Abstract
Cerebral injuries can trigger stress-related cardiomyopathy. The extent of cerebral injury and the involvement of the insular cortex influence the incidence and extent of myocardial injury (MI), and drugs with proven neuroprotective and cardioprotective properties such as levosimendan might be beneficial. This hypothesis was addressed in a rat model of transient middle cerebral artery occlusion. Transient brain ischemia was induced for 60 min by intraluminal occlusion of the middle cerebral artery in 40 male Wistar rats. Treatment with levosimendan (24 µg/kg) was started briefly before reperfusion. Hemodynamic parameters were recorded and cerebral and MI quantified after 24 h. Levosimendan treatment significantly reduced cerebral infarct size in the cortex, but not in the striatal and insular regions. However, its effects on survival (28 vs. 45 %), incidence of MI (8 vs. 33 %) as indicated by a troponin I (sTnI) threshold of 4.8 µg/L and large insular infarcts of ≥10 mm3 (23 vs. 50 %) failed to reach statistical significance. Blood pressure demonstrated significant differences related to insular infarct size during reperfusion. Levosimendan demonstrated no relevant effects on markers of MI (sTnI = 1.5 ± 2.8 vs. 5.3 ± 7.2 µg/L, P = 0.121). Insular infarct size could be identified as the only predictor of MI (odds ratio = 1.86, P = 0.037). In conclusion, the current investigation confirmed insular infarct size as a predictor of MI and source of hemodynamic compromise, but failed to demonstrate an effect of levosimendan on MI trigged by brain ischemia. A hardly protectable insular region might explain this.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several types of brain injuries, especially stroke and subarachnoid hemorrhage (SAH), can trigger cardiac injury and impede myocardial function (Samuels 2007). Although the exact mechanism is still unknown, elevated circulating catecholamine, cardiac autonomic damage and accelerated cardiac sympathetic tone were assumed to be the major source, leading to the term “stress cardiomyopathy.” This syndrome is also known as “broken heart syndrome” or Takotsubo cardiomyopathy and is represented by “neurogenic stunned myocardium” (Guglin and Novotorova 2011). The diagnostic criteria include transient regional wall motion abnormalities in the mid segments of the left ventricle with or without apical involvement exceeding the perfusion area of one major coronary vessel, presence of severe stressors, ST-segment abnormalities, elevated serum troponin levels and absence of coronary disease, pheochromocytoma or myocarditis (Iltumur et al. 2006). Because the prognosis of the patients was crucially influenced by fatal cardiac events, such as myocardial infarction (19 %), heart failure (9 %) and sudden death (22 %) (Prosser et al. 2007; Rincon et al. 2008), the treatment of cardiac insufficiency and cardioprotective therapeutic regimes might improve outcomes.
The calcium sensitizer levosimendan has not only cardioprotective properties similar to volatile anesthetics or opioids (Metzsch et al. 2007; Hein et al. 2009) but is also useful for the treatment of severe heart failure, especially in catecholamine-resistant shock (Werdan et al. 2012). In contrast to other positive inotropic agents, it bypasses β-adrenergic receptors and phosphodiesterase type III and thus does not increase myocardial oxygen consumption or trigger proapoptotic signaling pathways (Talan et al. 2011). Its neuroprotective properties (Katircioglu et al. 2008; Lafci et al. 2008; Hein et al. 2013) might support this action; the incidence and extent of myocardial injury (MI) correlated with cerebral infarct size (Iltumur et al. 2006). Therefore, we tested the hypothesis that levosimendan could reduce the incidence and extent of MI triggered by experimental stroke either by limiting neurological injury or by directly protecting the heart against neurogenically induced stress. Hemodynamic responses and markers of MI were investigated in a rat model of transient middle cerebral artery occlusion (MCAO) with and without levosimendan treatment and were analyzed with respect to the extent of cerebral injury.
Materials and methods
Animals
Recordings, serum samples and tissue specimens were taken from a previous study on the effects of levosimendan on cerebral reperfusion injury in a rat MCAO model (Hein et al. 2013). Selection of data followed different criteria, and additional analyses were performed (quantification of insular infarct volume, heart rate variability and MI). Forty male Wistar rats (Charles River, Sulzfeld, Germany) with verifiable cerebral infarction after MCAO and bodyweight between 350 and 450 g could be included retrospectively. Additional six sham-operated animals were used as negative controls for gene expression analyses. All procedures were in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 85-23, revised in 1996) and were approved by the Governmental Animal Care and Use Committee of the State Nordrhine Westfalia (No. 8.87-50.10.37.09.258; Landesamt für Natur-, Umwelt- und Verbraucherschutz Nordrhein-Westfalen, Recklinghausen, Germany).
Surgical procedures
As described previously, anesthesia was induced by an intraperitoneal (i.p.) injection of 100 mg/kg S-ketamine (Ketanest S, Pfizer, New York, USA) and 10 mg/kg xylazine (Xylazin 2 %, Medistar, Ascheberg, Germany) and maintained by repetitive i.p. injections of 20 mg/kg S-ketamine. After oral intubation, the animals were mechanically ventilated and monitored with electrocardiogram, pulse oximetry and invasive measurement of blood pressure. Cerebral ischemia was induced for 60 min by insertion of a filament through the left common and internal carotid artery to occlude the origin of the left middle cerebral artery, which was validated by a reduction in blood flow in the parietal cortex to 20–30 % of baseline values, as measured by laser Doppler (VP10M200ST/P10d, Moor Instruments, Devon, UK). SAH would induce a further decrease in blood flow (Schmid-Elsaesser et al. 1998). After 30 min of reperfusion, all catheters were removed, the vessels were ligated, and all wounds were closed after local application of 0.2 % ropivacaine (Naropin, AstraZeneca, Plankstadt, Germany). The animals received intraperitoneal 20 mg/kg metamizole (Novalgin, Sanofi Aventis, Frankfurt, Germany) as pain prophylaxis and were extubated when spontaneous breathing and righting reflex returned.
Experimental groups
Forty animals were randomized to the treatment or control group using an envelope system and received either an i.v. bolus infusion of 24 µg/kg levosimendan (Simdax, Orion Pharma, Espoo, Finland) or an equal amount of saline (NaCl) over the time course of 20 min, starting 5 min prior to the onset of reperfusion. Six sham-operated animals were used as controls.
Hemodynamics
A data acquisition system (PowerLab, ADInstruments, Spechbach, Germany) was used to monitor and record the arterial pressure, electrocardiogram, cerebral blood flow and body temperature. Heart rate (HR), mean arterial blood pressure (MAP) and parameters of heart rate variability (HRV) were analyzed from recordings of 3, 10 and 20 min before ischemia, as well as every 10 min after induction of ischemia and for the first 30 min of reperfusion (LabChart 7.3.7 Pro, ADInstruments, Spechbach/Germany). To quantify HRV, the spectral powers of a low-frequency region (0.195–2.5 Hz) and high-frequency region (0.605–2.5 Hz) were used to describe changes in sympathetic and parasympathetic activity. Both values were normalized to total power minus the very-low-frequency component below 0.195 Hz (HF/TP, LF/TP) (Cheung et al. 1997).
Quantification of neurological injury
At 24 h after the onset of reperfusion, the rats were killed after intraperitoneal injection of 100 mg/kg thiopental (Trapanal, Nycomed, Konstanz, Germany), and serum samples were collected before transcardial perfusion with 100 mL ice-cold Ringer’s solution. Immediately after the procedure, the head was detached and the brain removed and cut into seven 2-mm cross sections, which were stained with 1 % 2.3.5-triphenyltetrazolium chloride (TTC, SERVA, Antwerpen, Belgium) for 15 min at 37 °C to differentiate between the ischemic lesion and the viable tissue. After digitalization, the infarcted areas in the cortex and striatum were calculated by planimetry (ImageJ 1.42, National institutes of Health, Bethesda, MD/USA) and corrected for edema volume as the difference between left and right hemispheric volumes, as described previously (Ryang et al. 2011; Bleilevens et al. 2013). Involvement of the insular cortex was further quantified after its boundaries were marked according to the Paxinos brain atlas (Paxinos and Watson 2007; Min et al. 2009).
Quantification of myocardial injury
Troponin I (sTnI), which was assayed with a commercially available ELISA Kit (2010-2-HS, Life Diagnostics Inc., West Chester, PA, USA), was used as a serum marker of MI and for the definition of relevant MI a priori: The threshold value of 4.8 µg/L from a previous investigation in rats to predict myocardial infarction was used in this analysis (Vietta et al. 2013).
The heart of each animal was dissected immediately after transcardial perfusion, and left ventricular specimens were snap-frozen in liquid nitrogen and stored at −80 °C until quantification of RNA content of different markers. As described earlier, the polymerase chain reaction (PCR) was performed on a PCR system (StepOne-Plus, Applied Biosystems, Carlsbad, CA/USA) (Roehl et al. 2013) using specific TaqMan probes (Applied Biosystems) for the following genes related to heart failure, calcium signaling and inflammation: connective tissue growth factor (CTGF, Rn00573960_g1), (BNP, Rn00676450_g1), osteopontin (OPN, Rn00563571_m1), sarco/endoplasmic reticulum Ca 2+-ATPase 2 (SERCA2, Rn01499544_m1), ryanodine receptor-2 (RyR2, Rn01470303_m1), adenylate cyclase-7 (AdCy7, Rn01538054_m1), tumor necrosis factor α (TNFα, Rn00562055_m1) and interleukin 6 (IL6, Rn01410330_m1). Relative quantities (RQ) of targets were calculated according to the 2−ΔΔCt method and normalized to the reference gene glyceraldehyde-3-phosphate dehydrogenase (Rn99999916_s1) and relative to expression in hearts from non-diseased rats (StepOne Software v2.3, Applied Biosystems) (Livak and Schmittgen 2001). To compare gene expression between groups, the ratio of corresponding RQ values was calculated.
Statistical analysis
Animals that did not survive were excluded from the analysis. In contrast to the original investigation, all animals with cerebral infarction were included independent of size and pattern or cerebral perfusion profiles (Hein et al. 2013). Indices of the extent of neurological injury and treatment were tested for their predictive value by a univariate logistic regression analysis. Receiver operator curves (ROC) were used to define threshold values for significant predictors of MI to form subgroups and thus describe infarct size independent of any cardioprotective effects of levosimendan. The effect of levosimendan on survival, incidence of MI and excess of threshold values of infarct sizes were analyzed using Fisher’s exact test. To describe the effects of levosimendan on neurological and cardiac injury, additional groups were formed according to thresholds for MI and infarct size, respectively. Time-dependent changes of hemodynamic variables are displayed as the mean plus or minus the standard error of the mean (SEM) to improve clarity. The median and its interquartile range as well as scattered dot plots were used to describe and plot the results for parameters of neurological or cardiac injury. Significant differences between groups were analyzed using univariate analysis of variance and variance analysis for repeated measurements with contrast analysis between consecutive time points. Differences for RQ values relative to sham-operated animals were tested for significance using the Wilcoxon signed-ranks test. We consider an RQ significant when there is a minimum of a twofold change. P values ≤0.05 were considered statistically significant (SPSS Statistics 22, IBM, Ehningen, Germany).
Results
Of the 40 rats, 22 were randomized to the NaCl group and 18 to the levosimendan group. Ten animals in the control group (45 %) and 5 in the levosimendan group (28 %) did not survive 24 h after MCAO (P = 0.33). Within the surviving animals, there was no significant difference in the occurrence of MI between groups (P = 0.16): In four animals from the control group (33.3 %) and in one from the levosimendan group (7.7 %), sTnI levels exceeded 4.8 µg/L (Fig. 1). Cumulative dosage of S-ketamine after induction was comparable in the NaCl (41.7 ± 21.7 mg) and levosimendan groups (37.0 ± 21.4 mg). No SAH could be detected on animals that survived.
Neurological injury
After forming additional groups according to MI, it could be demonstrated that treatment with levosimendan significantly reduced only the cortical fraction of cerebral infarction from 163 (45–162) mm3 to 74 (12–122) mm3 (P = 0.033). No effect on striatal or insular infarct volume and edema was found (Fig. 2). Additionally, the infarcted volume of the insular region was larger in animals with MI (13.4, 10.8–15.4 mm3) in comparison with animals without injury (7.0, 1.1–9.8 mm3).
Logistic regression determined only insular infarct volume as a predictor of MI, not edema or striatal or cortical infarct volume. Similarly, treatment with levosimendan failed significance (Table 1).
Using ROC analysis, a threshold of ≥10 mm3 for insular infarct volume could be defined to predict a significant MI with a sensitivity of 100 % and specificity of 80 % (AUC = 0.92, P = 0.004). Thus, animals could be classified into subgroups with large (≥10 mm3) or small (<10 mm3) insular infarcts for further analysis of treatment effects on the incidence of significant neuronal injury and hemodynamics. Six out of 12 animals demonstrated large insular infarcts in the control group (50 %), as did 3 out of 13 in the levosimendan group (23 %) (P = 0.226, Fig. 1).
Hemodynamic effects
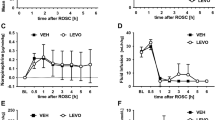
The threshold values for insular infarct size were used for additional grouping of hemodynamic parameters in order to describe infarct size and levosimendan-dependent effects. HR increased significantly over time (P = 0.002), with no differences between groups. Contrast analysis demonstrated a significant effect during early ischemia (10 min) and reperfusion (0 and 20 min). A significant decrease in MAP could be observed 10 min after induction of ischemia (P = 0.001) and immediately after reperfusion (P = 0.005). The effect of time on MAP was different between animals with large or small insular infarcts (P = 0.014). This difference became obvious during reperfusion. Although MAP remains low in animals with large insular infarcts, a significant increase could be measured in the other animals after 30 min (P = 0.009). No effect of levosimendan could be demonstrated (Fig. 3). Whereas LF/TP decreased significantly with the induction of cerebral ischemia (P = 0.01), an increase could be observed at reperfusion (P = 0.04). Although this effect seemed to be more pronounced in untreated animals with large insular infarcts, no significant differences between or within groups could be detected. HF/TP decreased in all animals over time (P < 0.001), especially immediately at reperfusion (P = 0.047). Levosimendan or infarct size did not influence the time course (Fig. 3).
Hemodynamics: effect of insular infarct size and treatment (NaCl, levosimendan) on heart rate (HR), mean arterial pressure (MAP) and low-frequency (LF) and high-frequency (HF) power normalized to total power (TP) (mean ± SEM, P values from variance analysis for repeated measurements: effect of time and interaction of time and insular infarct size (vol), *P < 0.05 versus insular infarcts ≥10 mm3, # P < 0.05 versus consecutive time points)
Myocardial injury
To demonstrate the effects of levosimendan on MI independent of cerebral infarct size, a grouping for treatment and insular infarct size was performed. Animals with large insular infarcts were found to have significantly higher sTnI values (P = 0.002), but levosimendan failed (P = 0.121) to demonstrate an effect (Fig. 4).
Markers of myocardial injury: effect of levosimendan on serum levels of troponin I (sTnI) and relative gene expression values (RQ) of brain natriuretic peptide (BNP), connective tissue growth factor (CTGF) and tumor necrosis factor α (TNFα) after MCAO compared to saline-treated (NaCl) animals after grouping for insular infarct volume [median with interquartile range, P values from ANOVA and Wilcoxon signed-rank test: significant effect of group (Pgroup), insular infarct volume (Pvol) and compared to sham (Psham)]
An increased myocardial expression of genes related to heart failure after MCAO could be detected only for BNP (2.2-fold, P = 0.001, Fig. 4). Following treatment with levosimendan, CTGF expression was reduced by 1.6-fold relative to vehicle-treated animals (P = 0.017, Fig. 4). No differences between small and large insular infarcts or for OPN could be detected (Table 2).
Of the calcium-handling genes, only the expression of AdCy7 was down-regulated after MCAO by a factor of 1.2 (P = 0.021). There were no differences detected between groups for the expression of SERCA2 or RyR2 (Table 2).
Inflammatory markers in the myocardium were observed to be regulated differently between groups. Whereas only IL6 was significantly up-regulated (by 2.5-fold) after MCAO (P = 0.007, Table 2), the expression of TNFα was 1.4-fold lower after levosimendan treatment (P = 0.034) and 1.3-fold lower in the myocardium of animals with large insular infarcts (P = 0.047) relative to vehicle treatment and small insular infarcts, respectively (Fig. 4).
Discussion
Whereas previous studies demonstrated the neuro- and cardioprotective effects of levosimendan, this work illustrates that the incidence and extent of MI after ischemic stroke were influenced only by the degree of neurological injury and not by levosimendan. Only the insular infarct volume could be identified as a predictor of MI. Animals with insular infarct volumes of ≥10 mm3 demonstrated higher values of sTnI and lower MAP after cerebral ischemia. Although levosimendan reduced cortical infarct volume, it failed to influence the incidence and extent of MCAO-triggered MI. Whereas MCAO induced a significant MI in 20 % of the animals, the gene expression of different markers of cardiac injury remained normal or was only marginally up-regulated (BNP, IL6) after 24 h.
Although no significant effects of levosimendan could be demonstrated, a trend toward a lower incidence of myocardial injuries (8 vs. 33 %) and larger insular infarcts (23 vs. 50 %) could be observed. The potential mechanisms of action are assumed to involve a reduction of neurological injury, reducing the risk for cardiac damage and a direct protection of the heart against neurogenic stress.
As described previously, levosimendan was able to reduce neuronal injury after transient ischemia (Katircioglu et al. 2008; Lafci et al. 2008; Hein et al. 2013). As this effect was additionally dependent on the timing and extent of reperfusion (Bleilevens et al. 2013) and no animals were excluded related to cerebral perfusion profiles as in the associated investigation (Hein et al. 2013), the effect of levosimendan reached statistical significance on infarct size only in the cortex in this analysis. Nevertheless, the involvement of the insular cortex appeared to be crucial for subsequent MI. The incidence and extent of structural and functional cardiac compromise increased with progressive effects on the left insular cortical region (Cheshire and Saper 2006; Iltumur et al. 2006). Because the left insular cortex influences the sympathetic/parasympathetic balance, catecholamine surge and subsequent myocytolysis and cardiac dysfunction might be related to severe damage to this region (Min et al. 2009). Indeed, insular infarct size was proven to be a predictor of MI, and an increase in sympathetic and decrease in parasympathetic tone could be observed during reperfusion, as described previously (Cheung et al. 1997; Dutsch et al. 2007). The continuous increase in HR is a consequence of this vegetative imbalance. Although HRV and MAP were affected early during ischemia, relevant changes occurred only during reperfusion, as demonstrated by a significant effect of insular infarct size on MAP. The lower blood pressure in animals with large insular infarcts during reperfusion might indicate a persistent functional compromise. However, it should be mentioned that a lower blood pressure during reperfusion might aggravate neurological injury and thus trigger a vicious circle, although cerebral blood flow as measured by laser Doppler flow was not significantly different between groups (data not shown).
Levosimendan failed to influence the insular infarct size and thus the incidence and extent of MI and hemodynamic responses. Although the insula might be part of the penumbra at the lower margin of the ischemic area, it remains unclear why neuroprotection by levosimendan failed in this region of the cortex. As described earlier, the largest decrease in blood flow during MCAO could be observed in the lower part of the frontoparietal cortex. This poorly perfused region was more frequently infarcted when compared with other regions and was thus more susceptible to ischemia, which could hardly be salvaged by reperfusion or protected by pharmacological agents (Memezawa et al. 1992).
Furthermore, a direct action of levosimendan on the myocardium, that is, cerebral infarct size independent, should be possible. Previous studies have described distinct regulation of genes related to calcium signaling, heart failure and inflammation in Takotsubo cardiomyopathy (Izumi et al. 2009; Nef et al. 2009; Pirzer et al. 2012). Upregulation of heart-failure-related genes (BNP, CTGF, OPN), differential regulation of the calcium-handling-related genes, such as RyR2 (down), SERCA2 (down) and AdCy7 (up), and increased expression of IL6 have been described. In this first report on experimental stroke-related cardiac injury, significant at least twofold regulation could only be detected after 24 h for BNP and IL6, although there were differences between groups for CTGF and TNFα but not calcium-handling-related genes. In theory, levosimendan should influence the expression of genes related to inflammation, calcium handling and heart failure (Louhelainen et al. 2009), but it fails to demonstrate relevant effects in the current investigation.
This leads us to the following major limitation of this study: It was an analysis to expand upon a prior investigation, it had insufficient power and important tests are absent, particularly an analysis of cardiac function after 24 h. The irregular distribution of animals between groups (only three large insular infarcts and one observed MI in the levosimendan group) limits the analytical possibilities. Thus, levosimendan failed to show significant effects on outcome parameters, and further investigations are needed.
However, other important aspects of experimental stroke research could be proven and should be highlighted: Cerebral injury might provoke MI, which will influence the outcome, if it is not monitored. Causes of death and elevated serum levels of brain-specific acidic protein S-100β might be misinterpreted. Levels of S-100β are related to infarct size in myocardial ischemia reperfusion injury (Cai et al. 2011) and correlated with TnI levels in the current investigation (R = 0.673, P = 0.001). Furthermore, retrospective analysis of rats subjected to MCAO (Bleilevens et al. 2013) under isoflurane anesthesia demonstrated a low incidence of myocardial injuries (1 out of 18). Thus, positive effects on survival during isoflurane anesthesia compared to S-ketamine might be related to fewer cardiac complications. Therefore, monitoring of cardiac function, continuous registration of blood pressure or ECG and immediately autopsy in case of premature death should be proposed.
In conclusion, the current investigation proved the connection between the involvement of the insular cortex and MI, but failed to provide evidence for the cardioprotective properties of levosimendan on stroke-induced MI. This result might be related both to the fact that the insula region could hardly be protected and to the low observable MI early after MCAO. Further studies with higher power are needed for a better judgment. Despite these limitations, levosimendan may still be a promising drug for the treatment of stroke-related cardiomyopathy, with effects beyond its reversal of hemodynamic compromise.
References
Bleilevens C, Roehl AB, Goetzenich A et al (2013) Effect of anesthesia and cerebral blood flow on neuronal injury in a rat middle cerebral artery occlusion (MCAO) model. Exp Brain Res 224:155–164
Cai XY, Lu L, Wang YN et al (2011) Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia–reperfusion injury. Atherosclerosis 217:536–542
Cheshire William P Jr, Saper CB (2006) The insular cortex and cardiac response to stroke. Neurology 66:1296–1297
Cheung RT, Hachinski VC, Cechetto DF (1997) Cardiovascular response to stress after middle cerebral artery occlusion in rats. Brain Res 747:181–188
Dutsch M, Burger M, Dorfler C, Schwab S, Hilz MJ (2007) Cardiovascular autonomic function in poststroke patients. Neurology 69:2249–2255
Guglin M, Novotorova I (2011) Neurogenic stunned myocardium and takotsubo cardiomyopathy are the same syndrome: a pooled analysis. Congest Heart Fail 17:127–132
Hein M, Roehl AB, Baumert JH, Scherer K, Steendijk P, Rossaint R (2009) Anti-ischemic effects of inotropic agents in experimental right ventricular infarction. Acta Anaesthesiol Scand 53:941–948
Hein M, Zoremba N, Bleilevens C, Bruells C, Rossaint R, Roehl AB (2013) Levosimendan limits reperfusion injury in a rat middle cerebral artery occlusion (MCAO) model. BMC Neurol 13:106
Iltumur K, Yavavli A, Apak I, Ariturk Z, Toprak N (2006) Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am Heart J 151:1115–1122
Izumi Y, Okatani H, Shiota M et al (2009) Effects of metoprolol on epinephrine-induced takotsubo-like left ventricular dysfunction in non-human primates. Hypertens Res 32:339–346
Katircioglu SF, Seren M, Parlar AI et al (2008) Levosimendan effect on spinal cord ischemia-reperfusion injury following aortic clamping. J Card Surg 23:44–48
Lafci B, Yasa H, Ilhan G et al (2008) Protection of the spinal cord from ischemia: comparative effects of levosimendan and iloprost. Eur Surg Res 41:1–7
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25:402–408
Louhelainen M, Vahtola E, Forsten H et al (2009) Oral levosimendan prevents postinfarct heart failure and cardiac remodeling in diabetic Goto-Kakizaki rats. J Hypertens 27:2094–2107
Memezawa H, Minamisawa H, Smith ML, Siesjo BK (1992) Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res 89:67–78
Metzsch C, Liao Q, Steen S, Algotsson L (2007) Levosimendan cardioprotection reduces the metabolic response during temporary regional coronary occlusion in an open chest pig model. Acta Anaesthesiol Scand 51:86–93
Min J, Farooq MU, Greenberg E et al (2009) Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke 40:2560–2563
Nef HM, Mollmann H, Troidl C et al (2009) Abnormalities in intracellular Ca2+ regulation contribute to the pathomechanism of Tako-Tsubo cardiomyopathy. Eur Heart J 30:2155–2164
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. London, Burlington, MA, Academic. http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10360087
Pirzer R, Elmas E, Haghi D et al (2012) Platelet and monocyte activity markers and mediators of inflammation in Takotsubo cardiomyopathy. Heart Vessels 27:186–192
Prosser J, MacGregor L, Lees KR, Diener H, Hacke W, Davis S (2007) Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 38:2295–2302
Rincon F, Dhamoon M, Moon Y et al (2008) Stroke location and association with fatal cardiac outcomes: northern Manhattan study (NOMAS). Stroke 39:2425–2431
Roehl AB, Funcke S, Becker MM et al (2013) Xenon and isoflurane reduce left ventricular remodeling after myocardial infarction in the rat. Anesthesiology 118:1385–1394
Ryang Y, Dang J, Kipp M et al (2011) Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci 12:113
Samuels MA (2007) The brain-heart connection. Circulation 116:77–84
Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ (1998) A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 29:2162–2170
Talan MI, Ahmet I, Xiao RP, Lakatta EG (2011) Beta(2) AR agonists in treatment of chronic heart failure: long path to translation. J Mol Cell Cardiol 51:529–533
Vietta GG, Andrades ME, Dall’alba R et al (2013) Early use of cardiac troponin-I and echocardiography imaging for prediction of myocardial infarction size in Wistar rats. Life Sci 93:139–144
Werdan K, Ruß M, Buerke M, Delle-Karth G, Geppert A, Schöndube FA (2012) Cardiogenic shock due to myocardial infarction: diagnosis, monitoring and treatment: a German–Austrian S3 guideline. Dtsch Arztebl Int 109:343–351
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bleilevens, C., Roehl, A.B., Zoremba, N. et al. Insular infarct size but not levosimendan influenced myocardial injury triggered by cerebral ischemia in rats. Exp Brain Res 233, 149–156 (2015). https://doi.org/10.1007/s00221-014-4096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4096-5