Abstract
Wax printing is the most widely used method for fabricating microfluidic paper-based analytical devices (μPADs), but it still suffers from disadvantages like discontinuation of wax printers and need for additional equipment for heating treatment. To address these issues, this work initially describes a new class of wax printing approach for high-precision, batch fabrication of μPADs using a household 3D printer. It only involves a one patterning step of printing polyethylene wax into rice paper body. Under optimized parameters, a fabrication resolution, namely the minimum hydrophilic channel width, down to ~189 ± 30 μm could be achieved. In addition, the analytical applicability of such polyethylene wax-patterned μPADs was demonstrated well with enhanced colorimetric detection of dopamine as a model analyte by combining metal-organic framework (MOF) based nanoenzymes (ZIF-67) with a smartphone (for portable quantitative readout). The developed nanosensor could linearly detect dopamine over a concentration range from 10 to 1000 μM, with a detection limit of ca. 2.75 μM (3σ). The recovery results for analyzing several real samples (i.e., pig feed, chicken feed, pork and human serum) were between 91.82 and 102.79%, further validating its good detection accuracy for potential practical applications in food safety and medical diagnosis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (2-(3,4-dihydroxyphenyl) ethylamine) is one of the most essential catecholamine neurotransmitters in both central and peripheral nervous system [1]. It plays a critical role in regulation and control of multiple biological activities and cognitive functions, such as movement, behavior, sleep, memory, and learning [2]. Abnormal concentrations of dopamine are considered to be an indicator of many diseases, including cardiovascular disease, schizophrenia, depression, Alzheimer’s, Parkinson’s, Huntington’s, epilepsy, and tumors [3,4,5]. Moreover, dopamine hydrochloride (precursor substance of dopamine) has been made lean meat powder added to animal feed for helping increase skeletal muscle mass and inhibit fat production [6,7,8]. However, its excess residues in animal tissues would further cause great harm to consumers’ health. Therefore, accurate detection of dopamine levels in human body fluids (e.g., serum) and animal feeds is of great importance for medical diagnosis and food safety.
So far, monitoring dopamine concentration in different samples has been completed by either traditional techniques such as gas chromatography (GC) [9], high performance liquid chromatography (HPLC) [10], capillary electrophoresis (CE) [11], and mass spectrometry (MS) [12], or several recently-reported chemical and biological analytical methods like fluorescence analysis [13], UV-vis absorption spectrometry [14], electrochemical sensing [15, 16], and surface-enhanced Raman scattering (SERS) [17]. These methods have their own advantages and disadvantages. For instance, while GC, HPLC, CE, MS, and SERS could offer outstanding sensitivity and specificity, they require expensive instrumentation and well-trained operators that may be not practical in poor-resource small labs. Although fluorescence or UV-vis absorption spectrometry is easily operated, large volumes of samples and reagents are typically consumed. Electrochemical methods are very sensitive for dopamine measurement, but usually suffer from sophisticated modification of working electrodes [15, 16].
In order to address these issues, some alternative efforts have been devoted to the development of simple, cost-effective colorimetric assays of dopamine using wax-patterned microfluidic paper-based analytical devices (μPADs) that are especially suited for field-based analysis as well as personal healthcare [18, 19]. In fact, the µPADs, which possess many attractive features such as cheap paper substrates, low-volume reagents and samples, capillary action-driven liquid flowing, and portability, have been widely used to design a variety of portable assays for ions, small molecules, macromolecules, or cancer cells [20,21,22]. The vast majority of existing µPADs reported previously are primarily created via “printing and heating” procedures with a wax printer (i.e., Xerox Phase 8400) and an additional heating equipment like an oven. However, such type of wax printer has been discontinued by the manufacturer in 2017. In response to this challenge, recently, several groups have utilized household three-dimensional (3D) printers instead of the wax printer for efficiently prototyping μPADs [23,24,25], but where either two steps of printing and heating operations or professional customized modifications of key printer components are still required.
In this work, a single-step wax printing strategy is initially described for μPADs’ fabrication with a commercially available, home-use, integrated 3D printer. Their utility is further demonstrated well with the development of a colorimetric assay for detecting dopamine as a model analyte by combining metal-organic framework (MOF) nanoenzymes with smartphone readout. MOFs, a family of emerging nanomaterials consisting of metal ions/clusters and organic ligands, have received growing interest for various analytical applications in recent years, owing to their simple synthetic process, high product yield, stable and porous crystal structure, abundant active sites, and adjustable nanoenzyme activity [26, 27]. However, up to now, there is no study on MOF nanoenzyme-enhanced colorimetric dopamine assay on μPADs.
Figure 1 shows the schematic procedures for the proposed 3D printer-assisted one-step wax printing method and smartphone-based dopamine detection on MOF-loaded μPADs. The new fabrication method only involves a single patterning step of printing hydrophobic polyethylene wax into hydrophilic paper body according to pre-designed device patterns. The effects of main printing factors (e.g., nozzle temperature, discharge volume, printing speed, workbench’s temperature, nozzle’s distance from workbench) have been studied in detail. Under optimal conditions, wax-patterned μPADs (i.e., circular microzone arrays) are fabricated for immobilizing MOFs of ZIF-67. Upon the introduction of dopamine sample, the nanoenzymes would catalyze para-coupling reaction between the analyte and 4-aminoantipyrine (4-AAP) to produce a purple product in the paper body, which could be further imaged by a smartphone for quantitative analysis. The main experimental conditions affecting the dopamine-4-AAP reactions are optimized, including MOF concentration, 4-AAP concentration, and reaction temperature and pH. The analytical performance of the proposed dopamine assay is investigated in terms of linear concentration range, detection limit, and specificity. In addition, its detection accuracy and practicality is also demonstrated by carrying out recovery of dopamine in real complex samples including pig feed, chicken feed, pork, and human serum.
Experimental section
Reagents and instruments
Dopamine hydrochloride and 4-aminoantipyrine (4-AAP) were purchased from Sigma-Aldrich Co., Ltd. (Shanghai, China). Disodium hydrogen phosphate, monosodium phosphate, dimethylimidazole, and cobalt nitrate were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). Methanol was purchased from Xilong Scientific Co., Ltd. (Guangdong, China). Polyethylene wax (type 102; melting range: 90–120 ℃) was purchased from Shanyi Plasticizing Co., Ltd. (Dongguan, China). Rice paper was purchased from Yutai Xuanzhi Art Co., Ltd. (Anhui, China). The used buffer is 0.2 M phosphate-buffered saline solution (PBS, pH 7.8). Hydrophilic polycarbonate nanoporous membranes (Whatman, ~0.22 μm in average pore size) were obtained from GE 25 Healthcare Life Sciences. All reagents used were of analytical grade without further purification. Feed samples were purchased from Aonong Feed Trading Co., Ltd. (Guilin, China). Pork samples were purchased from the local market. Human serum samples from healthy volunteers were collected from the university hospital. Unless otherwise specified, all solutions were prepared using deionized water (resistance >18.2 MΩ·cm at 25°C).
3D printer (G5) was purchased from Chuangxiang 3D Technology Co., Ltd. (Shenzhen, China). The ultrapure water system (UPS-II-20L) was obtained from Chengdu Yuejun Technology Co., Ltd. (Chengdu, China). The water contact angle measuring instrument (JC-2000) was obtained from Shanghai Zhongchen Digital Technology Equipment Co., Ltd. (Shanghai, China). X-ray diffraction (XRD) data were recorded on an X-ray diffraction automated diffractometer from Malvern Panalytical Co., Ltd. (X’Pert3 Power, UK) using CuKα radiation from 5° to 55° at a scan rate of 5°/min. The surface morphologies of ZIF-67 were investigated using a JSM-5600 LV scanning electron microscope (SEM, JEOL, Japan) and dynamic light scattering instrument from Malvern Instruments Co., Ltd. (Zetasizer Nano ZS90, UK).
Fabrication of μPADs
The fabrication process is shown in Fig. 1. Millimeter-sized polyethylene wax particles were chosen as model raw hydrophobic materials. Firstly, the 3D modeling software embedded in the 3D printer was utilized to transform the 2D design pattern of the μPAD’s barriers into a 3D model. Subsequently, the model was exported in the (.stl) file format. This file was then imported into the slicing software, which cut the model along the z-axis into multiple layers. The resulting layers were exported in turn in the (.gcode) file format. Then, the printer with a hot nozzle and a hot workbench that had been adjusted to appropriate working temperatures directly printed these patterns on the surface of a piece of rice paper. After cooling down to room temperature, wax microstructures patterned in the paper body would act as hydrophobic barriers to produce millimeter or sub-millimeter-sized hydrophilic microzones or channels in the resultant μPADs.
Synthesis of MOFs
MOF nanoenzyme (ZIF-67) was synthesized using a previously reported method with slight modifications [28]. In brief, 1.45 g of cobalt nitrate hexahydrate and 1.64 g of 2-methylimidazole were dissolved in 100 mL of methanol, respectively. Subsequently, the 2-methylimidazole solution was slowly added into the cobalt nitrate solution. The reaction mixture would immediately turn purple. Stirring the reaction solution lasted over 0.5 h, flowing by static aging of 12 h. The resulting mixture was centrifuged to obtain ZIF-67 precipitates which were further washed three times with methanol. Their vacuum drying was additionally performed at 70 ℃ for 8 h to obtain a powder product.
Dopamine detection
For the dopamine assay, polyethylene wax-patterned circular microzone array μPADs (diameter ~8 mm) were fabricated by the proposed 3D printing method. Then, 2.5 μL of ZIF-67 solution (250 μg/mL) and 2.5 μL of 4-AAP solution (10 mg/mL) were added onto each circular microzone. After the two types of reagents were dried at room temperature in the equipment dark, the as-prepared μPADs were stored in vacuum bags before use. For each assay run, 5 μL of dopamine sample was added onto one circular microzone and in turn incubated for 30 min. During the incubation, ZIF-67 MOF would catalyze para-coupling of dopamine by 4-AAP leading to the production of a purple red of quinone imine product in the μPAD. For further quantitative measurement, after loading such μPAD into a home-made fully enclosed device (for providing stable environmental factors like steady distances for LED light illumination and photography), a smartphone with fixed parameters (ISO, 100; WB, 6500K; Shutter, 1/90) was utilized to capture the image of colorimetric result. The average grayscale (AG) value of each image was additionally measured with ImageJ software on the computer according to the following processes: (1) open the colorimetric image and convert it into an 8-bit format in the RGB color model; (2) use the Rectangle tool to select the area to be measured; (3) select the analysis function to obtain the AG value. The change in the ΔAG values (ΔAG = AGsample − AGblank) obtained from the dopamine sample and blank sample (i.e., buffer without the analyte) was calculated for quantitative concentration analysis. Moreover, to conduct selective (specific) experiments, the same analytical procedures were carried out to detect PO43-, SO42-, NO3-, Cl-, Li+, Na+, Zn2+, Ca2+, Pb2+, Hg2+, Fe3+, lysine (Lys), tryptophan (Trp), methionine (Met), ascorbic acid (AA), and soy protein isolate (SPI) instead of dopamine [13, 14, 29].
Real sample analysis
The pre-treatment of real samples was performed according to previous methods [16, 30] or the approach recommended by the Chinese National Feed Industry Standardization Technical Committee [31]. For the feed samples, 5 g of pig feed or chicken feed was crushed and ground and then uniformly mixed with 10 mL of PBS (pH 7.8). After the mixture was centrifuged at 3500 r/min for 5 min, it was filtered by a nanoporous membrane with an average pore size of ~0.22 μm for further analysis with the μPADs. For the pork sample, 5 g of fresh pork was mixed with 10 mL of PBS (pH 7.8) and in turn minced and homogenized into a fine paste. The resultant paste was then uniformly mixed with 10 mL of 1 M ZnCl2, followed by incubation at room temperature for 10 min to allow for protein precipitation. Subsequently, the mixture was centrifuged at 3000 r/min for 15 min, and then it was filtered by a nanoporous membrane for further analysis using the μPADs. Before the detection of human serum, the sample was mixed with an equal volume of PBS (pH 7.8) and then filtered through a nanoporous membrane to minimize the impact of potential interferences.
Results and discussion
Fabrication of μPADs based on 3D printing
As mentioned earlier, wax printing is the most widely used method for the μPAD fabrication. However, it may suffer from several shortcomings, such as the discontinuation of wax printers, the requirement of additional equipment for heating treatment, etc. In response to these challenges, herein this work attempted to utilize 3D printing technology to develop an alternative, single-step, universal wax printing method. That is, a commercially available, cheap, household 3D printer was adopted for directly printing polyethylene wax into rice paper body according to pre-designed device patterns (Fig. 1).
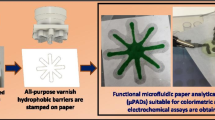
First of all, taking two dragon-shaped patterns as an example (Fig. 2A), the feasibility of fabricating μPADs in rice paper via 3D printing was investigated. Figure 2B shows clearly that although the dragon-shaped patterns had extremely complex configurations, gray outlines of polyethylene wax-patterned microstructures formed in the white rice paper were almost identical to their original designs. This indicated that the developed new method possessed a high-fidelity wax printing (patterning) process in addition to reliable pattern design. Moreover, the color and appearance of the patterned polyethylene wax outline on the back of the paper device were almost indistinguishable from their front views (Fig. 2C). This indirectly implied that after polyethylene wax particles had been heated and melted by the printer’s nozzle, the resultant hot wax liquid could quickly, vertically, and completely penetrate through the contacted paper body placed on the hot workbench during the printing process. It is noted that since bare rice paper had hydrophilic porous microstructures, its water contact angle measured was as low as 0° (Fig. 2D, top). On the other hand, due to the excellent hydrophobicity of polyethylene wax, the polymer-patterned paper region exhibited significantly increased wettability showing a water contact angle of up to 88.1° (Fig. 2D, bottom). As a result, the gray wax microstructures patterned in the rice paper body could serve as excellent hydrophobic barriers to guide the flowing of red ink in hydrophilic channels via capillary action (Fig. 2E). These patterned microstructures of polyethylene wax also showed outstanding stability. For instance, even after intense mechanical kneading, they still maintained an effective hydrophobic barrier function to prevent the overflow of red ink solution (Fig. S1, Supplementary Material).
A Pattern design of “double dragons playing with one pearl.” B Front and C back views of the resultant polyethylene wax-patterned paper created by the proposed wax printing method. D Contact angles of water droplets measured on the surfaces of hydrophilic paper (top) and hydrophobic polyethylene wax (bottom). E Capillary action-driven flowing of red ink in the hydrophilic channel of the dragon-shaped wax-patterned paper
Then, after verifying the feasibility of patterning polyethylene wax in rice paper with 3D printing, the effects of main experimental parameters (e.g., nozzle temperature, discharge volume, printing speed, workbench’s temperature, and nozzle’s distance from workbench) on patterning behaviors of polyethylene wax in rice paper body were investigated (Fig. 3). The resulting patterned hydrophilic regions were characterized using red ink. Single-channel μPADs, each of which consisted of the most commonly used three shapes of hydrophilic functional microzones (i.e., circular, rectangular, and triangular), were chosen as device models to study comprehensively the effects of different experimental conditions. For 3D printing, the nozzle temperature, discharge volume, and printing speed played a decisive role in outflow of molten polyethylene wax, namely the amount of the patterned polymer acting as hydrophobic barrier per unit area of rice paper that was inversely proportional to the actual hydrophilic channel size in the final device. As shown in Fig. 3, either the nozzle temperature increased from 82 to 102 °C (Fig. 3A) or the discharge volume increased from 200 to 600% (Fig. 3B), the correspondingly increased outflow of wax liquid allowed more amount of hydrophobic polymer barrier to be formed per unit area of rice paper, resulting in a decreasing trend in the sizes of all circular, rectangular, and triangular hydrophilic microregions of μPADs. However, Fig. 3C indicated that the amount of molten wax negatively depended on the printing speed. That is, as the printing speed increased from 40 to 120 mm/s, the sizes of circular, rectangular, or triangular hydrophilic microzones of the resultant paper devices gradually increased. Appropriate outflow of molten polyethylene wax was beneficial for building complete hydrophobic barriers throughout vertical rice paper body to restrict flowing of hydrophilic liquids like red ink. Considering the fabrication cost and efficiency, the nozzle temperature, discharge volume, and printing speed were optimized to be 87 ℃, 400%, and 100 mm/s, respectively.
Single-channel μPADs consisting of circular, rectangular, and triangular hydrophilic microzones fabricated with different parameters of 3D printer: A nozzle temperature (℃); B wax’s discharge volume (%); C printing speed (mm/s); D workbench’s temperature (℃); and E nozzle’s distance from workbench (cm). F Eight-channel μPAD with different hydrophilic channel width designs changing from 100 to 1400 μm that was characterized by red ink, and G the correlation curve describing relationship between the designed and actual measured values of the channel widths. Each error bar represents a standard deviation across three replicate experiments
Besides the three printing parameters mentioned above, the workbench’s temperature and nozzle’s distance from workbench also showed a great effect on the patterning of polyethylene wax in rice paper. Figure 3D clearly shows that when the workbench’s temperature was too low, i.e., 60 ℃, the molten polyethylene wax flowing out of the hot nozzle might not effectively, vertically penetrate the paper body to form a complete hydrophobic barrier because of its rapid solidification after printing on the paper surface, leading to the overflow of red ink from the residual hydrophilic zones (Fig. 3D, image 2). When the workbench’s temperature continued to rise to 70 or 80 ℃, the paper body could be heated and maintained at suitable temperatures. As a consequence, when the molten wax liquid was printed on the hot paper surface, it would continuously penetrate the entire paper body to produce a complete hydrophobic barrier for restricting the ink flow in the designed hydrophilic area (Fig. 3D, images 3 and 4). However, if the workbench’s temperature exceeded the polymer’s melting point (~90 ℃), the polyethylene wax microstructures patterned in the corresponding paper device would melt to erode its hydrophilic regions (Fig. 3D, image 5). The recommended workbench’s temperature was selected as 70 ℃ (which enabled production of μPAD with more ideal resolution of hydrophobic barrier in comparison with 80 ℃; Fig. 3D, images 3 and 4).
Figure 3E reveals that the nozzle’s distance from workbench also showed a significant effect on the patterning of polyethylene wax in the paper body. Too high of nozzle’s distance such as −1.70 and −1.74 cm only led to formation of incomplete hydrophobic wax barriers in the paper body that partially prevented overflow of red ink (Fig. 3E, images 1 and 2). In contrast, too low of nozzle’s distance would reduce the outline resolution of patterned polyethylene wax microstructures, e.g., slight deformation was observed in the circular microzone produced at −1.86 cm (Fig. 3E, image 5). The nozzle’s distance of −1.82 cm, with which complete, well-defined hydrophobic barriers of polyethylene wax could be patterned (created) in the paper body (Fig. 3E, image 4), was thus selected as the optimal factor for following device fabrication.
Next, under these optimized experimental conditions, the fabrication resolution of the new method (i.e., the minimum hydrophilic channel width) and the correlation between the designed hydrophilic channel width and the actual width were examined (Fig. 3F). A tooth-shaped eight-channel μPAD was designed, with theoretical widths set up to be 100, 200, 400, 600, 800, 1000, 1200, and 1400 μm. The minimum hydrophilic channel width was defined as the minimum actual width that the red ink could flow through. One can find from Fig. 3F that when the design widths were in the large range of 800–1400 μm, the ink solution could freely flow to the ends of four corresponding hydrophilic channels (Fig. 3F, channels 5–8). Moreover, as the design width was reduced from 800 to 600 and 400 μm, the red solution only flowed through 3/4 and 2/3 lengths of the resulting hydrophilic channels, respectively (Fig. 3F, channels 3 and 4). The reason why the red ink did not flow through the entire lengths of the two hydrophilic channels might be owing to insufficient volume of the colored solution used. It was also experimentally observed that the ink flowing rates increased from 0.13 ± 0.003 to 0.47 ± 0.033 mm/s for hydrophilic channels prepared with design widths ranging from 400 to 1000 μm (Fig. S2, Supplementary Material). However, the flowing rates decreased from 0.47 ± 0.033 to 0.30 ± 0.019 mm/s for channels with design widths expanded from 1000 to 1400 μm, which may be attributed to increased ink cross-flowing in the wider channels. The relative standard deviation for these flow rates were estimated to be about 5.98% (n = 6). In addition, with small design widths like 200 and 100 μm, incomplete hydrophilic channels were formed because of their serious destructions caused by the melting flow of polyethylene wax during the in situ printing process (Fig. 3F, channels 1 and 2). The actual width that was measured from the hydrophilic channel obtained from the design width of 400 μm was approximately 189 ± 30 μm (Fig. 3F), which was also the fabrication resolution of the 3D printing method developed herein. Figure 3G further shows a linear relationship between the actual widths of the hydrophilic channels that the red ink flew through and their design values, which could be characterized by a correlation equation of y = 0.8399x − 178.1510 (R2 = 0.9818). Moreover, the relative standard deviations obtained for the heights (201.86 ± 5.84 μm) and widths (983.77 ± 22.40 μm) of these different wax microstructures (barriers) were ca. 2.93% (n = 6) and 2.28% (n = 6), respectively, suggesting relatively good repeatability of the new fabrication method. In comparison with the classical wax-printer-based printing method and some other representative 3D printer-based ones, the developed technique could allow for one-step printing fabrication of μPADs with satisfactory resolution by using a commercially available 3D printer without any modifications of printer components (Table S1, Supplementary Material).
Smartphone-based visual quantitative detection of dopamine with microzone array μPADs and MOFs
To investigate potential of polyethylene wax-patterned μPADs for chemical and biological analysis, circular microzone arrays were fabricated to design a novel MOF nanoenzyme-enhanced colorimetric assay of dopamine (model analyte) with portable smartphone readout. The working principle of the dopamine assay method was illustrated in Fig. 1. Firstly, the successful synthesis of MOFs of ZIF-67 was verified using SEM to characterize their micromorphologies. Figure 4A clearly reveals that most MOFs possessed fine octahedral microstructures with average particle size of approximately 600 nm, which was consistent with that reported in previous study [32] as well the result of particle size analysis (Fig. 4B). XRD data further confirmed the excellent crystalline structures of these nanoenzyme products (Fig. 4C).
Characterization results of ZIF-67 products: A SEM image (inset shows the magnified image of one particle; scale bar, 300 nm); B dynamic light scattering particle size analysis; and C XRD analysis. D UV-vis spectra recorded from different (reaction) solutions: (a) 4-AAP (1 mg/mL); (b) dopamine (250 μM); (c) ZIF-67 (50 μg/mL); (d) 4-AAP (1 mg/mL) and dopamine (250 μM); (e) dopamine (250 μM), 4-AAP (1 mg/mL), and ZIF-67 (50 μg/mL); (f) ZIF-67 (250 μg/mL) and 4-AAP (10 mg/mL) on μPAD; and (g) dopamine (250 μM), 4-AAP (10 mg/mL), and ZIF-67 (250 μg/mL) on μPAD. Inset shows colorimetric results recorded from corresponding liquid-phase solutions and μPADs (circular microzones)
Subsequently, UV-vis spectroscopy was used to study catalytic performance of these MOF nanoenzymes. Figure 4D shows that no significant absorption responses were measured in the colorless solutions of 4-AAP (image a; curve a), dopamine (image b; curve b), and ZIF-67 (image c; curve c). After the 4-AAP was incubated with the dopamine sample, a colorless mixture was obtained (Fig. 4D, image d). However, a slight increase in absorbance during the wavelength range of 400–550 nm was observed in its UV-vis spectrum (Fig. 4D, curve d). This might be due to the fact that small amounts of dopamine and 4-AAP had been oxidized to dopaquinone and antipyrine-NH• in the presence of alkaline environment and dissolved oxygen, respectively. As a result, the quinone imine substance was formed from the two products via the para-coupling reaction. Interestingly, after the ZIF-67 was added, a reaction solution with a far darker purple red color was obtained (Fig. 4D, image e), indicating more colored products of quinone imine presumably due to the enhanced oxidation of dopamine and 4-AAP catalyzed by the nanoenzyme’s activity of oxidase and peroxidase allowing for production of a large amount of reactive oxygen species. As a consequence, the absorption peak intensity of quinone imine at around 500 nm significantly increased by 10 times (Fig. 4D, curve e). These results offered direct experimental evidence for the outstanding performance of ZIF-67 MOFs serving as high-quality nanoenzymes that were able to efficiently catalyze the para-coupling reactions between dopamine and 4-AAP.
In addition to the solution phase, it was experimentally found that the MOF nanoenzymes could also catalyze such color reaction in polyethylene wax-patterned μPAD. The surface of the circular microzone device immobilized with ZIF-67 and 4-AAP maintained the original white color of paper body (Fig. 4D, image f). After they reacted with dopamine, the surface color of the device turned into clear purple red because of the formation of quinone imine product (Fig. 4D, image g). In theory, the color intensity on the μPAD’s surface was proportional to the amount of quinone imine produced which positively relied on the dopamine level in sample. Therefore, equipment-free quantitative analysis could be realized with a smartphone to image the colored results. The reason for choosing the smartphone (rather than an expensive, complex, desktop UV-vis spectrophotometer) for signal readout was based on the fact that it could provide many unique inherent advantages in terms of cost, portability, user-friendliness, and so on.
Then, in order to obtain the highest signal-to-noise ratio, the main experimental parameters that might affect the dopamine-4-AAP reactions were optimized, including MOF concentration, 4-AAP concentration, temperature, and pH (Figs. S3-6, Supplementary Material). The results showed that an assay run for a dopamine sample could be quickly completed in 30 min when MOF concentration of 250 μg/mL, 4-AAP concentration of 10 mg/mL, temperature of 25°C, and pH of 7.8, respectively.
Under the optimized experimental conditions above, μPADs loaded with MOF and 4-AAP were used for detecting a series of dopamine samples containing different analyte concentrations to test analytical performance of the new method. As shown sin Fig. 5A, as the dopamine concentration increased from 0 to 2000 μM, the intensity of purple red color observed on the surface of corresponding paper device increased due to the enlarged amount of colored products of para-coupling reaction generated. ΔAG values (ΔAG = AGsample − AGblank) measured from these colorimetric results additionally displayed positive dopamine level-dependent responses (Fig. 5B) and were linear to the analyte concentrations ranging from 10 to 1000 μM. The calibration curve displayed two linear parts which could be described by two regression equations of y1 = 7.8931x1 − 4.5069 (R2 = 0.9976) and y2 = 41.2287x2 − 74.5875 (R2 = 0.9865) for the dopamine level ranges of 10–125 μM and 125–1000 μM, respectively. According to the 3σ rule, the limit of detection (LOD) for dopamine was estimated to be approximately 2.75 μM with the first equation. In comparison with some other representative colorimetrical dopamine assays, the new method developed herein could achieve comparable or even better analytical performance like lower LOD (Table 1).
A Colorimetric results recorded from the detection of 0–2000 μM dopamine samples with a 2 × 6 circular microzone array. B Calibration curve describing the relationship between the changes of ΔAG values (ΔAG = AGsample - AGblank) measured from the corresponding μPADs used in the above assays and the logarithmic values of analyte concentrations (LogCdopamine). Each error bar represents a standard deviation across three replicate experiments
Moreover, detection specificity (selectivity) of the new method was investigated by detecting blank sample (buffer without the analyte), 250 μM dopamine, and other 16 types of non-specific substances (i.e., including Li+, Na+, Zn2+, Ca2+, Pb2+, Hg2+, and Fe3+ cations; Cl-, NO3-, SO42-, and PO43- anions; Lys, Trp, and Met amino acids; AA and SPI). From Fig. 6A, it can be seen that colored products were only observed on the surface of the paper device used in the dopamine analysis; its AG value was measured to be 195.7 (Fig. 6B). On the other hand, assaying the blank sample or any other non-specific sample yielded almost indistinguishable colorimetric results with AG values about 220.2, indicating the absence of quinone imine substances products formed in the relevant μPADs. The data implied that the proposed method possessed good analytical specificity.
A Colorimetric results recorded from the detection of different samples (dopamine, DA; 250 μM for each substance tested) with a 2 × 9 circular microzone array. B AG values measured from the corresponding μPADs used in above assays. Each error bar represents a standard deviation across three replicate experiments
The ability to real sample analysis is also one key performance criterion of an analytical method in addition to sensitivity and specificity. Our new method was further applied for the detection of dopamine in pig feed, chicken feed, pork, and human serum samples. Table S2 in Supplementary Material showed that the obtained recovery result was between 91.82 and 102.79%. The relative standard deviation was less than 3.98%. The high recovery and low detection deviation suggested that the MOF-enhanced dopamine assay possessed excellent analytical accuracy and practicality and in turn has the potential for reliable determination of dopamine in real complex samples like feed, animal meat, and human fluid.
Conclusion
This study has successfully developed a new wax printing method for one-step, batch fabrication of various μPADs. Only a household 3D printer was adopted to print high-precision, patterned microstructures of polyethylene wax into rice paper body as hydrophobic barriers achieving the fabrication resolution (the minimum hydrophilic channel width) down to ~189 ± 30 μm. The developed 3D printing method is truly simple and low-cost and is thus expected to be widely applied especially in resource-poor small laboratories for the μPADs’ preparation and on-site uses. The utility of the resulting polyethylene wax-patterned μPADs (circular microzone array) was demonstrated well with the MOF nanoenzyme-enhanced visual quantitative detection of micromolar dopamine in artificial buffer samples as well as real complex samples (e.g., feed, pork and human serum) with smartphone readout. With advantages of simplicity, low cost, portability, and acceptable sensitivity and specificity, the proposed dopamine assay thus holds great promise particularly for point-of-need applications in fields including food safety and medical diagnosis. Some of future work will focus on (1) further optimization of fabrication parameters including nozzle size to achieve more desirable fabrication resolution and repeatability and (2) development of more complex assays like ELISA with newly designed μPADs.
References
Channer B, Matt SM, Nickoloff-Bybel EA, Pappa V, Agarwal Y, Wickman J, Gaskill PJ. Dopamine, immunity, and disease. Pharmacol Rev. 2023;75:62–158. https://doi.org/10.1124/pharmrev.122.000618.
Li M, Zhou L, Sun X, Yang Y, Zhang C, Wang T, Fu F. Dopamine, a co-regulatory component, bridges the central nervous system and the immune system. Biomed Pharmacother. 2022;145:112458. https://doi.org/10.1016/j.biopha.2021.112458.
Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–42. https://doi.org/10.1038/nrn983.
Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. https://doi.org/10.1016/j.biopsych.2016.07.014.
Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids. 2006;32(2):213–24. https://doi.org/10.1007/s00726-006-0409-8.
Walker P, Rhubart-Berg P, McKenzie S, Kelling K, Lawrence RS. Public health implications of meat production and consumption. Public Health Nutr. 2005;8:348–56. https://doi.org/10.1079/phn2005727.
Li Z, Zhou X, Wang K, Zou X, Shi J, Huang X, Holmes M. A novel sensor for determination of dopamine in meat based on ZnO-decorated reduced graphene oxide composites. Innov Food Sci Emerging Technol. 2015;31:196–203. https://doi.org/10.1016/j.ifset.2015.06.011.
Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15:2276–84. https://doi.org/10.1093/hmg/ddl152.
Hows ME, Lacroix L, Heidbreder C, Organ AJ, Shah AJ. High-performance liquid chromatography/tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples. J Neurosci Methods. 2004;138:123–32. https://doi.org/10.1016/j.jneumeth.2004.03.021.
Gu H, Varner EL, Groskreutz SR, Michael AC, Weber SG. In vivo monitoring of dopamine by microdialysis with 1 min temporal resolution using online capillary liquid chromatography with electrochemical detection. Anal Chem. 2015;87:6088–94. https://doi.org/10.1021/acs.analchem.5b00633.
Solinova V, Zakova L, Jiracek J, Kasicka V. Pressure assisted partial filling affinity capillary electrophoresis employed for determination of binding constants of human insulin hexamer complexes with serotonin, dopamine, arginine, and phenol. Anal Chim Acta. 2019;1052:170–8. https://doi.org/10.1016/j.aca.2018.11.026.
Gonzalez-Salamo J, Socas-Rodriguez B, Hernandez-Borges J, Rodriguez-Delgado MA. Determination of phthalic acid esters in water samples using core-shell poly(dopamine) magnetic nanoparticles and gas chromatography tandem mass spectrometry. J Chromatogr A. 2017;1530:35–44. https://doi.org/10.1016/j.chroma.2017.11.013.
Li X, Liu X, Liu Y, Gao R, Wu X, Gao X. Highly sensitive detection of dopamine based on gold nanoflowers enhanced-Tb(III) fluorescence. Talanta. 2022;249:123700. https://doi.org/10.1016/j.talanta.2022.123700.
Wen D, Liu W, Herrmann AK, Haubold D, Holzschuh M, Simon F, Eychmuller A. Simple and sensitive colorimetric detection of dopamine based on assembly of cyclodextrin-modified Au nanoparticles. Small. 2016;12:2439–42. https://doi.org/10.1002/smll.201503874.
Guan H, Liu B, Gong D, Peng B, Han B, Zhang N. Direct electrochemical enhanced detection of dopamine based on peroxidase-like activity of Fe3O4@Au composite nanoparticles. Microchem J. 2021;164:105943. https://doi.org/10.1016/j.microc.2021.105943.
Lu C, Ma Y, Cao Y, Huang Q, Wang J. Fluorine doping-induced oxygen vacancy-rich TiO2 via contact activation for signal boosting in electrochemical sensing. Chem Eng J. 2023;468:143598. https://doi.org/10.1016/j.cej.2023.143598.
Choi Y, Jeon CS, Kim KB, Kim HJ, Pyun SH, Park YM. Quantitative detection of dopamine in human serum with surface-enhanced Raman scattering (SERS) of constrained vibrational mode. Talanta. 2023;260:124590. https://doi.org/10.1016/j.talanta.2023.124590.
Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5. https://doi.org/10.1021/ac901071p.
Noviana E, Ozer T, Carrell CS, Link JS, McMahon C, Jang I, Henry CS. Microfluidic paper-based analytical devices: from design to applications. Chem Rev. 2021;121:11835–85. https://doi.org/10.1021/acs.chemrev.0c01335.
Jing W, Qiang S, Jia Z, Shi QH, Meng X, Yu M, Ma H, Zhao K, Dai Y. Smartphone-integrated nanozymes sensor array for high throughput recognition of organophosphorus pesticides. Sens Actuators B Chem. 2023;389:133857. https://doi.org/10.1016/j.snb.2023.133857.
Akarapipad P, Kaarj K, Breshears LE, Sosnowski K, Baker J, Nguyen BT, Eades C, Uhrlaub CL, Quirk J, Nikolich-Žugich J, Worobey M, Yoon JY. Smartphone-based sensitive detection of SARS-CoV-2 from saline gargle samples via flow profile analysis on a paper microfluidic chip. Biosens Bioelectron. 2022;207:114192. https://doi.org/10.1016/j.bios.2022.114192.
Xiao W, Gao Y, Zhang Y, Li J, Liu Z, Nie J, Li J. Enhanced 3D paper-based devices with a personal glucose meter for highly sensitive and portable biosensing of silver ion. Biosens Bioelectron. 2019;137:154–60. https://doi.org/10.1016/j.bios.2019.05.003.
Chiang CK, Kurniawan A, Kao CY, Wang MJ. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta. 2019;194:837–45. https://doi.org/10.1016/j.talanta.2018.10.104.
Suvanasuthi R, Chimnaronk S, Promptmas C. 3D printed hydrophobic barriers in a paper-based biosensor for point-of-care detection of dengue virus serotypes. Talanta. 2022;237:122962. https://doi.org/10.1016/j.talanta.2021.122962.
Chen C, Meng H, Guo T, Deshpande S, Chen H. Development of paper microfluidics with 3D-printed PDMS barriers for flow control. ACS Appl Mater Interfaces. 2022;14:40286–96. https://doi.org/10.1021/acsami.2c08541.
Yang GL, Jiang XL, Xu H, Zhao B. Applications of MOFs as luminescent sensors for environmental pollutants. Small. 2021;17(22):e2005327. https://doi.org/10.1002/smll.202005327.
Lv M, Zhou W, Tavakoli H, Bautista C, Xia J, Wang Z, Li X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens Bioelectron. 2021;176:112947. https://doi.org/10.1016/j.bios.2020.112947.
Wang Z, Zhou X, Li Y, Huang Z, Han J, Xie G, Liu J. Sensing ATP: Zeolitic imidazolate framework-67 is superior to aptamers for target recognition. Anal Chem. 2021;93:7707–13. https://doi.org/10.1021/acs.analchem.1c00976.
Louleb M, Latrous L, Ríos Á, Zougagh M, Rodríguez-Castellón E, Algarra M, Soto J. Detection of dopamine in human fluids using N-doped carbon dots. ACS Appl Nano Mater. 2020;3:8004–11. https://doi.org/10.1021/acsanm.0c01461.
Kogularasu S, Akilarasan M, Chen SM, Chen TW, Lou BS. Urea-based morphological engineering of ZnO; for the biosensing enhancement towards dopamine and uric acid in food and biological samples. Mater Chem Phys. 2019;227:5–11. https://doi.org/10.1016/j.matchemphys.2019.01.041.
National food safety standard for the determination of dopamine hydrochloride in feed by high performance liquid chromatography (GB 21036-2007) (in Chinese), General Administration for Quality Supervision Inspection and Quarantine, 2007.
Saliba D, Ammar M, Rammal M, Al-Ghoul M, Hmadeh M. Crystal growth of ZIF-8, ZIF-67, and their mixed-metal derivatives. J Am Chem Soc. 2018;140:1812–23. https://doi.org/10.1021/jacs.7b11589.
Heidary O, Akhond M, Hemmateenejad B. A microfluidic paper-based analytical device for iodometric titration of ascorbic acid and dopamine. Microchem J. 2022;182:107886. https://doi.org/10.1016/j.microc.2022.107886.
Liu C, Gomez FA, Miao Y, Cui P, Lee W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta. 2019;194:171–6. https://doi.org/10.1016/j.talanta.2018.10.039.
Faizul ZM, Chen PC, Yeh YC, Lin PH, Xu MY. Engineering a monolithic 3D paper-based analytical device (μPAD) by stereolithography 3D printing and sequential digital masks for efficient 3D mixing and dopamine detection. Sens Actuators A. 2022;347:113991. https://doi.org/10.1016/j.sna.2022.113991.
Tran TD, Nguyen PT, Le TN, Kim MI. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens Bioelectron. 2021;182:113187. https://doi.org/10.1016/j.bios.2021.113187.
Chau PBK, Dang TV, Kim MI. Highly crystalline oxidase-like MnOOH nanowire-incorporated paper dipstick for one-step colorimetric detection of dopamine. Chemosensors. 2023;11(7):382. https://doi.org/10.3390/chemosensors11070382.
Rahman MA, Pal RK, Islam N, Freeman R, Berthiaume F, Mazzeo A, Ashraf A. A facile graphene conductive polymer paper based biosensor for dopamine, TNF-alpha, and IL-6 detection. Sensors. 2023;23(19):8115. https://doi.org/10.3390/s23198115.
Funding
This work was financially supported by the Central Government-Guided Local Science and Technology Development Project (No. GuikeZY20198006), Guangxi Science and Technology Major Project (No. GuikeAA23023006), Guangxi Scholarship Fund of Guangxi Education Department (No. Guijiaoren-2019-5), and National Natural Science Foundation of China (Nos. 21874032 and 21765007).
Author information
Authors and Affiliations
Contributions
Yongkang Yan: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft. Xueer Huang: Conceptualization, data curation, investigation, methodology, software, visualization, writing—original draft. Lili Yuan: Formal analysis, investigation, methodology, validation, visualization. Yiyue Tang: Data curation, investigation, methodology, software, visualization. Wenli Zhu: Methodology, resources, writing—original draft. Hancong Du: Methodology, visualization, writing—original draft. Jinfang Nie: Methodology, funding acquisition, project administration, supervision writing—review and editing. Lang Zhang: Methodology, funding acquisition, project administration, supervision, writing—review and editing. Shan Liao: Methodology, validation, visualization, writing—original draft. Xuehui Tang: Methodology, investigation, resources. Yun Zhang: Methodology, funding acquisition, project administration, supervision, writing—review and editing
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the human serum samples in this study were collected from the Aliated Hospital of Guilin University of Technology with a protocol approved by the Review Board and Ethical Committee of Guilin University of Technology. We ensured that all serum donors were fully informed about the purpose of the donation and they agreed to conduct this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, Y., Huang, X., Yuan, L. et al. Single-step batch fabrication of microfluidic paper-based analytical devices with a 3D printer and their applications in nanoenzyme-enhanced visual detection of dopamine. Anal Bioanal Chem 416, 4131–4141 (2024). https://doi.org/10.1007/s00216-024-05337-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-024-05337-2