Abstract
Therapeutic drug monitoring is essential for ensuring the efficacy and safety of medications. This study introduces a streamlined approach that combines pipette-tip solid-phase extraction (PT-SPE) with matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), facilitating rapid and high-throughput monitoring of drug concentrations. As a demonstration, this method was applied to the extraction and quantification of antidepressants in serum. Utilizing Zip-Tip C18, the method enabled the extraction of antidepressants from complex biological matrices in less than 2 min, with the subsequent MALDI-MS analysis yielding results in just 1 min. Optimal extraction recoveries were achieved using a sampling solution at pH 9.0 and a 10 μL ethanol desorption solution containing 0.1% phosphoric acid. For MALDI analysis, 2,5-dihydroxybenzoic acid was identified as the most effective matrix for producing the highest signal intensity. The quantification strategy exhibited robust linearities (R2 ≥ 0.997) and satisfactory limits of quantification, ranging from 0.05 to 0.5 μg/mL for a suite of antidepressants. The application for monitoring dynamic concentration changes of antidepressants in rat serum emphasized the method’s efficacy. This strategy offers the advantages of high throughput, minimal sample usage, environmental sustainability, and simplicity, providing ideas and a reference basis for the subsequent development of methods for therapeutic drug monitoring.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, pharmacotherapy remains the primary approach for managing various diseases, leading to the widespread use of numerous drugs in clinical settings. However, it is important to acknowledge that many drugs have notable adverse effects and limited efficacy [1]. Given the narrow therapeutic window and potential side effects of certain medications, it is crucial to maintain drug concentrations within a therapeutic range to minimize toxicity risks [2]. This necessitates continuous therapeutic drug monitoring (TDM) to track drug concentration fluctuations in the patient’s body, thereby preventing medical non-responsiveness, overdose intoxication, complications, or noncompliance to the greatest extent possible [3].
Recently, numerous methods for detecting drug concentrations in biological fluids have been developed [4]. Chromatography-based methods have become mainstream due to their sensitivity, selectivity, and ability to simultaneously detect multiple substances [5]. Examples include high-performance liquid chromatography (HPLC) [6, 7], matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) [8], gas chromatography-mass spectrometry (GC–MS) [9], and liquid chromatography-mass spectrometry (LC–MS) [10,11,12,13]. However, these methods often involve laborious sample preparation, extensive use of organic solvents, or lengthy analysis times, rendering them impractical for emergency monitoring in clinical settings. Therefore, it is essential to refine these methods and develop a rapid, convenient, eco-friendly, and point-of-care method for TDM.
With the advent of MALDI technologies, their high throughput, spectral simplicity, low sample consumption, salt tolerance, and ease of operation [14] have led to their increased use in analyzing small molecule drugs [15, 16]. Yet, the sensitivity of MALDI is generally lower than that of LC–MS, which may result in undetected trace drug amounts in humans due to complex sample matrices, potentially missing critical information for clinical diagnosis and treatment.
To capitalize on the strengths and address the limitations of MALDI, pipette-tip solid-phase extraction (PT-SPE) was combined with MALDI MS to establish a rapid, convenient, and sensitive strategy for drug detection in rat serum. As a demonstration, the serum concentrations of five antidepressants were analyzed using this approach. Zip-Tip C18 pipette tips were utilized for the extraction of antidepressants and removal of impurities from complex serum matrices through hydrophobic interactions, thereby enriching the analytes and significantly enhancing sensitivity. In sum, this approach simplified the extraction process to mere aspiration and dispensing with a pipettor, eliminating the need for complex sample preparation and thus saving time and reducing costs. Moreover, the type and volume of desorption solvent, the type of acid in the desorption solvent, and the pH of the sampling solution and matrices were optimized. The linearity, accuracy, and precision of the method were evaluated, and the strategy combining MALDI and PT-SPE was successfully applied to rapidly quantify drugs in serum from rats treated with antidepressants.
Experimental section
Chemicals and materials
LC–MS grade methanol (MeOH), ethanol (EtOH), isopropyl alcohol (IPA), acetonitrile (ACN), acetone (AC), formic acid (FA), acetic acid (AA), trifluoroacetic acid (TFA), and phosphoric acid (PHA) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Analytical-grade sodium phosphate monobasic (NaH2PO4, ≥ 99.0%), sodium phosphate dibasic (Na2HPO4, ≥ 99.0%), and fluoxetine hydrochloride (FLX, ≥ 99%) as well as the internal standards fluoxetine-d6 (FLX-d6) and imipramine hydrochloride (IMP, ≥ 98%) were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Mirtazapine (MIP, ≥ 98%) and amfebutamone hydrochloride (AMF, ≥ 98%) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Venlafaxine hydrochloride (VEN, ≥ 98%) was purchased from Rhawn Reagent Co., Ltd. (Shanghai, China). Trans-2-[3-(4-tertbutylphenyl)-2-methyl-2-propenylidene] malononitrile (DCTB), 2,5-dihydroxybenzoic acid (DHB), 2,3-diaminonaphthalene (DAN), and α-cyano-4-hydroxycinnamic acid (CHCA) were purchased from TCI Chemical Trading Co., Ltd. (Shanghai, China).
Pure water (H2O) was sourced from Wahaha Group Co., Ltd. (Zhengzhou, China). Zip-Tip C18 was acquired from Merck Millipore Co., Ltd. (Billerica, MA, USA). A pH meter was purchased from Mettler Toledo Technology Co., Ltd. (Greifensee, Switzerland) and calibrated with buffers at pH values of 4.00, 6.86, and 9.18 before use.
A stock solution of each antidepressant was prepared in MeOH at a concentration of 1.0 mg/mL. A mixed standard solution of antidepressants at 100 μg/mL was prepared in MeOH. Working solutions with desired concentrations were prepared by stepwise dilution of the mixed standard solution (100 μg/mL) with H2O.
Each analyte should use a corresponding isotopic internal standard to correct for signal fluctuations, which is a well-recognized fact in mass spectrometry quantification. However, isotopic internal standards are known to be expensive and have long lead times. Therefore, in this study, a single internal standard for method development and validation was used to reduce analytical costs. The FLX-d6 internal standard’s m/z value is free from interference and is not influenced by background peaks resulting from the fragmentation and cluster signals of the organic matrix. Hence, FLX-d6 was used as the isotope-labeled internal standard to normalize signal variation.
For the preparation of the MALDI matrix solutions, concentrations of 10.0 mg/mL were achieved by dissolving the matrices in a solvent composed of 50% ACN with 0.1% TFA. To ensure stability and prevent degradation, these solutions were stored in an environment shielded from light, within a refrigerator maintained at 4 °C. The matrix solutions were freshly prepared on a weekly basis to maintain their efficacy and integrity.
Sample collection and pretreatment
Blank blood samples were collected from adult Sprague–Dawley (SD) rats. The actual blood samples for preparation were obtained from SD rats that had been orally administered antidepressant medications: Adult SD rats weighing approximately 200–300 g were randomly assigned to the venlafaxine, fluoxetine [17], imipramine [18], mirtazapine [19], and amfebutamone [20] groups. The specific dosages and solvents used are shown in Table 1. Blood samples were drawn at 0.25, 0.5, 1, 2, and 4 h after drug administration. The blood samples were collected in non-anticoagulant tubes. After centrifugation at 4000 rpm for 10 min at 4 °C, the resulting supernatants, which constitute the serum, were carefully extracted and stored at 4 °C for no more than 48 h before use. The collection and utilization of blood samples were carried out in strict adherence to ethical guidelines for the care and use of laboratory animals. These procedures were approved by the Ethics Committee of Zhengzhou University and executed in collaboration with the First Affiliated Hospital of Zhengzhou University.
To mitigate the viscosity of the serum and to control its pH value, the serum samples underwent a dilution process. This was achieved by employing a one-to-one dilution with phosphate-buffered saline (PBS, 50 mM, pH 9.0) solution. The diluted sample solution was then transferred for PT-SPE.
Pipette-tip solid-phase extraction
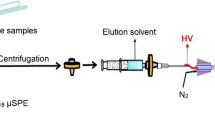
The commercially available Zip-Tip C18 was securely affixed to a modified 1-mL pipette tip, with approximately 0.5 cm trimmed from the end to facilitate its attachment to a pipettor: 1-mL pipette tip with the tip cut (about 0.5 cm from the tip) was tightly inserted into the Zip-Tip C18 according to the schematic diagram on the left side of Fig. 1. This assembly created a PT-SPE device for efficient sample processing. The setup enabled the extraction of up to 1 mL of sample solution by using the pipettor’s control button to aspirate and dispense the sample solution.
The PT-SPE process was conducted following a five-step procedure, as illustrated in Fig. 1, which included activation, equilibration, sample loading, washing, and elution: (1) activating the Zip-Tip C18 with 30 μL of acetonitrile through five cycles of aspiration and dispersion; (2) equilibrating the Zip-Tip C18 using 30 μL of PBS (50 mM, pH 9.0) in five cycles; (3) loading the sample by aspirating and dispensing 100 μL of a PBS-diluted solution (50 μL rat serum was diluted one-to-one by 50 μL PBS) five times, enriching the analytes on the C18 material; (4) washing the Zip-Tip C18 with 30 μL of PBS through five cycles to remove potential impurities; (5) eluting the analytes with 10 μL of a desorption solution in five cycles. The eluate was then prepared for mass spectrometry analysis.
LC–MS analysis conditions
The LC–MS analysis was conducted using a Therm Q Exactive Orbitrap mass spectrometer. The liquid chromatographic separation was performed on an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters, USA) using a binary gradient system. This system consisted of water with 0.1% formic acid (mobile phase A) and 100% acetonitrile with 0.1% formic acid (mobile phase B). The gradient program was as follows: 0–1 min 95%, 1–9 min 95–0%, 9–13 min 0%, 13–13.1 min 0–95%, and 13.1–15 min 95% (% of component A was given). The MS parameters used were as follows: capillary temperature, 320 °C; aux gas flow rate, 10 Arb; sheath gas flow rate, 40 Arb; spray voltage, 3500 V; mass range (m/z), 80–1200; full MS resolution, 70,000.
MALDI-TOF MS analysis conditions
A MALDI-TOF MS system (QuanTOF II) with a 355-nm Nd:YAG laser (IntelliBio, Qingdao, China) was used. MS calibration was performed using DHB matrix ions (m/z 137.024, 155.034, 154.027, 273.04) and a Peptide Calibration Standard, Bradykinin Fragment 1–7 (m/z 756.85). Mass spectra were acquired in the positive-ion reflector mode with a m/z scan range of 50–1000. Mass spectra were collected by summing 1000 laser shots, and data processing was performed using ProteoWizard 3.0.4472 software.
For MALDI-TOF MS analysis, the sample eluent was deposited on the stainless steel target probe using the dried droplet method. 1 μL of sample eluent and 2 μL of DHB solution (10 mg/mL, made in 50% ACN) were mixed on the target and air-dried before detection.
Results and discussion
Evaluation of PT-SPE enrichment and MALDI-MS detection feasibility
More recently, Zip-Tip C18 has been used primarily for protein purification and extraction in proteomics. Accordingly, its enrichment and purification effects have been demonstrated in a series of studies [21, 22]. And the Zip-Tip C18 is a commercially available and easily accessible product, making the method readily implementable. Hence, Zip-Tip C18 was used to extract antidepressants from complex biological samples in this study. The feasibility and performance of the proposed PT-SPE method were verified by applying it to extract and detect five antidepressants in serum (10 μg/mL) by MALDI MS.
Upon direct detection (Fig. 2B), the antidepressant effects in spiked serum samples were completely suppressed in the presence of large amounts of interfering substances. After PT-SPE (Fig. 2C), the interferences in spiked rat serum samples were removed, and the antidepressants were successfully detected according to the spectrum of the standard solution (Fig. 2A). This was because Zip-Tip C18 captured the antidepressants due to hydrophobic interactions, while the interference was left in the serum, as expected. More importantly, by comparing the spectra, the responses of the analytes were amplified many times after PT-SPE, enabling the detection of lower concentrations of antidepressants via MALDI.
Optimization of extraction and detection conditions
To establish the optimal extraction conditions, various factors that could potentially affect the extraction recovery were meticulously optimized. Specifically, standard aqueous solutions containing the five antidepressants at a concentration of 50.0 ng/mL were employed to fine-tune the extraction parameters, focusing on achieving the highest average extraction recoveries. Considering the analysis using MALDI, where signal reproducibility can be notably poor without internal standard calibration [23], the optimization process was predominantly conducted using LC–MS. LC–MS is widely recognized for its superior reproducibility, making it a more reliable method for this phase of optimization. To align with the LC–MS analysis requirements, the desorption solvent was diluted to 100 μL with H2O. The concentration used for the optimization in LC–MS was 50 ng/mL. Considering that the detection sensitivity of MALDI analysis is much lower than that of LC–MS, we aimed to demonstrate that the conditions optimized at 50 ng/mL in LC–MS are also applicable to higher concentrations. To support this, we examined the extraction recovery at 0.5 µg/mL and 5 µg/mL and compared them with the extraction recovery obtained at 50 ng/mL under the optimized conditions. The results showed comparable extraction recovery rates, indicating that the optimized conditions are suitable across a range of concentrations (Fig. S1).
This approach allowed for a thorough investigation into the most suitable types and volumes of desorption solvents, as well as the optimal pH for the sampling solution. Moreover, the study extended to evaluating the impact of different acids added to the desorption solvent and the choice of matrix for MALDI. These factors are critical as they can influence the desorption/ionization efficiency in MALDI, potentially affecting the detection sensitivity and overall analytical performance.
pH of the sample solution
Antidepressants are generally basic and hydrophobic compounds with pKa values ranging from 9.4 to 9.8 [6, 24]. Thus, the charge of the analytes is influenced by the pH of the sample solution, which further impacts the ability of Zip-Tip C18 to extract and enrich antidepressants. Therefore, it is necessary to investigate and select an appropriate pH for the sample solution. As shown in Fig. 3A, the extraction recoveries of the antidepressants gradually increased as the pH of the sample solutions increased from 3.0 to 9.0, and the curve tended to flatten when the pH of the sampling solution continued to increase. The possible reasons are as follows: when the pH of the sample solution was lower than the pKa of the antidepressant itself, the antidepressants mainly existed in a protonated form and were more soluble in polar sample solutions. In contrast, when the pH was higher than the pKa of the antidepressant itself, the antidepressant was not easily dissociated and mainly existed in a molecular state; the antidepressant was less soluble in polar sample solutions and could be easily enriched by Zip-Tip C18. When the pH exceeded 9.0, the drugs were in a molecular state to the greatest extent, which means that antidepressants could be fully enriched by Zip-Tip C18. Thus, a pH of 9.0 for the sample solution was selected for the subsequent experiments.
Types of desorption solvents
A suitable desorption solvent should be able to maximize the elution of analytes and be compatible with subsequent MALDI analysis. Considering the convenience and feasibility of the experiment, commonly used and available organic solvents, including MeOH, ACN, EtOH, AC, and IPA, were evaluated and the extraction recoveries were compared. As shown in Fig. 3B, there was no single desorption solvent with the greatest effect on any one antidepressant. For example, the most suitable desorption solvent was ACN for FLX, while it was EtOH for VEN. In addition, there was no significant difference in the extraction recoveries among these desorption solvents. Considering that EtOH is environmentally friendly and has lower toxicity compared to other solvents, it was selected as the final desorption solvent.
Volume of desorption solvents
A smaller volume of desorption solvent is preferred for enhanced sensitivity. However, using too little solvent might result in incomplete desorption of the analytes. Balancing sensitivity with complete desorption requires careful examination of the solvent volume. Since the desorption solvent is used directly in subsequent analyses without further concentration or enrichment steps, a higher concentration of analytes within the solvent facilitates improved detection sensitivity. Therefore, in addition to extraction recoveries, the peak areas of the analytes were also compared to determine the optimal desorption efficiency. The volume of EtOH was investigated within the range of 5–25 μL. The findings, as illustrated in Fig. 3C, revealed that a volume of 10 μL of EtOH yielded optimal results. Based on this evidence, 10 μL of EtOH was selected for use in all subsequent experiments.
The types of acid added in desorption solvent
Incorporating acids into the desorption solvent serves a dual purpose. On the one hand, it encourages a greater proportion of the analytes to exist in an ionized state, which facilitates easier desorption. On the other hand, acid additives can enhance the ionization of analytes, leading to improved sensitivity in detection. Consequently, the selection of the most appropriate acid additive for the MALDI MS analysis of antidepressants was determined by evaluating the signal intensity of the analytes by MALDI MS when various acid additives were used, including 0.1% TFA, 0.1% FA, 0.1% AA, and 0.1% PHA [25]. As illustrated in Fig. 3D, the analyte intensities for VEN, FLX, IMP, and AMF reached their peak levels when 0.1% PHA was employed as the additive. In the case of MIP, the variation in intensity across different acid additives was less pronounced. Based on these observations, PHA was selected as the preferred acid additive for this analytical method.
Selection of matrices
In MALDI MS, the matrix acts as an energy mediator that assists in the ionization and desorption of the analyte [25]. The critical role of the matrix is widely recognized, and selecting an appropriate matrix can enhance detection specificity and sensitivity [26]. Accordingly, four commonly used matrices—CHCA, DHB, DAN, and DCTB—were evaluated to determine the optimal matrix for the analysis of antidepressants in MALDI. As depicted in Fig. 3E, the highest intensity of antidepressants was observed when DHB (10 mg/mL) was employed as the matrix, indicating that DHB was the most suitable matrix for the five antidepressants studied. Additionally, DHB was characterized by lower background signals, which further contributes to improved selectivity in the analytical experiments.
Method validation
Utilizing the optimized conditions established earlier, the capability of the proposed method to rapidly quantify antidepressants in serum was assessed. This evaluation covered aspects such as linearity, inter- and intraday precision, and recovery. The spiked rat serum samples were prepared following these principles: The stock solutions of antidepressants (1 mg/mL) were diluted with H2O to obtain working solutions at various concentrations. Aliquots of these working solutions were then added to blank rat serum to achieve final concentrations covering the desired linearity range. Then, the spiked rat serum samples were incubated at 37 °C for 20 min before dilutions. It was ensured that the volume of the spiking solution did not exceed 10% of the rat serum volume, and this additional volume was accounted for in subsequent dilutions. Calibration curves were generated from the mean peak intensity ratios of analytes to FLX-d6 (analyte/internal standard) across various concentrations in MALDI. The data presented in Table 2 demonstrated that the method exhibited commendable linearity (R2 ≥ 0.997) in the range of 0.5–100 μg/mL (AMF), 0.05–10 μg/mL (MIP), 0.1–20 μg/mL (VEN), 0.25–50 μg/mL (IMP), and 0.5–100 μg/mL (FLX). Moreover, the limits of quantification for the method were established at 0.5, 0.05, 0.1, 0.25, and 0.5 μg/mL for AMF, MIP, VEN, IMP, and FLX, respectively.
The method’s precision was gauged through intraday and interday relative standard deviations (RSDs), while its accuracy was ascertained by evaluating the relative recoveries. Pooled rat serum samples (50.0 μL) spiked with three distinct concentrations of the antidepressants (AMF: 2.0, 10.0, and 50.0 μg/mL; VEN: 0.4, 2.0, and 10.0 μg/mL; FLX: 2.0, 10.0, and 50.0 μg/mL; IMP: 1.0, 5.0, and 25.0 μg/mL; MIP: 0.2, 1.0, and 5.0 μg/mL) underwent analysis. The intraday RSDs were determined by separately analyzing three samples, while the interday RSDs were obtained from analyses conducted over 3 days. The relative recovery rates were then calculated by comparing the measured spiked concentrations to the actual spiked concentrations. As outlined in Table 3, both RSDs and recovery rates ranged from 0.8 to 7.5% and 92.4 to 111.9%, respectively. These findings suggest that the accuracy and precision of the method, which integrates MALDI with PT-SPE, are satisfactory.
Application to actual rat serum samples
The efficacy of the established method was assessed through the analysis of drug concentrations in serum samples from rats treated with antidepressants. Blood samples were extracted from the orbital plexus of the rats post-administration of the antidepressants. The results, as illustrated in the accompanying graph, showed the concentration profiles of the antidepressants over time. The concentrations of antidepressants were measured at various time points—0.25, 0.5, 1, 2, and 4 h post-dosing (Table S1). The results showed in Fig. 4 clearly revealed that each drug exhibited a distinct concentration–time curve, aligning with the expected metabolic rates of the antidepressants in vivo [27,28,29]. The method successfully quantified the antidepressants, highlighting its applicability for pharmacokinetic studies and the potential for therapeutic drug monitoring in clinical settings.
Evaluation of method’s greenness
Environmental sustainability is becoming increasingly pivotal in the field of analytical chemistry. In this context, the “greenness” of an analytical method refers to its environmental impact, considering factors such as the use of chemicals, energy consumption, and waste production. To comprehensively assess the environmental footprint of the proposed analytical method, six distinguished greenness assessment tools were utilized. Each of these tools offers a unique perspective on the various aspects of the method’s greenness, ranging from the choice of reagents to the efficiency and waste reduction in the analytical process. The combined use of these tools provides a holistic evaluation of the method’s sustainability and adherence to the principles of green analytical chemistry.
The Green Analytical Procedure Index (GAPI) [30] evaluates the green character of an entire analytical methodology, encompassing sampling, transport, storage, sample preparation, and final determination. The GAPI uses a pictogram with a color scale to represent the greenness of each stage of the analytical process. According to the GAPI results illustrated in Fig. 5A, the pictogram contained eight green, three yellow, and four red areas, reflecting the method’s overall excellent greenness.
The evaluation of method greenness by five greenness assessment tools: (A) the Green Analytical Procedure Index (GAPI), (B) the Analytical GREEnness (AGREE), (C) Analytical Greenness Metric for Sample Preparation (AGREEprep), (D) Blue Applicability Grade Index (BAGI), and (E) Sample Preparation Metric of Sustainability (SPMS)
The Analytical GREEnness (AGREE) calculator is based on the 12 principles of green analytical chemistry and allows for the weighting of different criteria [31]. The clock-like graph produced by the AGREE tool (Fig. 5B) displayed a central score of 0.76, surpassing the 0.6 threshold, which signified that the method was considered green.
The AGREE metric specifically for sample preparation (AGREEprep) emphasizes ten categories impacting sample preparation [32]. Figure 5C shows the colorful round pictogram representing the AGREEprep assessment of the established method. The inner circle was green, and the overall score was 0.73, indicating a strong green performance in sample preparation.
The Blue Applicability Grade Index (BAGI) complements existing green metrics by focusing on the practical aspects of an analytical method [33]. The asteroid pictogram displayed in Fig. 5D, with its color hue scale and the number inside the BAGI pictogram, indicated the method’s excellent applicability.
As an additional tool, the Sample Preparation Metric of Sustainability (SPMS) assesses the greenness of sample preparation techniques [34]. The clock diagram in Fig. 5E used colors to signify performance levels, ranging from green for “successful” to red for “inadequate.” The central large square in the diagram indicated that the global numeric score for the sample preparation method was 7.47.
Lastly, the Analytical Eco-Scale (AES) [35] assigns penalty points to various stages of the analytical process. The AES calculation (Table S2) resulted in a score of 90, which was above the 75 benchmark for excellent green analysis.
In summary, the evaluations by these six greenness assessment tools collectively indicate that the method employed in this study was exceptionally green.
Method comparison
Currently, a multitude of strategies for the quantification of antidepressants in rat serum exist. A comparative analysis was conducted to assess the efficacy of the method described in this study relative to those documented in the literature (Table 4). While chromatography-based methods like LC-UV or LC–MS have demonstrated advantages in detection sensitivity, they are not without significant drawbacks. The main challenges of these strategies include complex sample pretreatment, lengthy chromatographic separation, and the consumption of large volumes of organic mobile phases, which results in substantial waste.
In response to these challenges, MALDI MS has come to the fore. For instance, in 2016, Watanabe et al. [8] utilized MALDI MS for the analysis of six tricyclic antidepressants in blood and urine samples. However, their methodology required multiple preparation steps such as centrifugation, liquid–liquid extraction, additional centrifugation, and evaporation, extending the sample preparation time to over 30 min. Moreover, the use of highly toxic 1-chlorobutane as the extraction solvent posed environmental and safety concerns.
The method introduced in this study leverages commercially available Zip-Tip C18 for PT-SPE combined with MALDI MS detection. The entire sample preparation is streamlined to a mere aspirating/dispensing action with a pipettor, completing in under 2 min. This approach not only expedites the preparation but also enhances sensitivity through sample purification and enrichment. Subsequent MALDI MS analysis can process a sample within 1 min, facilitating rapid, high-throughput detection. The entire procedure requires only 30 μL of ACN and 10 μL of EtOH, highlighting its green and environmentally friendly nature, while also being user-friendly for laboratory personnel. The PT-SPE process can be further automated using a liquid handling station with multichannel pipettors or automated liquid handling systems, enabling automated and high-throughput sample processing. Overall, this method, characterized by high analytical throughput, minimal sample consumption, eco-friendly, and simplicity, holds great promise for potential clinical applications.
Conclusions
This study has developed a rapid, uncomplicated, eco-friendly, and high-throughput method for the analysis of various antidepressants in serum. Using Zip-Tip C18, the antidepressants were extracted from complex biological matrices with exceptional ease. The entire extraction process, consisting merely of aspirating and dispensing the solution, was completed in under 2 min. Subsequent MALDI MS analysis provided fast and high-throughput results within 1 min. The method exhibited excellent linearity (R2 ≥ 0.997) along with satisfactory accuracy (relative recoveries ranging from 92.4 to 111.9%) and precision (RSD ≤ 7.5%). When applied to actual serum samples for the detection of antidepressant concentrations, the method proved to be efficient. Looking ahead, this study serves as a proof of concept by utilizing commercial Zip-Tips to achieve rapid sample preparation, which are relatively expensive (~ $5–6 each). Future research could explore the use of more affordable pipette tips, as there are numerous reports in the literature on simple and inexpensive pipette-tip extraction methods. Additionally, while MALDI-TOF–MS was employed, which has a high initial cost, it is robust and durable, requires minimal consumables, and is user-friendly. Apart from the instrument cost, the analysis costs are relatively low. Therefore, the method developed in this study, with the potential use of low-cost pipette-tip extraction tips, shows promise for cost-effective analysis. But the method has lower sensitivity, precision, and accuracy compared to traditional LC–MS methods, so the method presented in this study is not suitable for applications requiring high sensitivity detection. In sum, the strategy outlined requires minimal sample preparation and markedly reduces the time needed for analysis. It offers high analytical throughput, minimal sample consumption, eco-friendliness, and simplicity, providing ideas and a reference basis for the subsequent development of methods for therapeutic drug monitoring. Additionally, given the simplicity and rapidity of our method, it is particularly advantageous in situations such as acute overdose of antidepressants, where quick detection of drug concentration is essential.
References
Mayneris-Perxachs J, Castells-Nobau A, Arnoriaga-Rodriguez M, Martin M, de la Vega-Correa L, Zapata C, Burokas A, Blasco G, Coll C, Escrichs A, Biarnes C, Moreno-Navarrete JM, Puig J, Garre-Olmo J, Ramos R, Pedraza S, Brugada R, Vilanova JC, Serena J, Gich J, Ramio-Torrenta L, Perez-Brocal V, Moya A, Pamplona R, Sol J, Jove M, Ricart W, Portero-Otin M, Deco G, Maldonado R, Fernandez-Real JM. Microbiota alterations in proline metabolism impact depression. Cell Metab. 2022;34(5):681-701 e10. https://doi.org/10.1016/j.cmet.2022.04.001.
Guzinski M, Lindner E, Pendley B, Chaum E. Electrochemical sensor for tricyclic antidepressants with low nanomolar detection limit: Quantitative Determination of Amitriptyline and Nortriptyline in blood. Talanta. 2022;239:123072. https://doi.org/10.1016/j.talanta.2021.123072.
Sarikaya M, Ulusoy HI, Morgul U, Ulusoy S, Tartaglia A, Yilmaz E, Soylak M, Locatelli M, Kabir A. Sensitive determination of Fluoxetine and Citalopram antidepressants in urine and wastewater samples by liquid chromatography coupled with photodiode array detector. J Chromatogr A. 2021;1648:462215. https://doi.org/10.1016/j.chroma.2021.462215.
Chen L, Zhang Y, Zhang Y-X, Wang W-L, Sun D-M, Li P-Y, Feng X-S, Tan Y. Pretreatment and analysis techniques development of TKIs in biological samples for pharmacokinetic studies and therapeutic drug monitoring. J Pharm Anal. 2023. https://doi.org/10.1016/j.jpha.2023.11.006.
Chen D, Zhang JX, Cui WQ, Zhang JW, Wu DQ, Yu XR, Luo YB, Jiang XY, Zhu FP, Hussain D, Xu X. A simultaneous extraction/derivatization strategy coupled with liquid chromatography-tandem mass spectrometry for the determination of free catecholamines in biological fluids. J Chromatogr A. 2021;1654:462474. https://doi.org/10.1016/j.chroma.2021.462474.
Xu XL, Wang B, Li WX, Wu JY, Yuan H, Xu X, Chen D. In-pipette-tip natural-feather-supported liquid microextraction for conveniently extracting hydrophobic compounds in aqueous samples: A proof-of-concept study. Microchem J. 2023;185:108274. https://doi.org/10.1016/j.microc.2022.108274.
Montemurro M, De Zan MM, Robles JC. Optimized high performance liquid chromatography-ultraviolet detection method using core-shell particles for the therapeutic monitoring of methotrexate. J Pharm Anal. 2016;6(2):103–11. https://doi.org/10.1016/j.jpha.2015.12.001.
Nozawa H, Minakata K, Yamagishi I, Hasegawa K, Suzuki M, Gonmori K, Suzuki O, Watanabe K. Simultaneous determination of cyclic antidepressants and their related drugs and the estimation of new metabolites in human whole blood and urine by MALDI-QTOF-mass spectrometry. Forensic Toxicol. 2016;34(2):244–55. https://doi.org/10.1007/s11419-016-0313-1.
Rana S, Uralets VP, Ross W. A new method for simultaneous determination of cyclic antidepressants and their metabolites in urine using enzymatic hydrolysis and fast GC-MS. J Anal Toxicol. 2008;32(5):355–63. https://doi.org/10.1093/jat/32.5.355.
Lopez-Rabunal A, Lendoiro E, Concheiro M, Lopez-Rivadulla M, Cruz A, de-Castro-Rios A. LC-MS-MS Method for the Determination of Antidepressants and Benzodiazepines in Meconium. J Anal Toxicol. 2020;44(6):580-588. https://doi.org/10.1093/jat/bkaa012.
Thu NQ, Tien NTN, Yen NTH, Duong TH, Long NP, Nguyen HT. Push forward LC-MS-based therapeutic drug monitoring and pharmacometabolomics for anti-tuberculosis precision dosing and comprehensive clinical management. J Pharm Anal. 2024;14(1):16–38. https://doi.org/10.1016/j.jpha.2023.09.009.
Looby N, Roszkowska A, Yu M, Rios-Gomez G, Pipkin M, Bojko B, Cypel M, Pawliszyn J. In vivo solid phase microextraction for therapeutic monitoring and pharmacometabolomic fingerprinting of lung during in vivo lung perfusion of FOLFO. J Pharm Anal. 2023;13(10):1195–204. https://doi.org/10.1016/j.jpha.2023.04.005.
Yuan HY, Yu SH, Chai GH, Liu JT, Zhou Q. An LC-MS/MS method for simultaneous analysis of the cystic fibrosis therapeutic drugs colistin, ivacaftor and ciprofloxacin. J Pharm Anal. 2021;11(6):732–8. https://doi.org/10.1016/j.jpha.2021.02.004.
Chen J, Huang H, Ouyang D, Lin J, Chen Z, Cai Z, Lin Z. A reactive matrix for in situ chemical derivatisation and specific detection of cis-diol compounds by matrix-assisted laser desorption/ionisation mass spectrometry. Analyst. 2023;148(21):5402–6. https://doi.org/10.1039/d3an01400b.
Wang Y, Hummon AB. Quantification of Irinotecan in Single Spheroids Using Internal Standards by MALDI Mass Spectrometry Imaging. Anal Chem. 2023;95(24):9227–36. https://doi.org/10.1021/acs.analchem.3c00699.
Tang W, Zhang Y, Li P, Li B. Evaluation of Intestinal Drug Absorption and Interaction Using Quadruple Single-Pass Intestinal Perfusion Coupled with Mass Spectrometry Imaging. Anal Chem. 2023;95(6):3218–27. https://doi.org/10.1021/acs.analchem.2c03767.
Guo S, Li K, Chen Y, Li B. Unraveling the drug distribution in brain enabled by MALDI MS imaging with laser-assisted chemical transfer. Acta Pharm Sin B. 2022;12(4):2120–6. https://doi.org/10.1016/j.apsb.2021.11.007.
Bielawski A, Zelek-Molik A, Rafa-Zablocka K, Kowalska M, Gruca P, Papp M, Nalepa I. Elevated Expression of HSP72 in the Prefrontal Cortex and Hippocampus of Rats Subjected to Chronic Mild Stress and Treated with Imipramine. Int J Mol Sci. 2023;25(1):243. https://doi.org/10.3390/ijms25010243.
Barbosa-Mendez S, Leff P, Arias-Caballero A, Hernandez-Miramontes R, Heinze G, Salazar-Juarez A. Mirtazapine attenuates cocaine seeking in rats. J Psychiatr Res. 2017;92:38–46. https://doi.org/10.1016/j.jpsychires.2017.03.021.
Pahlavani H, Masoudi M, Khoshroo N, Kakhki S, Mahdi Rezavanimehr M, Ghari A, Beheshti F. Vitamin B(12) reversed anxiety and depression induced by adolescent nicotine withdrawal through alteration the inflammatory, oxidative and serotoninergic profiles in male rats. Biochem Pharmacol. 2023;217:115832. https://doi.org/10.1016/j.bcp.2023.115832.
Balizs G, Weise C, Rozycki C, Opialla T, Sawada S, Zagon J, Lampen A. Determination of osteocalcin in meat and bone meal of bovine and porcine origin using matrix-assisted laser desorption ionization/time-of-flight mass spectrometry and high-resolution hybrid mass spectrometry. Anal Chim Acta. 2011;693(1–2):89–99. https://doi.org/10.1016/j.aca.2011.03.027.
Wu S, Yang K, Liang Z, Zhang L, Zhang Y. Urea free and more efficient sample preparation method for mass spectrometry based protein identification via combining the formic acid-assisted chemical cleavage and trypsin digestion. Talanta. 2011;86:429–35. https://doi.org/10.1016/j.talanta.2011.08.052.
Bu XM, Zhao WD, Zhang MY, Wu DQ, Wu JY, Xu X, Chen D. Matrix-assisted laser desorption/ionization high-resolution mass spectrometry for high-throughput analysis of androgenic steroid adulteration in traditional Chinese medicine based on d0/d5-Girard’s reagent P labeling. Talanta. 2023;253:124006. https://doi.org/10.1016/j.talanta.2022.124006.
Chen D, Liu FL, Rong Y, Qi MH, Li YY, Shi XZ, Xie Y, Xu X. Coupling in-syringe kapok fiber-supported liquid-phase microextraction with flow injection-mass spectrometry for rapid and green biofluid analysis: Determination of antidepressants as an example. J Pharm Biomed Anal. 2023;229:115380. https://doi.org/10.1016/j.jpba.2023.115380.
Choi H, Lee D, Kim Y, Nguyen HQ, Han S, Kim J. Effects of Matrices and Additives on Multiple Charge Formation of Proteins in MALDI-MS Analysis. J Am Soc Mass Spectrom. 2019;30(7):1174–8. https://doi.org/10.1007/s13361-019-02213-7.
Zhou Q, Fulop A, Hopf C. Recent developments of novel matrices and on-tissue chemical derivatization reagents for MALDI-MSI. Anal Bioanal Chem. 2021;413(10):2599–617. https://doi.org/10.1007/s00216-020-03023-7.
Sirot EJ, Harenberg S, Vandel P, Lima CAM, Perrenoud P, Kemmerling K, Zullino DF, Hilleret H, Crettol S, Jonzier-Perey M, Golay KP, Brocard M, Eap CB, Baumann P. Multicenter Study on the Clinical Effectiveness, Pharmacokinetics, and Pharmacogenetics of Mirtazapine in Depression. J Clin Psychopharm. 2012;32(5):622–9. https://doi.org/10.1097/JCP.0b013e3182664d98.
Chen FF, Jiang H, Xu J, Wang SH, Meng DR, Geng PW, Dai DP, Zhou Q, Zhou YF. In Vitro and In Vivo Rat Model Assessments of the Effects of Vonoprazan on the Pharmacokinetics of Venlafaxine. Drug Des Dev Ther. 2020;14:4815–24. https://doi.org/10.2147/Dddt.S276704.
Wolker LHW, Veltri CA, Pearman K, Lozoya M, Norris JW. Pharmacokinetics of fluoxetine in horses following oral administration. J Vet Pharmacol Ther. 2022;45(1):63–8. https://doi.org/10.1111/jvp.13029.
Plotka-Wasylka J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta. 2018;181:204–9. https://doi.org/10.1016/j.talanta.2018.01.013.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE-Analytical GREEnness Metric Approach and Software. Anal Chem. 2020;92(14):10076–82. https://doi.org/10.1021/acs.analchem.0c01887.
Wojnowski W, Tobiszewski M, Pena-Pereira F, Psillakis E. AGREEprep – Analytical greenness metric for sample preparation. TrAC. 2022;149:116553. https://doi.org/10.1016/j.trac.2022.116553.
Manousi N, Wojnowski W, Płotka-Wasylka J, Samanidou V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green Chem. 2023;25(19):7598–604. https://doi.org/10.1039/d3gc02347h.
Gonzalez-Martin R, Gutierrez-Serpa A, Pino V, Sajid M. A tool to assess analytical sample preparation procedures: Sample preparation metric of sustainability. J Chromatogr A. 2023;1707:464291. https://doi.org/10.1016/j.chroma.2023.464291.
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC. 2012;37:61–72. https://doi.org/10.1016/j.trac.2012.03.013.
Han WC, Zhang HJ, Chen JB, Chen YY, Wang WJ, Liu YW, Yang P, Yuan DD, Chen D. A green and rapid deep eutectic solvent dispersed liquid-liquid microextraction with magnetic particles-assisted retrieval method: Proof-of-concept for the determination of antidepressants in biofluids. J Mol Liq. 2024;395:123875. https://doi.org/10.1016/j.molliq.2023.123875.
Locatelli M, Covone S, Rosato E, Bonelli M, Savini F, Furton KG, Gazioglu I, D’Ovidio C, Kabir A, Tartaglia A. Analysis of seven selected antidepressant drugs in post-mortem samples using fabric phase sorptive extraction followed by high performance liquid chromatography-photodiode array detection. Forensic Chem. 2022;31:100460. https://doi.org/10.1016/j.forc.2022.100460.
Lioupi A, Kabir A, Furton KG, Samanidou V. Fabric phase sorptive extraction for the isolation of five common antidepressants from human urine prior to HPLC-DAD analysis. J Chromatogr B. 2019;1118–1119:171–9. https://doi.org/10.1016/j.jchromb.2019.04.045.
Azadkish K, Shokrollahi A, Rezayat MR, Rastgar M. Development of dispersive liquid-liquid microextraction with solid-phase evaporation as a novel hyphenated method prior to ion mobility spectrometry and its application for trace analysis of fluoxetine. Anal Bioanal Chem. 2023;415(14):2665–76. https://doi.org/10.1007/s00216-023-04665-z.
Oliveira AF, de Figueiredo EC, Dos Santos-Neto AJ. Analysis of fluoxetine and norfluoxetine in human plasma by liquid-phase microextraction and injection port derivatization GC-MS. J Pharm Biomed Anal. 2013;73:53–8. https://doi.org/10.1016/j.jpba.2012.04.006.
Ma W, Gao X, Guo H, Chen W. Determination of 13 antidepressants in blood by UPLC-MS/MS with supported liquid extraction pretreatment. J Chromatogr B. 2021;1171:122608. https://doi.org/10.1016/j.jchromb.2021.122608.
H.D. de Faria, A.T. Silveira, B.C. do Prado, J.L.M. Nacif, M.A. Rosa, J.D.R. Dos Santos, P. Santos, E.C. Figueiredo, I. Martins. Online biological sample preparation with restricted access hybrid carbon nanotubes for determination of anti-smoking drugs. J Chromatogr A. 2022;1669:462931. https://doi.org/10.1016/j.chroma.2022.462931.
Funding
This work was supported by grants from the National Key R&D Program of China (2021YFC2401105), the National Natural Science Foundation of China (No. 82374018, No. 82003921), the China Postdoctoral Science Foundation (2021M702937, 2023M733256), and the Henan Provincial Science and Technology Research Project (242102311184).
Author information
Authors and Affiliations
Contributions
Zhi Sun: writing—original draft. Fangfang Wang: writing—original draft, methodology. Wenxuan Li: methodology. Ruobing Ren: methodology. Peipei Zhou: methodology. Qingquan Jia: methodology. Lingguo Zhao: methodology. Di Chen: conceptualization, writing—review and editing. Lihua Zuo: writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
The collection and utilization of blood samples were carried out in strict adherence to ethical guidelines for the care and use of laboratory animals. The study was approved by the Ethics Committee of Zhengzhou University and executed in collaboration with the First Affiliated Hospital of Zhengzhou University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z., Wang, F., Li, W. et al. Pipette-tip solid-phase extraction coupled with matrix-assisted laser desorption/ionization mass spectrometry enables rapid and high-throughput analysis of antidepressants in rat serum. Anal Bioanal Chem 416, 5013–5023 (2024). https://doi.org/10.1007/s00216-024-05439-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-024-05439-x