Abstract
Motor imagery (MI), the mental rehearsal of motor tasks, has promise as a therapy in post-stroke rehabilitation. The potential effectiveness of MI is attributed to the facilitation of plasticity in numerous brain regions akin to those recruited for physical practice. It is suggested, however, that MI relies more heavily on regions commonly affected post-stroke, including left hemisphere parietal regions involved in visuospatial processes. However, the impact of parietal damage on MI-based skill acquisition that underlies rehabilitation remains unclear. Here, we examine the contribution of the left inferior parietal lobule (IPL) to MI using inhibitory transcranial magnetic stimulation (TMS) and an MI-based implicit sequence learning (ISL) paradigm. Participants (N = 27) completed the MI-based ISL paradigm after receiving continuous theta burst stimulation to the left IPL (TMS), or with the coil angled away from the scalp (sham). Reaction time differences (dRT) and effect sizes between implicit and random sequences assessed success of MI-based learning. Mean dRT for the sham group was 36.1 ± 28.2 ms (d = 0.71). Mean dRT in the TMS group was 7.7 ± 38.5 ms (d = 0.11). These results indicate that inhibition of the left IPL impaired MI-based learning. We conclude that the IPL and likely the visuospatial processes it mediates are critical for MI performance and thus MI-based skill acquisition or learning. Ultimately, these findings have implications for the use of MI in post-stroke rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motor imagery (MI), the mental rehearsal of a motor task (Jeannerod 1995), has been shown to be a useful adjunct to physical practice (PP) to aid skill acquisition in numerous domains (Wulf et al. 2010; Moran et al. 2012). Recent work from our laboratory has demonstrated that MI, independent of PP, is an effective means of facilitating the learning of implicit perceptual-motor skills (Kraeutner et al. 2015). Establishing that MI alone can effectively drive learning lends support to its use in disciplines wherein PP is not always possible, including rehabilitation following neurological injury such as stroke (Sharma et al. 2006, 2009; Johansson 2011). Due to the reported parallels in brain activation (Zhang et al. 2012; Hétu et al. 2013; Kraeutner et al. 2014), MI is thought to drive brain plasticity akin to that of PP, thus providing the basis for why MI is effective as a form of practice (Jeannerod 1995, 2001; Wulf et al. 2010). What is not entirely clear in the literature examining MI is how stroke-related brain damage impacts its effectiveness as a modality for skill acquisition. Determining how brain damage impacts on MI-based skill acquisition is critical to understanding its role in post-stroke rehabilitation.
Damage to parietal regions, commonly observed post-stroke, is suggested to affect the ability to perform MI (McInnes et al. 2015). Buch et al. (2012), studying stroke-related lesions, sought to assess structural and functional morphology relating to imagery of a grasping task. Connectivity between premotor and posterior parietal regions was shown to correspond with successful task performance. Thus, the authors suggest that parietal integrity may be necessary for MI (Buch et al. 2012). Other work has shown that damage to the parietal cortex impairs the generation of movement representations via MI, evidenced by increased time to imagine versus execute a movement (i.e., mental chronometry; Sirigu et al. 1996). Lastly, parietal cortex damage was recently shown to impair or altogether prevent the performance of MI, based on the findings of 23 studies that provided a measure of ability to perform MI (McInnes et al. 2015). As it seems the ability to perform MI is impaired following parietal cortex damage, it stands to reason that so to would MI-based skill acquisition. While these aforementioned studies show MI to be compromised to varying degrees following parietal cortex damage, all assessed MI ability using subjective rating scales rather than measuring the outcome of MI, which in most instances is skill acquisition or learning. As such, it has not been possible to identify the effect of parietal cortex damage on MI-based skill acquisition. Further, the results of these lesion-based studies are difficult to interpret due to the limited number of cases and the variability in lesion location and size (Rorden and Karnath 2004).
Results from neuroimaging studies support the involvement of the parietal cortex in MI, demonstrating that regions within the parietal cortex are activated to a greater extent in MI relative to PP (Burianová et al. 2013; Hétu et al. 2013). While parietal cortex involvement in MI is attributed to the recruitment of stored motor representations (Cooke et al. 2003; Hétu et al. 2013), the left parietal cortex specifically, including the inferior parietal lobule (IPL), is suggested to be involved in processes related to motor attention that are critical for movement selection, planning, and visuospatial integration, due to its involvement in the dorsal visual pathway (Rushworth et al. 2001, 2003; Rizzolatti and Matelli 2003). Indeed, the left IPL has been shown to be active during MI in numerous studies (for review see Hétu et al. 2013). For instance, a study involving MI and PP of simple finger movements using both hands demonstrated that the left IPL was more involved in MI compared to PP regardless of the hand that was imagined. Further (Kawamichi et al. 1998), investigating the time course of activation patterns during an MI-based hand rotation task, demonstrated that the IPL was a critical region of information processing between visual and premotor areas. Taken together, the evidence suggests that the left IPL plays a key role in MI, although the nature of this role as well as the impact of damage to the IPL in MI-based skill acquisition remains unknown.
Based on the above-noted evidence, damage to regions within the parietal cortex (notably the IPL) may limit the effectiveness of MI for skill acquisition, rendering it impractical for aiding functional recovery after stroke. While it is difficult to link impairment in MI ability to lesion location in patient populations owing to the high degree of variability, a technique called repetitive transcranial magnetic stimulation (rTMS) permits the modulation of cortical activity in non-disabled individuals (Miyawaki et al. 2012). Continuous theta burst stimulation (cTBS), a form of rTMS that results in transient inhibition of the targeted brain area, is being used in an increasing number of studies as this protocol uses fewer pulses over a shorter duration of stimulation (typically 40 s) compared to typical low-frequency rTMS paradigms (Chen et al. 2003; Oberman et al. 2011). While cTBS and other TMS protocols represent an ideal approach to probing the role of the parietal cortex in MI, few studies have employed these techniques (de Vries et al. 2009; Lebon et al. 2012).
The current study thus seeks to identify the effect of damage to the IPL on MI-based skill acquisition through the use of non-invasive brain stimulation to induce a ‘virtual lesion’ prior to MI-based practice of a novel skill. Skill acquisition will be determined using an MI-based implicit sequence learning (ISL) task, for which we have previously demonstrated learning via MI without prior PP, whereby faster reaction times (RTs) to a practiced sequence indicate successful learning (Kraeutner et al. 2015). If the IPL is indeed critical to MI performance or MI-based skill acquisition, inhibition via brain stimulation should impair learning. As such, we hypothesize that following inhibition of the left IPL, skill acquisition will be impaired relative to those receiving sham or no stimulation. Demonstrating that inhibition of the left IPL diminishes MI-based skill acquisition will provide important information related to how MI should be applied in post-stroke rehabilitation. Importantly, these results provide an opportunity to explore the nature of the role of the left IPL in MI-based skill acquisition.
Method
Participants
Twenty-seven right-handed participants (17 females, aged 21.9 ± 4.1) naïve to any form of brain stimulation took part in the study. Handedness was demonstrated by a score of ≥40 on the Edinburgh Handedness Inventory (Oldfield 1971). All were healthy and free of neurological disorder, and each provided written informed consent. All participants self-reported to have normal hearing and verbally confirmed they understood the instructions prior to the study onset, and each was free of contraindications to TMS (Rossi et al. 2009). The study received approval from the Research Ethics Board of the Capital District Health Authority. Prior to the onset of the study, participants were randomized into a sham or TMS group. To contextualize the findings, data from the sham and TMS groups were compared to that of a control group (N = 24; 16 females; aged 23.6 ± 5.3 years; Kraeutner et al. 2015) collected as part of a previous study that compared MI-based practice of the ISL paradigm to PP of the same task. Participants in this control group completed the experimental procedure as outlined below with the exception of TMS.
Experimental task
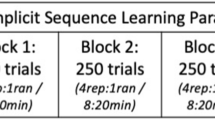
The experiment involved four training blocks of an MI-based ISL task followed by a physical test block (Fig. 1), as employed in Kraeutner et al. (2015). The task involved MI of button presses with the non-dominant (left) hand. All participants performed the task sitting at a chair in front of a computer screen oriented at eye level, with their left hand resting comfortably over the keyboard. Participants were oriented to four keys (V, C, X and Z) numbered 1–4 from right to left, representing the index, middle, ring and little finger, respectively, and were instructed to close their eyes and imagine themselves performing the button presses that were cued auditorily through noise-canceling headphones. An auditory tone was played, and their response was recorded if participants pressed a button during the training blocks. Each individual imagined key press event lasted 1.5 s based on the time separating consecutive auditory cues. Each training block consisted of 250 trials, with a 5-min rest block provided between each.
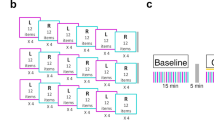
Illustration of the experimental setup depicting: a the timeline of the single experimental session. A reaction time (RT) test involving physical performance (i.e., key presses) of the ISL task and verbal report assessment followed MI-based practice and b the placement of targets shown via Brainsight 2™ including the grid placement over the motor cortex for hot spot localization, and localization of the IPL
Embedded within the training blocks was a repeated sequence (Goschke and Bolte 2012) that consisted of ten, non-repeating digits unique for each participant. The implicit sequence appeared 80 % of the time throughout each block (20 sequences total), while random sequences of equal length appeared 20 % of the time (5 sequences total). The order in which the sequences appeared was randomized. Participants were blind to the fact that sequences were incorporated within each block and were thus naive to the implicit learning nature of the task.
To ensure the absence of muscle activity during MI-based practice, the electromyogram (EMG) was obtained from the left flexor and extensor muscles of the digits (anterior and posterior aspects of the forearm, respectively). The EMG signal was acquired using self-adhering electrodes (1 × 3 cm; Q-Trace Gold; Kendall-LTP, USA) in a bipolar configuration with a 1-cm inter-electrode distance, sampled at 1000 Hz with a bandpass of 25–100 Hz (1902 and Power 1401; Cambridge Electronics Design, UK) and stored for off-line analysis.
Transcranial magnetic stimulation
Neuronavigated TMS was administered using a Brainsight 2™ (Rogue Research Inc., Montreal, Canada) navigation system and an air-cooled 70-mm figure-of-eight coil connected to a SuperRapid2Plus1 magnetic stimulator (Magstim, Whitland, UK). Prior to each TMS session, three anatomical landmarks (nasion, right and left preauricular points) were digitized for each participant and co-registered with a template brain (MNI152_T1_1mm) to facilitate accurate positioning and orientation of the TMS coil.
Resting motor threshold (RMT) was determined by measuring the peak-to-peak amplitude of motor-evoked potentials (MEPs) recorded via surface EMG overlying the right first dorsal interosseous (FDI) muscle. EMG was obtained using vendor-supplied hardware (Brainsight EMG Isolation Unit and Amplifier Pod). Briefly, a 5 × 5 grid with 7.5-mm spacing was overlaid on the template brain with the midpoint (location 2, 2) centered on the ‘hand knob’ of the left primary motor cortex (Fig. 1; Kleim et al. 2007). Stimulator output was set to 55 %, and points on the grid were stimulated starting from 2,2 with the coil positioned tangentially to the scalp with the handle at a 45° angle to the posterior, working outwards from the center in a counterclockwise manner to determine the location(s) that produced the highest amplitude MEPs for 5 out of 10 stimulations. Once the ‘hot spot’ was localized, the RMT was determined as the lowest stimulator output where a MEP of an amplitude of ≥50 μV was obtained on 5 out of 10 stimulations and confirmed by stimulating grid points around the hot spot again in a counterclockwise manner with the resultant stimulator output. Following determination of the RMT, inhibitory stimulation was delivered to the left IPL using a cTBS paradigm following established practices (Huang et al. 2005; Oberman et al. 2011). cTBS intensity was set at 90 % of RMT (Nyffeler et al. 2008, 2009) and delivered in bursts of three stimuli at 50 Hz pulses, repeated at intervals of 200 ms for a total of 600 pulses (Huang et al. 2005). Activation peaks from a study comparing activation during MI to PP of a similar button-press sequence task (Kraeutner et al. 2014) was used to localize IPL in MNI space (−36, −32, 34). The location of stimulation (i.e., the left IPL) across participants is shown in Fig. 1. To validate our target location, we co-registered a participant’s head to their anatomical MRI (obtained in previous work) and identified the IPL using the coordinates provided above. Results of this validation showed a high degree of similarity to localization on the template brain (see Fig. 1 Supplementary material). Participants receiving sham stimulation underwent the same procedures as that of the TMS group, with the exception that during cTBS, the TMS coil was placed over the vertex of the head and stimuli were delivered at 15 % of RMT.
Experimental procedure
Following informed consent and TMS screening, participants first completed the KVIQ (Malouin et al. 2007) to establish their ability to perform MI prior to the MI-based training. Ability to perform MI was based on achieving a score on the KVIQ within the range previously reported for healthy control subjects (Malouin et al. 2007). The KVIQ is an assessment of imagery ability that involves the performance of five body movements, followed by imagery of these movements. The KVIQ has high internal reliability and validity in both healthy controls and clinical populations (Malouin et al. 2007). Participants then underwent the TMS procedures as described above. Following administration of the cTBS, all participants completed a MI familiarization block (Fig. 1; Kraeutner et al. 2015). During this block, participants listened to an audio recording describing the type of MI to be performed (kinesthetic), and the task to be imagined. Kinesthetic MI was selected as this type of MI is proposed to better facilitate basic motor skill learning (Stinear et al. 2006). The audio recording emphasized the poly-sensory aspects of MI, directing the participants to attend to sensory information related to task performance, which has been shown to facilitate MI (Braun et al. 2008). Upon completion of the familiarization block, participants began the first of the four MI-based ISL training blocks.
Immediately following the MI-based training, participants performed two assessments to measure skill acquisition (Fig. 1). The first assessment was a RT test. Participants repeated a shortened block of the auditory-cued sequence task but were instructed to respond ‘as quickly as possible’ physically pressing the indicated key. In this test block, each cue was presented immediately following the previous response, and the implicit and random sequences of equal length appeared 10 times each (i.e., a 1:1 ratio) for a total of 200 trials. The order that the sequences appeared was again randomized, and an auditory tone was played if participants provided an incorrect response (e.g., pressed the ‘4’ key when the ‘2’ key was cued). Responses and the corresponding RTs were recorded for off-line analysis. As in the training blocks, participants remained naive to the implicit sequence nature of the task.
The second assessment was a verbal report, the purpose of which was to determine whether explicit or implicit learning had occurred. Participants were first informed that the purpose of the training was to teach them a 10-digit sequence. Participants were then asked to respond to the question ‘Do you think you learned a sequence during the training blocks?’ For ‘yes’ responses, participants were asked if they could report the sequence that they learned (i.e., the 10 consecutive numbers). For ‘no’ responses, participants were also asked if they could report the sequence to further confirm their negative response. Participants were instructed ‘it was okay if they did not think they learned a sequence.’
Data analysis
Data analysis followed procedures reported in our prior work (Kraeutner et al. 2015).
Identifying explicit learners
Participants that demonstrated explicit learning were excluded from further analysis. Specifically, participants that answered ‘yes’ to the question of whether or not they thought they learned a sequence and who correctly reported more than 50 % of the sequence (i.e., 5 consecutive sequence elements), were excluded from further analyses.
Response analysis
Analysis of the responses made during the MI training blocks was performed to identify and remove participants who had actually performed button presses and had thus experienced a degree of PP during the MI-based training. Participants that made responses >2 % (20/1000 responses total) of the time across all training blocks were excluded from further analyses.
EMG analysis
Analysis of the EMG data obtained during MI was performed to further identify and reject participants that demonstrated PP during the MI-based training (Kraeutner et al. 2015). Data were first rectified, and a 10-Hz low-pass filter was applied. The absence of activity in the left flexor and extensor muscles of the digits during MI was determined by calculating the average amplitude across 15-s envelopes of the EMG signal during each training block and comparing each to a 15-s envelope acquired during the familiarization block (during which participants were at rest). The EMG threshold was defined as the average rest amplitude plus 2 standard deviations. Participants were excluded from further analysis if >15 % of the comparisons exceeded the threshold.
Performance analysis
For the RT task, the first element of each sequence was omitted from analysis as per (Wohldmann et al. 2007) due to its role in perception versus motor execution (i.e., providing a cue to the movement about to be performed). RTs for trials that occurred before 100 ms and after 1300 ms were removed from analysis to control for anticipatory and outlier responses (Rüsseler et al. 2001). RTs for trials in which an incorrect response was provided were also removed from analysis. The RTs for all remaining trials as well as error rates were then averaged for both the implicit and random sequences for each individual. RT differences (dRT) between the implicit and random sequences (average RT random minus average RT implicit) were also calculated for the purposes of group comparison.
Group analysis
A 2 (sequence type) × 2 (group) mixed ANOVA was conducted to analyze the between condition effects of sequence type (implicit vs. random) and group (TMS vs. sham) on RT. An alpha value of p < 0.05 denoted significance. To assess learning within the groups, an effect size was computed for both groups comparing RTs of implicit to random sequence using the average standard deviation between the two sequence types. To characterize group differences, effect sizes were computed comparing dRTs of the TMS versus sham groups using the average standard deviation of dRT between groups. A secondary analysis was then conducted to compare the previously reported control group to the sham group by computing effect sizes comparing dRTs of the sham versus control group using the average standard deviation of dRT between groups. Throughout, mean values are reported followed by standard deviation.
Results
Following the criteria above, seven participants were excluded leaving a total of 20 participants in the behavioral analysis (9 and 11 in the TMS and sham groups, respectively). From the TMS group, two participants were excluded for demonstrating explicit knowledge as they accurately reported more than five consecutive sequence elements on the verbal report task. Data from one participant were further excluded from final analyses due to an error in the EMG calibration. Of the total remaining TMS group participants, the average number of responses made across all 1000 of the MI-based training trials was 1.44 ± 3.24. From the sham group, one participant was excluded for demonstrating explicit knowledge as they accurately reported more than five consecutive sequence elements on the verbal report task, and three participants were excluded due to the presence of muscle activity during the MI training that exceeded our threshold. Of the total remaining sham group participants, the average number of responses made across all 1000 of the MI-based training trials was 1.82 ± 3.92. As noted above, data from 24 participants that performed the same MI-based task were included to serve as a behavioral control group.
Imagery ability
As previously reported, for the MI group, the mean scores for visual and kinesthetic MI were 20.0 ± 4.7 and 19.3 ± 3.6, respectively (Kraeutner et al. 2015). For the sham group, the mean scores for visual and kinesthetic MI were 20.5 ± 3.8 and 20.2 ± 4.5, respectively. For the TMS group, the mean scores for visual and kinesthetic MI were 19.5 ± 3.6 and 17.9 ± 3.9, respectively. Values for visual and kinesthetic MI were within ranges previously reported for healthy controls (Malouin et al. 2007).
Reaction time
As reported previously, the control group produced mean RTs of 583 ± 84 and 632 ± 86 ms for the implicit and random sequences, respectively (Kraeutner et al. 2015). Mean error rate (%) for the implicit and random sequences within the control group was 1.92 ± 2.00 and 2.62 ± 2.68, respectively (Kraeutner et al. 2015). For the sham group, mean RT for the implicit and random sequences was 593 ± 50 and 629 ± 51 ms, respectively (Fig. 2). Mean error rate for the implicit and random sequences was 2.36 ± 2.77 and 2.64 ± 2.84, respectively. For the TMS group, mean RT for the implicit and random sequences was 626 ± 73 and 634 ± 62 ms, respectively (Fig. 2). Mean error rate for the implicit and random sequences was 2.56 ± 1.42 and 2.89 ± 2.76, respectively. Mean dRT and effect sizes between the sequence types for all groups are reported in Table 1.
Group comparisons
Overall, there was a significant main effect of sequence type [F(1,18) = 9.87, p = 0.0056], where RTs were significantly faster to sequence numbers than to random numbers. There was no main effect of group detected [F(1,18) = 0.55, p = 0.47]. While there was no significant interaction between sequence type and group [F(1,18) = 3.63, p = 0.073], the results were trending in this direction (further detailed below).
To characterize the amount of learning that resulted via MI, effect sizes (Kelley and Preacher 2012) within the TMS and sham groups were calculated comparing the RTs of the implicit and random sequences (Table 1). While the sham group resulted in similar differences between the implicit and random sequences, the TMS group showed no difference (Table 1). Thus, we further investigated observed RTs between the groups. A comparison of dRTs between the TMS and sham groups yielded an effect size of 0.84. A secondary analysis comparing the sham and control groups resulted in similar differences observed between the implicit and random sequences (within-group effect size for the control group is also depicted in Table 1). Further, a comparison of dRTs between the control and sham groups yielded an effect size of 0.34. Taken together with the within-group effects, we conclude the TMS group did not produce similar implicit sequence RTs as that of the sham group (Fig. 2), and that the sham group did not differ from the previously reported control group.
Discussion
The primary objective of this study was to examine the impact of left IPL damage on MI-based skill acquisition. Following MI-based practice, successful skill acquisition was observed in participants receiving sham TMS, but not in those receiving real TMS. Similar to that of the previously collected control group, RTs were decreased for the implicit compared to random sequences in the sham group, whereas no difference in RT was observed between the implicit and random sequences in the TMS group. Thus, we conclude that inhibition of the left IPL via cTBS impaired MI-based skill acquisition. Below we discuss these findings and their implications for the use of MI in skill acquisition.
The current findings demonstrate that inhibition of left IPL impaired MI-based skill acquisition. We assume then, consistent with previous lesion-based research, that inhibition of the left IPL via cTBS decreased MI ability, and in turn MI-based skill acquisition. As outlined above and based on the prior literature, it is not possible to wholly conclude that MI ability is impacted by damage to regions within the parietal cortex, as the performance of MI is assessed subjectively, with no robust, quantifiable measure of the outcome of MI performance (e.g., skill acquisition). Additionally, variability associated with lesion size and location further confounds the interpretation of previous findings. Here, we established that MI was affected following a virtual lesion to the left IPL, via performance of a robust learning paradigm that did not rely on subjective report. As MI has been previously shown to facilitate skill acquisition utilizing this learning paradigm (Kraeutner et al. 2015), and performance following sham stimulation was shown to parallel these results, we conclude that the left IPL is indeed critical to MI performance and, in turn, the effectiveness of MI as a modality for skill acquisition.
Impact of IPL damage to MI
As stated above, our results left us to conclude that the left IPL is critical to MI performance and thus MI-based skill acquisition. While damage to the left IPL may well hinder the effectiveness of MI as a modality of skill acquisition post-stroke, it was previously unclear whether the left IPL was critical to MI performance (i.e., the ability to do MI), or rather processes underlying learning that is mediated by MI. The evidence generated to date would largely suggest that it is impairment in MI ability that prevents learning via MI following IPL damage. First, although subjective in nature, there is a large body of the literature showing impaired MI ability in patients with parietal cortex lesions. When coupled with the current findings, the notion of impaired MI ability receives further validation in that we show an inability to learn via MI as assessed quantitatively using a robust learning paradigm that is independent of any PP. Second, neuroimaging work has shown that activity in the IPL during MI occurs prior to that of motor regions, in that the IPL first receives input from visual areas, followed by a bidirectional flow of information with premotor areas (Kawamichi et al. 1998). Thus, while the IPL is an important hub for information processing and transfer, the temporal order of activation during MI (i.e., prior to involvement of regions key to motor learning including pre- and primary motor regions; Hikosaka et al. 2002; Ungerleider et al. 2002; Doyon and Benali 2005), suggests a critical role in MI performance rather than motor learning per se.
In addition to that described above, other neuroimaging studies provide a means to understand the impact of IPL damage on MI ability. Research has demonstrated that while parietal regions are critical to explicit motor sequence learning, the network underlying implicit motor sequence learning relies on connectivity between the primary motor cortex, cerebellum and striatum (Destrebecqz et al. 2005; Marvel et al. 2007; Tzvi et al. 2014). It follows then that parietal damage does not impact upon implicit learning (in this case ISL), but rather the modality or substrate used to facilitate it. This evidence suggests that the impairment of MI-based learning observed in the current study was in fact due to an inability to perform MI following rTMS of the left IPL.
Role of IPL to MI
The current findings extend those of previous work that indicate parietal regions, including the left IPL, play a key role in MI ability and thus are more critical to MI-based practice relative to PP. Indeed, while similar brain regions are driven during MI-based practice as in PP (Burianová et al. 2013; Hétu et al. 2013; Kraeutner et al. 2014), MI is suggested to recruit a more widespread and bilateral network, including the consistent activation of left parietal regions (Burianová et al. 2013; Hétu et al. 2013). While the underlying mechanisms involved in MI-based learning remain largely unexplored, we turn to previous work to provide preliminary insight into the nature of the role of IPL in conjunction with the present findings.
Previous research suggests the parietal cortex, including the IPL, is involved in generating motor representations and movement selection (Rushworth et al. 2001, 2003). Specifically, regions in parietal cortex are thought to be responsible for coding properties of objects and intrinsically creating a sequence of movements to execute (Culham et al. 2006; Gallivan and Culham 2015). Arguably, however, the generation of a ‘motor map’ is equally important for both MI and PP. Thus, the consistent activation of the left IPL during MI may suggest that skill acquisition via MI involves additional processes less critical for skill acquisition via PP, and that these processes are modulated by the IPL.
Numerous studies have demonstrated the involvement of the IPL in visuospatial processes (Hilgetag et al. 2001; Corbetta and Shulman 2002; Rizzolatti and Matelli 2003; Chambers et al. 2007; Kitadono and Humphreys 2011). In fact, damage to this area often results in spatial attentional deficits (Behrmann 2004; Vandenberghe et al. 2012). As the IPL is a key structure in the dorsal visual pathway (responsible for visuospatial integration), it follows that these processes may be critical to MI. Further, research suggests that MI improves the implicit or cognitive aspect of the skill being practiced (i.e., MI consolidates the generated motor representation) without improving the actual execution component as, unlike PP, MI does not generate the feedback necessary to update the motor plan (Annett 1995). Thus, it follows that MI activates regions involved in visuospatial integration and motor attention that are critical to generating the motor representation and consolidating cognitive aspects of the skill more so than regions necessary for movement output, such as the primary motor cortex. Reviewed in the context of the present findings, the implicit nature of MI-based learning may provide an explanation as to why parietal regions, including the left IPL, are critical to MI. To this end, we suggest that these visuospatial processes may be more necessary for MI-based learning compared to skill acquisition occurring via PP.
Limitations
A limitation of the current study includes the possibility that cTBS of the left IPL decreased processes involved in actual motor execution, and thus, the RTs observed for the implicit sequence would be attributable to impairment in motor execution (i.e., actual button pressing) in the test block. No difference was observed, however, when comparing the baseline motor response across groups (i.e., RTs of random sequence; see Fig. 3). Thus, we conclude that actual motor execution was not impaired following inhibition of the left IPL, and that the lack of skill acquisition in the TMS group is attributed to the induced virtual lesion.
A second limitation is that data from our TMS group showed greater variability compared to the sham and control groups. In fact, one participant receiving real TMS demonstrated acquisition of the implicit skill. Previous research has demonstrated that some individuals, termed ‘non-responders,’ do not show inhibition following cTBS (López-Alonso et al. 2014). Thus, we suggest that the TMS participant who demonstrated successful skill acquisition was a non-responder. Due to the location of the stimulation site (i.e., a non-motor area), we were not able to assess cTBS effects via MEP amplitude to identify non-responders, but rather depended on behavioral outcomes. Importantly, while removal of this participant’s data from the analysis decreased variability (see Fig. 4), its inclusion still resulted in a robust effect.
Importantly, due to the nature of TMS, the exact spread of current within the brain is unable to be quantified (Wagner et al. 2004; Bolognini and Ro 2010), and thus, we cannot eliminate the possibility that TMS-induced effects were exclusive to the left IPL (for reviews on this topic see Sack 2006; Ruff et al. 2009). While sham stimulation was employed to control for placebo effects, no active control site was used as the effect of cTBS delivered to another brain region (i.e., an active control) would be unknown, thus adding a confound to the data. In light of this limitation, it is possible that other processes, such as working memory, could have been affected. Reviewing the current findings in the context of the previous literature discussing the neural networks underlying MI and the involvement of the IPL in visuospatial processes, it stands to reason that cTBS of the left IPL disrupted MI-based skill acquisition due to a dampening of the visuospatial processes necessary for MI-based skill acquisition to occur.
Conclusion
MI is shown to be a useful adjunct to PP in numerous domains and is emerging as a useful tool for neurorehabilitation post-stroke, yet it is unknown how stroke-related brain damage impacts on the effectiveness of MI as a modality of skill acquisition. The present study demonstrates that damage to the left IPL impairs acquisition of an implicit perceptual-motor skill through MI, thus providing direct evidence that this region is critical for MI performance and thus MI-based learning. The involvement of the IPL in visuospatial processes suggests that the IPL may contribute to generating the motor representation and consolidating cognitive aspects of the skill, and that these visuospatial processes may thus be more critical to skill acquisition via MI versus PP. As parietal cortex function is often impaired following stroke, these results have implications for the use of MI-based skill acquisition in post-stroke neurorehabilitation. Future work should investigate the mechanisms underlying MI ability.
References
Annett J (1995) Motor imagery: perception or action? Neuropsychologia 33:1395–1417
Behrmann M (2004) Parietal cortex and attention. Curr Opin Neurobiol 14:212–217. doi:10.1016/j.conb.2004.03.012
Bolognini N, Ro T (2010) Transcranial magnetic stimulation: disrupting neural activity to alter and assess brain function. J Neurosci 30:9647–9650. doi:10.1523/JNEUROSCI.1990-10.2010
Braun S, Kleynen M, Schols J et al (2008) Using mental practice in stroke rehabilitation: a framework. Clin Rehabil 22:579–591. doi:10.1177/0269215508090066
Buch ER, Modir Shanechi A, Fourkas AD et al (2012) Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain 135:596–614. doi:10.1093/brain/awr331
Burianová H, Marstaller L, Sowman P et al (2013) Multimodal functional imaging of motor imagery using a novel paradigm. NeuroImage 71:50–58. doi:10.1016/j.neuroimage.2013.01.001
Chambers C, Payne J, Mattingley J (2007) Parietal disruption impairs reflexive spatial attention within and between sensory modalities. Neuropsychologia 45:1715–1724. doi:10.1016/j.neuropsychologia.2007.01.001
Chen W-HH, Mima T, Siebner HR et al (2003) Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol 114:1628–1637
Cooke DF, Taylor CS, Moore T, Graziano MS (2003) Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA 100:6163–6168. doi:10.1073/pnas.1031751100
Corbetta M, Shulman G (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. doi:10.1038/nrn755
Culham J, Cavina-Pratesi C, Singhal A (2006) The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia 44:2668–2684. doi:10.1016/j.neuropsychologia.2005.11.003
de Vries P, de Jong B, Bohning D et al (2009) Changes in cerebral activations during movement execution and imagery after parietal cortex TMS interleaved with 3T MRI. Brain Res 1285:58–68. doi:10.1016/j.brainres.2009.06.006
Destrebecqz A, Peigneux P, Laureys S et al (2005) The neural correlates of implicit and explicit sequence learning: interacting networks revealed by the process dissociation procedure. Learn Mem 12:480–490. doi:10.1101/lm.95605
Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. doi:10.1016/j.conb.2005.03.004
Gallivan JP, Culham JC (2015) Neural coding within human brain areas involved in actions. Curr Opin Neurobiol 33:141–149. doi:10.1016/j.conb.2015.03.012
Goschke T, Bolte A (2012) On the modularity of implicit sequence learning: independent acquisition of spatial, symbolic, and manual sequences. Cogn Psychol 65:284–320. doi:10.1016/j.cogpsych.2012.04.002
Hétu S, Grégoire M, Saimpont A et al (2013) The neural network of motor imagery: an ALE meta-analysis. Neurosci Biobehav Rev 37:930–949. doi:10.1016/j.neubiorev.2013.03.017
Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12:217–222
Hilgetag C, Théoret H, Pascual-Leone A (2001) Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nat Neurosci 4:953–957. doi:10.1038/nn0901-953
Huang Y-ZZ, Edwards MJ, Rounis E et al (2005) Theta burst stimulation of the human motor cortex. Neuron 45:201–206. doi:10.1016/j.neuron.2004.12.033
Jeannerod M (1995) Mental imagery in the motor context. Neuropsychologia 33:1419–1432
Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage 14:S103–S109. doi:10.1006/nimg.2001.0832
Johansson B (2011) Current trends in stroke rehabilitation. A review with focus on brain plasticity: stroke rehabilitation. Acta Neurol Scand 123:147–159. doi:10.1111/j.1600-0404.2010.01417.x
Kawamichi H, Kikuchi Y, Endo H et al (1998) Temporal structure of implicit motor imagery in visual hand-shape discrimination as revealed by MEG. NeuroReport 9:1127–1132
Kelley K, Preacher K (2012) On effect size. Psychol Methods 17:137–152. doi:10.1037/a0028086
Kitadono K, Humphreys G (2011) Neuropsychological evidence for an interaction between endogenous visual and motor-based attention. Neurocase 17:323–331. doi:10.1080/13554794.2010.509322
Kleim J, Kleim E, Cramer S (2007) Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat Protoc 2:1675–1684. doi:10.1038/nprot.2007.206
Kraeutner S, Gionfriddo A, Bardouille T, Boe S (2014) Motor imagery-based brain activity parallels that of motor execution: evidence from magnetic source imaging of cortical oscillations. Brain Res 1588:81–91. doi:10.1016/j.brainres.2014.09.001
Kraeutner S, MacKenzie L, Westwood D, Boe S (2015) Characterising skill acquisition through motor imagery with no prior physical practice. J Exp Psychol. doi:10.1037/xhp0000148
Lebon F, Lotze M, Stinear C, Byblow W (2012) Task-dependent interaction between parietal and contralateral primary motor cortex during explicit versus implicit motor imagery. PLoS ONE 7:e37850. doi:10.1371/journal.pone.0037850
López-Alonso V, Cheeran B, Rio-Rodriguez D, Fernández-Del-Olmo M (2014) Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7:372–380 doi:10.1016/j.brs.2014.02.004
Malouin F, Richards C, Jackson P et al (2007) The kinesthetic and visual imagery questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther 31:20–29. doi:10.1097/01.NPT.0000260567.24122.64
Marvel C, Turner B, O’Leary D et al (2007) The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology 21:761. doi:10.1037/0894-4105.21.6.761-777
McInnes K, Friesen C, Boe S (2015) Specific brain lesions impair explicit motor imagery ability: a systematic review of the evidence. Arch Phys Med Rehabil 15:620–626. doi:10.1016/j.apmr.2015.07.012
Miyawaki Y, Shinozaki T, Okada M (2012) Spike suppression in a local cortical circuit induced by transcranial magnetic stimulation. J Comput Neurosci 33:405–419. doi:10.1007/s10827-012-0392-x
Moran A, Guillot A, MacIntyre T, Collet C (2012) Re-imagining motor imagery: building bridges between cognitive neuroscience and sport psychology: re-imagining motor imagery. Br J Psychol 103:224–247. doi:10.1111/j.2044-8295.2011.02068.x
Nyffeler T, Cazzoli D, Wurtz P et al (2008) Neglect-like visual exploration behaviour after theta burst transcranial magnetic stimulation of the right posterior parietal cortex. Eur J Neurosci 27:1809–1813. doi:10.1111/j.1460-9568.2008.06154.x
Nyffeler T, Cazzoli D, Hess CW, Müri RMM (2009) One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke 40:2791–2796. doi:10.1161/STROKEAHA.109.552323
Oberman L, Edwards D, Eldaief M, Pascual-Leone A (2011) Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J Clin Neurophysiol 28:67–74. doi:10.1097/WNP.0b013e318205135f
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. doi:10.1016/0028-3932(71)90067-4
Rizzolatti G, Matelli M (2003) Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res 153:146–157. doi:10.1007/s00221-003-1588-0
Rorden C, Karnath H-O (2004) Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 5:813–819. doi:10.1038/nrn1521
Rossi S, Hallett M, Rossini P, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. doi:10.1016/j.clinph.2009.08.016
Ruff CC, Driver J, Bestmann S (2009) Combining TMS and fMRI: from “virtual lesions” to functional-network accounts of cognition. Cortex 45:1043–1049. doi:10.1016/j.cortex.2008.10.012
Rushworth M, Krams M, Passingham R (2001) The Attentional Role of the Left Parietal Cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci 13:698–710. doi:10.1162/089892901750363244
Rushworth MF, Johansen-Berg H, Göbel S, Devlin J (2003) The left parietal and premotor cortices: motor attention and selection. NeuroImage 20:S89–S100. doi:10.1016/j.neuroimage.2003.09.011
Rüsseler J, Hennighausen E, Rösler F (2001) Response anticipation processes in the learning of a sensorimotor sequence. J Psychophysiol 15:95–105. doi:10.1027//0269-8803.15.2.95
Sack AT (2006) Transcranial magnetic stimulation, causal structure-function mapping and networks of functional relevance. Curr Opin Neurobiol 16:593–599. doi:10.1016/j.conb.2006.06.016
Sharma N, Pomeroy V, Baron J-C (2006) Motor imagery a backdoor to the motor system after stroke? Stroke 37:1941–1952. doi:10.1161/01.STR.0000226902.43357.fc
Sharma N, Baron J-C, Rowe J (2009) Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol 66:604–616. doi:10.1002/ana.21810
Sirigu A, Duhamel JR, Cohen L et al (1996) The mental representation of hand movements after parietal cortex damage. Science 273:1564–1568
Stinear C, Byblow W, Steyvers M et al (2006) Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res 168:157–164. doi:10.1007/s00221-005-0078-y
Tzvi E, Münte T, Krämer U (2014) Delineating the cortico-striatal-cerebellar network in implicit motor sequence learning. Neuroimage 94:222–230. doi:10.1016/j.neuroimage.2014.03.004
Ungerleider LG, Doyon J, Karni A (2002) Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78:553–564
Vandenberghe R, Molenberghs P, Gillebert C (2012) Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia 50:1092–1103. doi:10.1016/j.neuropsychologia.2011.12.016
Wagner TA, Zahn M, Grodzinsky AJ, Pascual-Leone A (2004) Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans Biomed Eng 51:1586–1598. doi:10.1109/TBME.2004.827925
Wohldmann EL, Healy AF, Lyle BEJ (2007) Pushing the limits of imagination: mental practice for learning sequences. J Exp Psychol Learn Mem Cogn 33:254–261. doi:10.1037/0278-7393.33.1.254
Wulf G, Shea C, Lewthwaite R (2010) Motor skill learning and performance: a review of influential factors: motor skill learning and performance. Med Educ 44:75–84. doi:10.1111/j.1365-2923.2009.03421.x
Zhang H, Xu L, Zhang R et al (2012) Parallel alterations of functional connectivity during execution and imagination after motor imagery learning. PLoS ONE 7:e36052. doi:10.1371/journal.pone.0036052
Acknowledgments
This work was supported by funding from the Heart and Stroke Foundation of Canada and Natural Sciences and Engineering Research Council (NSERC) awarded to SB. SK is supported by training awards from NSERC and the Nova Scotia Research and Innovation Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
221_2015_4472_MOESM1_ESM.tif
Supplementary Figure 1. Validation of the target site (IPL) in a participant using their anatomical MRI. The left panel shows target placement for localization of the hot spot and inferior parietal lobe (IPL) on a template brain, with localization of the IPL via MNI coordinates on a participants anatomical MRI shown in the middle and right panels.(TIFF 1073 kb)
Rights and permissions
About this article
Cite this article
Kraeutner, S.N., Keeler, L.T. & Boe, S.G. Motor imagery-based skill acquisition disrupted following rTMS of the inferior parietal lobule. Exp Brain Res 234, 397–407 (2016). https://doi.org/10.1007/s00221-015-4472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4472-9