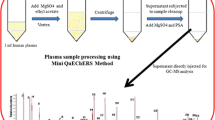
Abstract
Analysis of biofluids, such as plasma, can be used to investigate occupational pesticide exposure in the agricultural industry. Considering the chemical complexity and variability of plasma samples, any protocol for pesticide analysis should achieve efficient sample cleanup to minimize matrix effects and enhance method sensitivity through analyte pre-concentration. In this work, a high-throughput method was developed for analysis of 79 pesticides, commonly used in agricultural practices, in human plasma, using biocompatible solid-phase microextraction (SPME) coupled to liquid chromatography–tandem mass spectrometry. An SPME method was developed using a biocompatible hydrophilic–lipophilic balance/polyacrylonitrile (HLB/PAN) extraction phase and demonstrated negligible matrix effects. The performance of the developed SPME method was compared to a QuEChERS —Quick, Easy, Cheap, Effective, Rugged, and Safe— method, the most common sample preparation and cleanup approach for pesticide analysis in complex matrices. Comparable accuracy and precision were achieved for both methods, with accuracy values within 70–120% and relative standard deviation < 15%. Overall, the developed SPME and QuEChERS methods extracted 79 out of 82 monitored pesticides in human plasma. The SPME protocol demonstrated higher sensitivity than the QuEChERS method and a drastic reduction of matrix effects.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are widely used in agricultural production to prevent or reduce produce losses caused by pests [1]. They are also used to improve the quality of produce, including their appearance, which is often significant to consumers [2]. Despite their numerous benefits for agriculture, pesticides may pose serious health risks to farmers when they are directly exposed to pesticides during mixing and application, or working on treated fields. Furthermore, pesticide residues in food and water may also cause indirect health risks for the general population [3]. It is worth noting that farmers who directly get in contact with pesticides are more likely to suffer adverse effects from pesticide exposure than consumers who eat food contaminated with pesticide residues [1, 4]. This is because farmers are exposed more frequently to higher levels of pesticides through dermal absorption, ingestion, or inhalation [5]. Reports indicate that occupational exposure to pesticides can cause neurological disorders, poisoning, cancer, reproductive disruptions, respiratory problems, genotoxicity, and chronic kidney diseases among farm workers [1, 6]. The Environmental Protection Agency (EPA) has created the “Worker Protection Standards (WPS)” to protect workers from occupational exposure to agricultural pesticides [4]. In addition, there has been a great effort to regulate pesticide usage, under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) due to the unreasonable risk pesticides pose to people [7, 8]. It is critical that farmers who directly handle pesticides are properly trained and must wear protective equipment in compliance with government regulations to avoid exposure to harmful chemicals [9]. However, workers who engage in regular activities on the farm are often not well-trained and thus are at the greatest risk for pesticide exposure. Long-established agricultural industries, such as fruit and vegetable farms, have the experience and technology to minimize pesticide exposure to their workers. However, this is not the case for new and emerging industries, in particular the cannabis industry, [9]. As cannabis is still classified as a schedule 1 substance, regulatory guidance is much different from other agricultural products [9]. As reported in the Colorado survey of cannabis worker health, a significant number of workers experienced irritation to their skin (n = 33, 18%), headaches and dizziness (n = 27, 14%), and eye irritation (n = 25, 13%) after exposure to pesticides at the cultivation site [9]. Common pesticides used for cannabis cultivation are insecticides, acaricides, and fungicides. It has been noted that the majority of pesticide contamination occurs via the skin of workers during the harvesting, drying, trimming, and processing of cannabis foliage [9, 10]. As cannabis has been recently legalized in several states, inexperienced farmers are more likely to be exposed to pesticides since they are less familiar with pesticide safety equipment and procedures. Nevertheless, pesticide exposure can also occur during unsafe agricultural practices, especially in unregulated rural areas, due to a lack of knowledge of proper pesticide storage and handling [1]. It has been reported that chronic pesticide exposure in rural areas of China is associated with suicidal ideation, with exposure being exacerbated by the storage of pesticides in private households [11]. Therefore, assessing pesticide exposure is crucial for workers’ health and safety, to correct malpractices in pesticide storage and application, and prevent further exposure.
Biofluid analysis is a reliable method to determine whether a subject has been exposed to toxic chemicals [12, 13]. Various biofluids, including blood, saliva, plasma, and urine, have been used in previous studies to analyze humans’ exposure to toxic chemicals [14]. In addition to analyzing biofluids, nail and hair samples have also been tested to evaluate the effect of long-term exposure to various xenobiotics [15]. Blood or plasma tests are the most reliable way to determine the amount of pesticide within the body after exposure to pesticides. This is because, as reported, pesticide exposure in the fields typically occurs through the skin [9]. As soon as pesticides are absorbed through the skin, they pass into the bloodstream and circulate throughout the body [16]. Therefore, analysis of blood or plasma can provide useful information regarding pesticide exposure levels. To quantify pesticides in biological fluids, liquid or gas chromatography in combination with mass spectrometry is typically used [17]. Liquid chromatography/mass spectrometry (LC–MS) analysis of pesticides at ultra-trace levels from biofluids can be highly impacted by matrix effects. Matrix components co-eluting with analytes can enhance or suppress the analytical response by interfering with the ionization process in the ionization source [18], which makes quantification challenging [19]. Thus, an unbiased and comprehensive quantification of pesticides in biofluids such as plasma requires the development of more reliable, robust, and efficient analytical methods.
Used sample preparation methods for pesticide analysis in biofluids are solid-phase extraction (SPE) and quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction. Both methods have been extensively evaluated and validated in the literature and are widely accepted for biofluid analysis [20, 21]. These methods, however, can have several disadvantages, including labor-intense and prolonged workflows. In addition, SPE typically requires a large volume of organic solvent and clogging SPE cartridges due to complex matrix from biofluids can also be a problem [20]. To avoid clogging SPE cartridges, dilution of the samples and loading low sample volumes is recommended; however, these strategies may affect method sensitivity and require further pre-concentration of the extracts [22]. To this end, solid-phase microextraction (SPME) offers substantial advantages. With the use of biocompatible SPME extraction phases, direct immersion SPME (DI-SPME) in biofluids can be achieved avoiding biofouling of the extraction phase with matrix constituents [23]. To ensure matrix compatibility of SPME fibers for LC applications, polyacrylonitrile (PAN) is frequently used as the sorbent binder of the extraction phase [23]. Additionally, SPME provides advantages such as higher throughput, easy automation, and meets the requirements of green analytical chemistry.
This work provides an alternative and robust method to quantify pesticides, commonly used in most agricultural practices, including in the cannabis industry, at ultra-trace levels in biofluids. This was achieved by developing an SPME-LC–MS/MS method that can extract and analyze simultaneously a wide variety of pesticides from human plasma. The extraction phase chemistry was optimized to improve extraction coverage of pesticides with a wide range of log P values and molecular weights. To achieve pesticide quantification at part per trillion levels, plasma matrix modification was carried out. Additionally, the developed method was compared with QuEChERS, which is commonly used to extract pesticides from food and biological samples. In conducting this comparison, sample throughput, matrix effects, robust method evaluation (linearity, limit of quantitation (LOQ), accuracy, repeatability), and the environmental impact caused by the method were taken into consideration.
Materials and methods
Reagents and standards
The reference standards of Canadian cannabis pesticide mixtures (mixture number 1, 2, 3, 4, 5, and 6) were purchased from Chem Service (West Chester, PA). The physicochemical properties of the pesticides are listed in Table S1. The 82 pesticides originally selected for this study are the most commonly used pesticides during cannabis cultivation. Therefore, this list represents a comprehensive collection of pesticides that might be present in plasma after pesticide exposure in several agricultural practices, including the cannabis industry. The deuterated standards of dimethoate-D6, carbaryl-D7, malathion-D6, fenoxycarb-D3, coumaphos-D10, and pyridaben-D13 were purchased from Toronto Research Chemicals (North York, Ontario, Canada). Pooled human plasma preserved in sodium citrate was purchased from Innovative Research (MI, USA). HLB/PAN fibers for this study were provided by Millipore Sigma (Bellefonte, PA, USA). Fiber thickness and coating length were 45 μm and 1.0 cm, respectively. LC–MS grade methanol (MeOH), acetonitrile (ACN), water, ammonium formate, and formic acid were obtained from Fisher Scientific (Waltham, MA, USA). Phosphate-buffered saline (PBS) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Instrumentation and data processing
The LC–MS/MS pesticides analysis was performed using a Perkin Elmer QSight® LX50 binary pump UHPLC, autosampler, and column compartment (PerkinElmer Inc., Waltham, MA, USA) coupled to a triple quadrupole mass spectrometer Perkin Elmer QSight® 220 (PerkinElmer Inc. Waltham, MA, USA), operated in both positive and negative electrospray ionization (ESI) mode. Spectra were acquired in multiple reaction monitoring (MRM) mode. The optimized MRM transitions and operating conditions of the mass spectrometer for pesticide analysis are summarized in Table S2 and Table S3. Nitrogen gas flow for the ESI source, the laminar flow ion guide, and the collision cell was provided using a Parker/Balston nitrogen generator system (Parker Hannifin Corporation, Lancaster, NY, USA).
Chromatographic separation of pesticides was achieved using a 100 mm × 4.6 mm Raptor C18 column 2.7 μm (Restek Corporation, Bellefonte, PA, USA), at a flow rate of 0.8 mL min−1 with the column temperature at 30 °C. The chromatographic conditions optimized for pesticides are listed in Table S4. The chromatographic run time was 18 min. The gradient was applied with mobile phase A as 0.1% (v:v) formic acid and 2 mM ammonium formate in water/MeOH (98:2, v:v) and 0.1% (v:v) formic acid and 2 mM ammonium formate in MeOH/water (98:2, v:v) as mobile phase B. Data acquisition and processing were performed with Simplicity 3Q™ software (version 1.8.2006.12348) (PerkinElmer Inc., Waltham, MA USA). The SRM transition providing a higher signal intensity and specificity for each pesticide was selected as a quantifier, and the second most abundant transition was monitored as a qualifier. The peak area ratios of the pesticides and IS were plotted against the known concentration of pesticides to produce SPME calibration curves.
Standard preparation and solid-phase microextraction procedure
The concentration of individual pesticides in the stock solution was 100 µg mL−1. The working pesticide solution was prepared at the concentration of 10 µg mL−1 in MeOH and stored at − 20 ºC until use.
Sample preparation for method optimization, SPME calibration levels, and quality control samples
SPME method optimization was conducted using commercially available pooled human plasma. Ten mL of human plasma was spiked with 100 µL of pesticide working solution at 10 µg mL−1 to achieve individual pesticide final concentration of 100 µg L−1. The organic solvent content in the plasma during spiking was maintained at 1%, to avoid affecting the partition of the analytes between the sample and the extraction phase. All the spiked plasma samples were vortexed for 1 min and incubated overnight at 4 °C to allow binding equilibria between analytes and the plasma to take place. The plasma was then allowed to equilibrate at room temperature prior to further handling and extraction.
An SPME calibration curve was obtained by matrix-matched calibration in human plasma with internal standard signal correction. All the spiking mixtures were prepared in MeOH and stored at − 20 °C until use. To spike the 10 calibration levels, a series of spiking mixtures in the concentration range of 1.5 ng mL−1 to 15 µg mL−1 were prepared, to spike the same volume of pesticide solution in each plasma sample. To prepare calibration levels ranging from 0.01 to 100 ng mL−1 of pesticides in human plasma, 15 µL of the above-mentioned spiking solutions was transferred in 2.25 mL human plasma. Quality control (QC) levels at 0.025, 0.5, 15, and 70 ng mL−1 (accuracy levels) were prepared by spiking 2.25 mL of human plasma with 15 µL of spiking mixtures in a concentration range of 3.75 ng mL−1 to 10.5 µg mL−1. The deuterated pesticide standard mixtures were prepared at concentrations (2.4–12 µg mL−1) optimized to ensure adequate sensitivity. Each level of the calibration curve and QC samples was spiked with 7.5 µL of internal standard mixture. The final concentration of internal standards in plasma was in the range of 8–40 ng mL−1. The spiked plasma samples for each calibration and QC levels were diluted with PBS containing 10% ACN and 0.5 mol L−1 (NH4)2SO4 to achieve a 3:1 (v:v) dilution ratio of plasma: PBS solution. To obtain three replicates for each calibration and QC levels, diluted plasma was divided into 3 aliquots of 667 μL (500 µL of plasma + 167 µL of PBS solution), and then each aliquot was placed in an 800 μL plastic vial.
SPME fiber extraction procedure
The pesticides were extracted from the human plasma samples using HLB/PAN fibers. Prior to extraction, fibers were preconditioned with a solution 1:1 MeOH:water (v:v) for 10 min and then rinsed with ultrapure water for 10 s to remove any organic solvent residue on the extraction phase. The extraction was performed for 90 min at 1200 rpm at room temperature (20 °C ± 2 °C). After extraction, SPME fibers were rinsed with ultrapure water for 20 s to remove any loosely attached matrix components from the extraction phase surface. Then fiber desorption was conducted at 1200 rpm for 60 min in 120 µL of desorption solvent, consisting of a solution of ACN:MeOH:water at the volume ratio of 2:2:1. Immediately after desorption, the desorption solutions were stored at − 20 °C until analysis.
QuEChERS extraction procedure for calibration
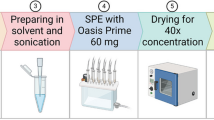
The QuEChERS calibration levels were prepared following the same procedure as the SPME calibration levels. Initially, 500 µL of spiked human plasma was diluted with 500 µL of PBS in an Eppendorf tube. Then the diluted plasma was transferred into a centrifuge tube containing 1 mL of ACN and 5 mg of MgSO4:NaOAc salt mixture in a w:w ratio of 4:1. To extract pesticides into ACN, the centrifuge tube containing diluted plasma, salts mixture, and ACN was vortexed for 3 min and then centrifuged at 20 °C and 5000 rpm for 13 min to allow liquid–solid-phase separation. The supernatant from the centrifuge tube was withdrawn and added into a dispersive SPE (d-SPE) tube containing C18 particles. Then the d-SPE tube was vortexed for 3 min and centrifuged at 20 °C and 1300 rpm for 6 min. The supernatant was transferred into an Eppendorf tube and centrifuged for another 6 min as a precaution to remove any residual C18 particles from the extract.
Calibration and method validation
The method validation for both SPME and QuEChERS was conducted in accordance with the Food and Drug Administration (FDA) guideline for biological samples analysis in terms of selectivity, a lower limit of quantitation (LLOQ), linearity, accuracy, and precision [24]. Matrix-matched calibration with internal standard correction was used for both methods. Weighted least-squares method with a weighting factor of 1/x was used to build up the linear regression of the calibration curve. QC samples were evaluated to calculate the accuracy and precision at intermediate concentrations within the linear dynamic range for all the pesticides. Both intra- and inter-day accuracy and precision were calculated by testing QC samples at 0.025, 0.5, 15, and 70 ng mL−1 (6 replicates for each concentration) for all pesticides over 6 days. The bias and precision of accuracy should be within ± 15% and < 15%. For each pesticide, the lowest calibration point achieving accuracy within ± 20% of the nominal value and coefficient of variation (CV) < 20% was considered the LLOQs.
Results and discussion
SPME method optimization
This study targeted multi-class pesticides with varied physical and chemical properties (molecular weight range from 162.20 to 760.02 g mol−1, log P range from − 0.43 to 6.62), as listed in Table S1. To ensure optimal extraction for all the analytes, an extraction phase with sufficient extraction coverage needs to be selected. To determine which extraction phase was best suited for pesticide extraction, fibers with different extraction phases chemistries, including HLB/PAN, C18/PAN, and (mixed mode) MM/PAN, were evaluated. In Fig. 1., the extraction performance of each extraction phase is shown for all pesticides, along with their log P values and retention time. Figure S1 shows further details on the extraction performance related to each extraction phase for all pesticides. The type and the amount of analytes extracted by the SPME fiber depend on the intermolecular interactions between the extraction phase and the analytes [1]. Based on the results obtained, the C18/PAN extraction phase extracted pesticides with higher lipophilicity due to van der Waals interactions between the C18 chain of the extraction phase and pesticides. Therefore, as shown in Fig. 1, extracted amounts by C18/PAN exhibit a positive correlation with log P of the pesticides. Thus, late-eluting analytes in reversed-phase chromatography using a C18 column were extracted more efficiently than early-eluting analytes that were extracted. In contrast, HLB/PAN provided balanced coverage for pesticides by extracting both early-eluting hydrophilic and late-eluting hydrophobic pesticides. In fact, HLB/PAN extraction phase is made up of a co-polymer containing divinylbenzene, which can interact pesticides via van der Waals and π–π interaction, and hydrophilic N-vinylpyrrolidinone, which can interact with pesticides via hydrogen bonds and dipole–dipole interactions. MM/PAN extraction phase consists of benzenesulfonic acid, which provides mainly ion exchange interactions and C8 groups that extract via van der Waals interactions. However, MM/PAN did not outperform HLB/PAN for the extraction of non-ionized pesticides. The MM/PAN and C18/PAN extraction phases showed similar extraction for moderately polar but non-ionized pesticides (Fig. 1). For example, carbaryl (pKa, 14.77; log P, 2.46), acetamiprid (pKa, 4.16; log P, 1.11), and carbofuran (pKa, 14.76; log P, 2.05) were not ionized at the pH of the experiment conducted; extraction from both MM/PAN and C18/PAN showed similar results, suggesting that these analytes are primarily extracted via hydrophobic groups of both extraction phases (C8 in MM/PAN and C18 in C18/PAN). In light of all these observations, HLB/PAN was considered for further optimization, as it avoids extraction discrimination between hydrophilic and hydrophobic analytes.
Extraction efficiency of pesticides extracted using HLB/PAN, C18/PAN, and MM/PAN extraction phases from PBS. Extractions were performed for 90 min, assuming equilibrium is reached for most of the targeted analytes. The correlations between extraction efficiency, log P values, and retention time of pesticides are also presented: (a) early-eluting pesticides, (b) late-eluting pesticides
Once the best extraction phase is selected, desorption conditions must be tuned to guarantee the quantitative desorption of all targeted analytes. Firstly, a suitable desorption solution was optimized to provide < 1% analytes carryover left on the extraction phase. Two organic solvents (ACN and MeOH) were tested as organic modifiers for the aqueous solutions evaluated as desorption media. Because ACN and MeOH can establish different intermolecular interactions with polar and non-polar pesticides, they were selected to be tested as desorption media [25, 26]. Figure S2 shows the recovery of pesticides in different desorption solutions, namely, ACN:MeOH:water (2:2:1, v:v:v), ACN:water (8:2, v:v), MeOH:water (8:2, v:v), and MeOH:water (1:1, v:v). A solution of ACN:MeOH:water at the volume ratio of 2:2:1 was selected as the optimal desorption solution for SPME since it showed overall higher recovery for all pesticides. Desorption time was tested from 5 to 180 min. Figure S3 shows the desorption time profile for all the pesticides tested. Following the first desorption, a second desorption was performed for 180 min to assess fiber carryover. No detectable analyte carryover was observed. A desorption time of 60 min was selected for further studies to ensure adequate response and better reproducibility for all the pesticides while maintaining the analytes carryover < 1%.
According to the literature, addition of 0.1% formic acid improves the stability of pesticide solutions due to the pH reduction [27]. Thus, we tested pesticide stability in the desorption solutions and assessed their potential degradation with and without addition of formic acid. Based on the results obtained (Fig. S4), all pesticides remained stable up to 3 weeks, both with and without formic acid in the desorption solution. Moreover, pesticide ESI ionization did not improve by adding formic acid to desorption solutions. Therefore, desorption was conducted without formic acid.
Subsequently, using the optimized desorption conditions, an extraction time profile was conducted from 500 µL of human plasma for 5–180 min using an agitation speed of 1200 rpm. Figure S5 shows the extraction time profile for all the tested pesticides. As the results in Fig. S5 show, only 12 pesticides achieved equilibrium by 90 min or earlier. Other pesticides did not achieve equilibrium within the extraction time tested. An extraction time of 90 min was selected for further study due to the acceptable analyte response, even though it is within pre-equilibrium range for some pesticides.
As analyte equilibrium is not achievable under a feasible time frame, and pesticide exposure needs to be often monitored at ultra-trace level, further investigation into improving method sensitivity was required. To achieve this, pesticides were extracted using dual SPME fibers (simultaneous extraction of samples with two SPME fibers) and compared with single-fiber extraction [28]. According to Fig. S6, dual fiber extraction increased the amount extracted for all pesticides by a factor of 2. It is advantageous to use two 1-cm fibers instead of one 2-cm fiber here, since this allows a smaller amount of desorption solution to be used to fully submerge the extraction phase. As a result, analyte pre-concentration is increased.
Matrix modification
Sample matrix modification is often applied for headspace SPME (HS-SPME) to facilitate the mass transport of free analytes from the sample to its headspace [29]. However, in DI-SPME, matrix modifications can be carried out to dissociate the targeted molecules from binding media and achieve higher free concentration of analytes in the sample to improve SPME recovery [29]. Common matrix modifications for plasma analysis reported in the literature include plasma dilution, salt and organic solvent addition [29]. Figure 2 illustrates the three steps used in this study to determine the optimal plasma modification procedure to enhance the method’s sensitivity. The first step was to evaluate the different ratios of plasma dilution with PBS buffer. PBS was selected for diluting the plasma because it has similar pH, osmolarity, and ionic concentration to human plasma [30]. Therefore, extraction conditions in diluted plasma were similar to those in unmodified human plasma. Moreover, plasma dilution with PBS may help to disrupt protein binding of analytes, resulting in higher free analyte concentration in the sample [14]. However, too much dilution can also decrease the free concentration of analytes; thus, it is critical to find the appropriate dilution ratio for this type of experiment. Therefore, plasma was diluted with PBS to volume ratios of plasma to PBS 3:1, 1:1, and 1:3, and the responses of analytes extracted from each diluted plasma and pure plasma were compared (Fig. 3, step 1). The plasma:PBS 3:1 (v:v) dilution ratio was selected as optimal to achieve acceptable response for the analytes and was utilized to conduct subsequent matrix modification steps involving organic modifiers and salts.
Schematic representation of matrix modification workflow comprised of three steps: (1) optimization of human plasma dilution with PBS; (2) optimization of the amount of organic modifier (ACN) added to the diluted human plasma; (3) optimization of the amount of salt ((NH4)2SO4) added to the diluted human plasma
Heat map showing the response of pesticides for three different steps of plasma modifications: step 1, comparison of the responses of pesticides when extracted from unmodified plasma and diluted plasma with PBS at different volume ratios; step 2, comparison of the responses of pesticides when extracted from unmodified plasma and diluted plasma containing different amounts of ACN; step 3, comparison of the responses of pesticides when extracted from unmodified plasma and diluted plasma containing different concentrations of (NH4)2SO4
The second step to matrix modification (Fig. 2, step 2), involved introducing an organic modifier, ACN, to the diluted plasma to improve pesticide extraction. ACN was selected as the organic modifier to improve the extraction of non-polar molecules more strongly bound to plasma components. However, direct spiking of ACN into pure plasma can cause sudden precipitation of both analytes and plasma proteins [31]. To avoid this effect, ACN was spiked in PBS at different concentrations, and the plasma was diluted using the ACN solution in PBS at the optimized dilution ratio (plasma:PBS, 3:1, v:v). For this experiment, three different ACN solutions (1%, 10%, 30% - v:v) in PBS were prepared and used to dilute the plasma samples to a ratio of 3:1 (v:v), achieving ACN concentrations in diluted plasma of 0.25%, 2.5%, and 7.5%, respectively. Figure 3, step 2, illustrates the comparison of pesticide responses between plasma with different concentrations of ACN and plasma without modification. As indicated by the results (Fig. 3, step 2), adding 10% ACN increased the efficiency of non-polar pesticide extraction by 40%, whereas it reduced the extraction of polar pesticides by 21% in comparison to unmodified plasma. To ensure adequate responses for both polar and non-polar pesticides, ACN percentage of 10% (2.5% ACN in diluted plasma) was selected as the optimal condition for further optimization. The third step of the matrix modification process involved optimizing the type and the amount of salts required to improve the pesticide recovery. Adding salt in solid form directly into plasma can affect its solubility and can increase the viscosity of the plasma. Increasing viscosity in the plasma sample decreases the efficiency of the mass transfer of analytes to the extraction phase [32, 33]. To avoid these complications, salt solutions at different concentrations were prepared in PBS and used to dilute the plasma according to optimized plasma dilution ratio selected in the first step.
Firstly, salts of different chemical compositions were tested for their effect on pesticide extraction from plasma. The amount of pesticides extracted with and without the addition of salts was evaluated using 0.25 mol L−1 solutions of NaCl, (NH4)2SO4, and Na2SO4 separately dissolved in PBS. Figure S7 shows the amount of pesticides extracted from plasma with and without the addition of salts. The addition of (NH4)2SO4 to plasma increased the extraction of all pesticides. Furthermore, different concentrations of (NH4)2SO4 in PBS were used to dilute the plasma to determine which concentration improves the pesticide extraction. According to the results shown in Fig. 3, step 3, 0.5 mol L−1 (NH4)2SO4 enhanced the extraction of early-eluting analytes (log P 0.3–1.1) by 21–40%. Further increases in salt concentration reduced the amount of pesticides extracted. Therefore, introducing 0.5 mol L−1 (NH4)2SO4 to the plasma compensated for the decrease in extraction efficiency for polar analytes due to the addition of ACN. In light of the results obtained for matrix modification experiments, PBS containing 10% ACN (2.5% in plasma) and 0.5 mol L−1 (NH4)2SO4 was used to dilute the plasma to the ratio of 3:1 (v:v), and these conditions were selected as optimal for further method validation.
Matrix effect evaluation for SPME and QuEChERS
Matrix effects were evaluated for both the developed SPME method and QuEChERS. Matrix effects (%) were calculated for the QuEChERS method according to the approach proposed by Matuszewski et al. [34]. For SPME, the method proposed by Matuszewski et al. was modified as follows: blank plasma was extracted using HLB/PAN fiber and desorbed into the optimized desorption solution (ACN:MeOH:water, 2:2:1, v:v:v). Then, the desorption solution was spiked post-extraction with the targeted analytes at 100 ng mL−1 and subjected to LC–MS analysis. Pesticide response (peak area) was compared with the peak areas obtained from a neat desorption solvent mixture spiked at the same concentration level. Matrix effects (%) were calculated using the following equation:
According to the equation, 0% indicates no matrix effects for analyte response, > 0% indicates response enhancement, and < 0% indicates response suppression. Matrix effects (%) on pesticides for the SPME method were calculated using both undiluted plasma and diluted plasma. Matrix effect (%) evaluation with diluted plasma was performed under the optimized conditions and with dual fiber extraction. For QuEChERS, it was necessary to dilute human plasma with PBS to a volume ratio of 1:1 to have sufficient sample volumes for the entire extraction procedure. Therefore, we only evaluated matrix effects for the QuEChERS method considering diluted plasma. Moreover, to also obtain a direct comparison to the QuEChERS method, matrix effects % were also calculated for SPME when using diluted plasma in ratio of 1:1 (v:v) with PBS. Figure 4 shows matrix effects (%) of each pesticide as a function of each pesticide’s retention time for both sample preparation methods. For SPME, matrix effects for both diluted and undiluted plasma were < 5% for most of the analytes. The QuEChERS method displayed matrix effects between 5 and 20% for most analytes, except bifenthrin (21.9%) and kinoprene (27.3%). For the QuEChERS method, most pesticides showed increased response due to matrix enhancement effects.
Method validation comparison of SPME and QuEChERS
The optimized SPME and QuEChERS methods were validated and compared for their ability to accurately quantify the pesticides targeted in this study in human plasma. SPME and QuEChERS methods were both capable of quantifying 79 pesticides; spiromesifen, spirodiclofen, and spirotetramat were not quantified due to their low response. Table 1 displays the main figures of merit for both SPME and QuEChERS methods, and pesticides are listed according to their retention time on a C18 chromatographic column. Additional figures of merit are reported in Table S5 in Supplementary Information. HLB/PAN SPME fibers were able to extract 20% of the total pesticides at the lowest concentration level tested (0.01 µg L−1). Conversely, only 6% of the total pesticides could be extracted by QuEChERS at the same calibration level. As shown in Table S6, acceptable accuracy and precision were achieved for both the SPME method and QuEChERS methods. Using SPME, 92% of pesticides provided accuracy values in the range of 70–120% and < 25% reproducibility (RSD %). Even though QuEChERS method showed higher matrix effects for most of the analytes, acceptable accuracy for the pesticides was observed at the range of 70–120%, proving that the correction of analytes’ response with ISD response was significant to mitigate the influence of the matrix. To minimize matrix effects on analytes, it was crucial to select internal standards that elute in different regions of the chromatogram. Matrix effects caused by matrix components co-eluting with analytes can be corrected by selecting internal standards with elution times representative of the entire chromatographic space. Six isotopically labeled internal standards were included in the study, and Fig. S8 shows their elution order across the entire chromatogram. Furthermore, the developed SPME method showed a broader linear dynamic range compared to QuEChERS for most of the pesticides.
Conclusions
This novel SPME-LC–MS/MS method enables simultaneous multi-residue analysis of 79 pesticides in human plasma at ultra-trace levels. The biocompatible HLB/PAN extraction phase demonstrated remarkable biocompatibility in the plasma with negligible or no matrix effect. As occupational exposure to pesticides can occur at varying concentration levels, it is critical for any developed method to quantify pesticides at low concentrations. LOQs for analytes ranged between 0.01 and 5 µg L−1 for the developed SPME protocol, with the more challenging polar pesticides efficiently extracted and quantitated from plasma. By contrast, the QuEChERS method showed higher matrix effects for most of the analytes. As a whole, LOQs were higher for the QuEChERS method; however, the more hydrophobic pesticides were better extracted with this sample preparation method. The SPME-LC–MS/MS method was proven capable of quantification of pesticides in human plasma at part per trillion levels. This method can be used in the agricultural industry to better monitor pesticide exposure for workers, especially for emerging sectors such as cannabis cultivation. Moreover, the developed SPME method produces less laboratory waste and consumes minimal amount of organic solvents compared to the QuEChERS method. A summary of practical aspects (sensitivity, throughput, matrix effects, solvent consumption, production of laboratory waste) of both the developed SPME and the QuEChERS methods is highlighted in Table S7. To meet the growing demands of regulatory agencies and routine analysis laboratories, sample throughput and method tunability is critical. The SPME method proposed can be automated to extract 96 samples simultaneously with 96 concepts autosamplers compatible to 96 well plates, allowing the preparation time per sample to be about 1.7 min. Furthermore, the SPME extraction phase chemistry can be easily tuned to enhance selectivity for different pesticide classes.
References
Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8:1402–19. https://doi.org/10.3390/ijerph8051402.
Alavanja MCR. Pesticides use and exposure extensive worldwide. Rev Environ Health. 2009;24:303–9. https://doi.org/10.1515/REVEH.2009.24.4.303.
Philippe V, Neveen A, Marwa A, Ahmad AYBasel. Occurrence of pesticide residues in fruits and vegetables for the Eastern Mediterranean Region and potential impact on public health. Food Control. 2021;119:107457. https://doi.org/10.1016/j.foodcont.2020.107457.
Curl CL, Beresford SAA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, Kaufman JD. Estimating pesticide exposure from dietary intake and organic food choices: the multi-ethnic study of atherosclerosis (MESA). Environ Health Perspect. 2014;123:475–83. https://doi.org/10.1289/ehp.1408197.
Damalas CA, Koutroubas SD. Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics. 2016;4:1–10. https://doi.org/10.3390/toxics4010001.
Dhananjayan V, Ravichandran B. Occupational health risk of farmers exposed to pesticides in agricultural activities. Curr Opin Environ Sci Heal. 2018;4:31–7. https://doi.org/10.1016/j.coesh.2018.07.005.
Fishel FM (2015) A summary of revisions to the worker protection. Edis 1–5.
Hornstein JS, Ellerhoff J (2018) Compliance with the worker protection standard. https://doi.org/10.31274/icm-180809-529.
Corva D, Meisel JS (2021) The Routledge Handbook of Post-Prohibition Cannabis Research.
Pinkhasova DV, Jameson LE, Conrow KD, Simeone MP, Davis AP, Wiegers TC, Mattingly CJ, Leung MCK. Regulatory status of pesticide residues in cannabis: implications to medical use in neurological diseases. Curr Res Toxicol. 2021;2:140–8. https://doi.org/10.1016/j.crtox.2021.02.007.
Zhang J, Stewart R, Phillips M, Shi Q, Prince M. Pesticide exposure and suicidal ideation in rural communities in Zhejiang province, China. Bull World Health Organ. 2009;87:746–53. https://doi.org/10.2471/BLT.08.054122.
Alves A, Kucharska A, Erratico C, Xu F, Den Hond E, Koppen G, Vanermen G, Covaci A, Voorspoels S. Human biomonitoring of emerging pollutants through non-invasive matrices: state of the art and future potential. Anal Bioanal Chem. 2014;406:4063–88. https://doi.org/10.1007/S00216-014-7748-1.
Panuwet P, Jr REH, Souza PED, Chen X, Radford A, Cohen JR, Marder ME, Kartavenka K, Barry P, Barr DB (2016) Biological matrix effects in quantitative tandem mass spectrometry-based analytical methods: advancing biomonitoring. 46:93–105. https://doi.org/10.1080/10408347.2014.980775.Biological.
Godage NH, Gionfriddo E. Biocompatible SPME coupled to GC/MS for analysis of xenobiotics in blood plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2022;1203:123308. https://doi.org/10.1016/j.jchromb.2022.123308.
Lehmann E, Oltramare C, NfonDibié JJ, Konaté Y, d Alencastro LF. Assessment of human exposure to pesticides by hair analysis: the case of vegetable-producing areas in Burkina Faso. Environ Int. 2018;111:317–31. https://doi.org/10.1016/j.envint.2017.10.025.
Baynes RE, Riviere JE (2010) Absorption. Hayes’ Handb Pestic Toxicol 877–892. https://doi.org/10.1016/B978-0-12-374367-1.00037-9.
Hernández F, Sancho JV, Pozo OJ. Critical review of the application of liquid chromatography/mass spectrometry to the determination of pesticide residues in biological samples. Anal Bioanal Chem. 2005;382:934–46. https://doi.org/10.1007/s00216-005-3185-5.
Jiang R, Xu J, Zhu F, Luan T, Zeng F, Shen Y, Ouyang G. Study of complex matrix effect on solid phase microextraction for biological sample analysis. J Chromatogr A. 2015;1411:34–40. https://doi.org/10.1016/j.chroma.2015.07.118.
Cichelli J, Doyle RM (2015) LC/MS/MS analysis of barbiturates in urine, oral fluid and blood. 5000.
Campíns-Falcó P, Sevillano-Cabeza A, Herráez-Hernández R, Molins-Legua C, Moliner-Martínez Y, Verdú-Andrés J. Solid-phase extraction and clean-up procedures in pharmaceutical analysis. Encycl Anal Chem. 2012. https://doi.org/10.1002/9780470027318.a1920.pub2.
Wilkowska A, Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125:803–12. https://doi.org/10.1016/j.foodchem.2010.09.094.
Simpson H, Berthemy A, Buhrman D, Burton R, Newton J, Kealy M, Wells D, Wu D. High throughput liquid chromatography/mass spectrometry bioanalysis using 96-well disk solid phase extraction plate for the sample preparation. Rapid Commun Mass Spectrom. 1998;12:75–82. https://doi.org/10.1002/(SICI)1097-0231(19980131)12:2%3c75::AID-RCM112%3e3.0.CO;2-C.
Godage NH, Gionfriddo E. A critical outlook on recent developments and applications of matrix compatible coatings for solid phase microextraction. TrAC - Trends Anal Chem. 2019;111:220–8. https://doi.org/10.1016/j.trac.2018.12.019.
(2018) Food and Drug Administration, Bioanalytical Method validation guidance.
Dutta S, Basu N, Mandal D. ESIPT in a binary mixture of non-polar and protic polar solvents : role of solvation dynamics. J Photochem Photobiol A Chem. 2022;435:114240. https://doi.org/10.1016/j.jphotochem.2022.114240.
Darvishnejad F, Raoof JB, Ghani M. MIL-101 (Cr) @ graphene oxide-reinforced hollow fiber solid-phase microextraction coupled with high-performance liquid chromatography to determine diazinon and chlorpyrifos in tomato, cucumber and agricultural water. Anal Chim Acta. 2020;1140:99–110. https://doi.org/10.1016/j.aca.2020.10.015.
Morales EL, Sinuco LDC, Ahumada DA (2020) Stabilization strategies for unstable pesticides in calibration reference materials. 17th IMEKO TC 10 EUROLAB Virtual Conf “Global Trends Testing, Diagnostics Insp 2030” 129–134.
Godage NH, Cudjoe E, Neupane R, Boddu SH, Bolla PK, Renukuntla J, Gionfriddo E (2020) Biocompatible SPME fibers for direct monitoring of nicotine and its metabolites at ultra trace concentration in rabbit plasma following the application of smoking cessation formulations. J Chromatogr A 1626:. https://doi.org/10.1016/j.chroma.2020.461333.
Risticevic S, Lord H, Górecki T, Arthur CL, Pawliszyn J. Protocol for solid-phase microextraction method development. Nat Protoc. 2010;5:122–39. https://doi.org/10.1038/nprot.2009.179.
Brandt JM, Brière LK, Marr J, MacDonald SJ, Bourne RB, Medley JB. Biochemical comparisons of osteoarthritic human synovial fluid with calf sera used in knee simulator wear testing. J Biomed Mater Res - Part A. 2010;94:961–71. https://doi.org/10.1002/jbm.a.32728.
Shiea J, Bhat SM, Su H, Kumar V, Lee CW, Wang CH (2020) Rapid quantification of acetaminophen in plasma using solid-phase microextraction coupled with thermal desorption electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 34: https://doi.org/10.1002/rcm.8564.
Abdel-Rehim M, Bielenstein M, Arvidsson T (2000) Evaluation of solid-phase microextraction in combination with gas chromatography (SPME-GC) as a tool for quantitative bioanalysis. J Microcolumn Sep 12:308–315. https://doi.org/10.1002/(SICI)1520-667X(2000)12:5<308::AID-MCS5>3.0.CO;2-F.
Prosen H, Zupančič-Kralj L. Solid-phase microextraction. TrAC - Trends Anal Chem. 1999;18:272–82. https://doi.org/10.1016/S0165-9936(98)00109-5.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem. 1998;70:882–9. https://doi.org/10.1021/ac971078+.
Funding
The authors thank the University of Toledo for funding and Supelco, MilliporeSigma, for providing the SPME devices tested in this work. T.C. acknowledges the Office of Undergraduate Research at the University of Toledo for providing funding to conduct this work through the Undergraduate Research Academic Year Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry 2023 with guest editors Zhi-Yuan Gu, Beatriz Jurado-Sánchez, Thomas H. Linz, Leandro Wang Hantao, Nongnoot Wongkaew, and Peng Wu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Godage, N.H., Cudjoe, E., Chau, T. et al. Quantitative determination of pesticides in human plasma using bio-SPME-LC–MS/MS: a robust tool to assess occupational exposure to pesticides. Anal Bioanal Chem 415, 4423–4434 (2023). https://doi.org/10.1007/s00216-023-04589-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04589-8