. Although the head and target are initially aligned, in the approximately 200 ms before the head starts to move, the target has moved as many as 20° (for target velocities of 100°/s). We have shown in this study that the position of the target relative to the head has significant effects on head movement, so head movements made when the head is initially aligned with the target will be driven by both the velocity of the target and the position error that accumulates during the latency period.
Overall, the pattern of behavior that we observed is consistent with the idea that head movements during pursuit are made to prevent the eyes from reaching the oculomotor limits and hindering further pursuit. Intuitively, observed head movements typically serve to bring the eyes nearer to the center of the orbits, although further examination reveals that the eyes do cover a range of positions during pursuit. For example, in Fig. 4 the eyes consistently move to the left of center while pursuing a target to the right, as the head overtakes the position of the target. Similarly, Fig. 5 similarly shows that the eyes are not immediately driven to the center, as the head makes only small movements on these trials. This suggests that head movements are serving a more complex function than to simply maintain the eyes strictly in the center of the oculomotor range, although this hypothesis is useful for predicting how the head and eyes will coordinate in a general way.
When designing the behavior tasks for this experiment, we payed special attention to ensuring that the endpoint of pursuit was not predictable. It has been well established that cognitive factors such as expectation and anticipation play a significant role in the generation of smooth pursuit eye movements as well as head movements (Barnes and Collins 2008; Ackerley and Barnes 2011). Further, monkeys can be trained to voluntarily pursue targets while maintaining the head in a relatively stable position (Suzuki et al. 2009). For our analysis, we wanted a dataset that could be analyzed on a trial-by-trial basis, in order to isolate the effects of the velocity and position of the target. A reward was only given to the monkeys for successfully following the target for the entire duration of the trial. Only gaze position was enforced once the target began to move, meaning the head was free to move in any way as long as gaze could remain on target. If we only varied the velocity of the target’s movement and not its endpoint, monkeys could use a strategy of immediately moving the head rapidly toward the endpoint, once the direction of motion was revealed, and then pursue the target using slower eye movements. Although this is an interesting behavior, we would not be able to draw any reliable conclusions about the influence of the target’s velocity or position on head movement. As our task is designed, the endpoint of the first trajectory is not predictable. Any strategy that relies on moving the head immediately toward the expected endpoint will fail because the reward is not given until the monkey successfully follows the target until the completion of the second trajectory.
It is likely that the unpredictability of our behavior tasks is biasing the behavior toward the most general strategy, and may be partly responsible for the differences in behavior we observe as compared to previous studies of gaze pursuit. Our design also required pursuit over much larger amplitudes and much higher velocities than previous studies. We intended this to put the monkeys in situations where making large head movements was advantageous or even required to complete the tasks. Other studies have kept pursuit amplitudes small, even when the head is free to move in order to facilitate direct comparison with head-fixed data, but compensate by training monkeys to pursue targets with their heads (Suzuki et al. 2009). Our methods are more comparable with behavior in the natural world, where visual targets do not always remain observable within the oculomotor limits, and can change direction and velocity at any time.
Nevertheless, since we have shown that gaze and head movements during pursuit can be dissociated, we can reassess some open questions in gaze pursuit coordination, such as the role of the vestibulo-ocular reflex (VOR). The activity of the VOR is often considered counterproductive to the goal of gaze pursuit, particularly when the velocity of the head matches the velocity of the target, and the eyes are largely stationary in the orbits. When this happens, VOR signals will drive the eyes in the opposite direction of pursuit and must therefore be countermanded in some way. Proposed methods for this include suppressing the VOR signal so that it does not affect eye movement or continuing to drive the eyes with a smooth pursuit signal that is canceled by the VOR. Evidence has been found to support both of these hypotheses. Head brake experiments have revealed a hidden pursuit command that is observed when the head suddenly stops. The eyes accelerate after head braking with a latency too short to be the result of a newly initiated command (Lanman et al. 1978). A similar experiment examining this sudden acceleration indicates that the eyes do not immediately reach the desired gaze velocity, suggesting that the smooth pursuit command is altered after head braking, meaning that the VOR is partially suppressed during pursuit (Huebner et al. 1992). Evidence for a labile VOR gain is prevalent in the literature, with the gain of the VOR changing with the existence of a visual target or with instructions to imagine a visual target in darkness (Barnes 1993).
Further evidence for VOR suppression during gaze pursuit comes from the examination of position-vestibular-pause (PVP) neurons in the vestibular nuclei, which have been proposed as a neural correlate of VOR suppression (Roy and Cullen 1998). These neurons pause their firing during active head movements (Roy and Cullen 2002), which has been suggested to allow monkeys to distinguish between afferent and reafferent vestibular signals (Cullen 2012). This is a challenging hypothesis for researchers to test experimentally because it predicts that the VOR will appear to be active when the head is moved by external perturbations, such as those employed in head braking experiments, but will still be suppressed for active, self-generated head movements. Earlier, a similar mechanism was proposed to account for the response of the VOR during gaze shifts (Freedman and Sparks 2000; Freedman 2001), and the best evidence for suppression of the VOR comes from studies on gaze shifts.
With head and gaze movements during gaze pursuit dissociated, we can consider how head movements influence ongoing pursuit without relying on external perturbation. We observe that during pursuit, the head often makes movements that are intentionally different from gaze velocity. Under these circumstances, the VOR is not counterproductive. When the head is driven by signals other than desired gaze velocity, suppressing the VOR would actually introduce additional challenges because it would allow signals unrelated to gaze to influence gaze pursuit. In our experiments, we observed head movements with peak velocities 2–3 times as fast as the target. Since these were made as an intentional component of accurate gaze pursuit, if the gain of the VOR were reduced during gaze pursuit, some other, yet proposed, mechanism would need to compensate for these head movements to reduce gaze pursuit velocity.
References
Ackerley R, Barnes GR (2011) The interaction of visual, vestibular and extra-retinal mechanisms in the control of head and gaze during head-free pursuit J Physiol. doi:10.1113/jphysiol.2010.199471
Barnes GR (1993) Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog Neurobiol 41:435–472
Barnes GR, Collins CJ (2008) Evidence for a link between the extra-retinal component of random-onset pursuit and the anticipatory pursuit of predictable object motion. J Neurophysiol 100:1135–1146. doi:10.1152/jn.00060.2008
Belton T, McCrea RA (2000) Role of the cerebellar flocculus region in the coordination of eye and head movements during gaze pursuit. J Neurophysiol 84:1614–1626
Cullen KE (2012) The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35:185–196. doi:10.1016/j.tins.2011.12.001
Dubrovsky AS, Cullen KE (2002) Gaze-, eye-, and head-movement dynamics during closed- and open-loop gaze pursuit. J Neurophysiol 87:859–875
Freedman EG (2001) Interactions between eye and head control signals can account for movement kinematics. Biol Cybern 84:453–462
Freedman EG (2008) Coordination of the eyes and head during visual orienting. Exp Brain Res 190:369–387. doi:10.1007/s00221-008-1504-8
Freedman EG, Sparks DL (1997) Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77:2328–2348
Freedman EG, Sparks DL (2000) Coordination of the eyes and head: movement kinematics. Exp Brain Res 131:22–32
Huebner WP, Leigh RJ, Seidman SH, Thomas CW, Billian C, DiScenna AO, Dell’Osso LF (1992) Experimental tests of a superposition hypothesis to explain the relationship between the vestibuloocular reflex and smooth pursuit during horizontal combined eye-head tracking in humans. J Neurophysiol 68:1775–1792
Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20:535–538
Lanman J, Bizzi E, Allum J (1978) The coordination of eye and head movement during smooth pursuit. Brain Res 153:39–53
Leigh RJ, Zee DS (2006) The neurology of eye movements. Oxford University Press. Table of contents only http://www.loc.gov/catdir/toc/ecip0517/2005022301.html Publisher description http://www.loc.gov/catdir/enhancements/fy0637/2005022301-d.html
Rashbass C (1961) The relationship between saccadic and smooth tracking eye movements. J Physiol 159:326–338
Roy JE, Cullen KE (1998) A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci 1:404–410. doi:10.1038/1619
Roy JE, Cullen KE (2002) Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87:2337–2357
Suzuki DA, Betelak KF, Yee RD (2009) Gaze pursuit responses in nucleus reticularis tegmenti pontis of head-unrestrained macaques. J Neurophysiol 101:460–473. doi:10.1152/jn.00615.2007
Wellenius GA, Cullen KE (2000) A comparison of head-unrestrained and head-restrained pursuit: influence of eye position and target velocity on latency. Exp Brain Res 133:139–155
Wickham J (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
C. Pallus, A., G. Freedman, E. Target position relative to the head is essential for predicting head movement during head-free gaze pursuit. Exp Brain Res 234, 2107–2121 (2016). https://doi.org/10.1007/s00221-016-4612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4612-x