Abstract
A high-throughput approach to detecting, quantifying, and characterizing microplastics (MPs) by shape, size, and polymer type using laser direct infrared (LDIR) spectroscopy in surface water samples is demonstrated. Three urban creeks were sampled for their MP content near Cincinnati, OH. A simple Fenton reaction was used to oxidize the surface water samples, and the water samples were filtered onto a gold-coated polyester membrane. Infrared (IR) analysis for polymer identification was conducted, with recoveries of 88.3% ± 1.2%. This method was able to quantify MPs down to a diameter of 20 µm, a size comparable to that of MPs quantified by other techniques such as Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. A shape-classifying algorithm was designed using the aspect ratio values of particles to categorize MPs as fibers, fibrous fragments, fragments, spherical fragments, or spheres. Cut-off values were identified from measurements of known sphere, fragment, and fibrous particles. About half of all environmental samples were classified as fragments while the other shapes accounted for the other half. A cut-off hit quality index (HQI) value of 0.7 was used to classify known and unidentified particles based on spectral matches to a reference library. Center for Marine Debris Research Polymer Kit 1.0 standards were analyzed by LDIR and compared to the given FTIR spectra by HQI, showing that LDIR obtains similar identifications as FTIR analysis. The simplicity and automation of the LDIR allows for quick, reproducible particle analysis, making LDIR attractive for high-throughput analysis of MPs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics (MPs) have become a topic of interest within the scientific community over the past decade, with publications exponentially increasing between the years 2004 and 2019 [1]. MP pollution is ubiquitous in the environment, with detection in various matrices including drinking water, surface water, stormwater, the atmosphere, lake and ocean sediments, and even rain [2,3,4,5,6,7,8,9]. Urban areas are more susceptible to MP pollution due to increased anthropogenic sources such as tire wear on roads, wastewater treatment plant effluents, and litter [4, 10, 11]. Thus, a focus on urban watershed management for MPs must be considered to keep waterways healthy, mitigate further transport of MPs to sediments and marine environments, and reduce the exposure of humans and ecosystems to MPs.
Due to the various degradation pathways (e.g., photolysis, microbial, thermal, etc.), many MPs in the environment are no longer considered virgin and may interact differently than their virgin counterparts [12]. MPs act as vectors for possible harmful organic compounds due to the sorption properties of polymers, leading to bioaccumulation and transport of both MPs and the sorbed organic contaminants [13,14,15,16,17]. Additives in the plastics, including fire retardants, UV stabilizers, plasticizers, and colorants, may also pose a risk to human and ecological health [18]. Due to the health concerns to both humans and the surrounding ecology, monitoring of MP pollution in water systems is vital and poses a unique challenge.
It is known that wastewater treatment facilities are a point source for MPs in watersheds due to the large volume of water that they treat and discharge into surface waters [19, 20]. The higher concentration of MPs in urban watersheds and the proximity to humans make studying urban rivers for MP pollution imperative. Rivers vary in MP pollution both spatially and temporally; thus, developing a high-throughput analysis method is required to monitor urban watersheds [10, 21]. Further, standardization of sampling, sample preparation, and analysis techniques for MPs between researchers is lacking, possibly due to different instrumentation and capabilities between laboratories [22]. MP samples in different matrices require different sample preparation steps, complicating standardization among all MP samples [23].
Laser direct infrared (LDIR) is a fully automated analysis method that can quantify and characterize MPs based on size, shape, and polymer identification in a fraction of the time that a conventional FTIR or Raman spectroscopy needs [24, 25]. Due to its automation, LDIR has been utilized in other studies to identify MPs less than 500 μm, reducing the user intensity of identifying MPs individually. It was also used to count large numbers of MPs as well as other natural particles that absorb in the infrared region of 975–1800 nm [26, 27]. LDIR utilizes a quantum cascade laser (QCL) as the IR source; QCL has been utilized in previous MP research [27, 28]; here, it focuses on individual particles while ignoring blank space, reducing the total analysis time. LDIR is able to quantify based on polymer count, an alternative to other quantitative methods such as pyr-GCMS, which yields concentrations based on weight (e.g., mg/kg or mg/L) [29, 30]. LDIR use for MP analysis in landfills was conducted; however, they did not utilize the LDIR to determine the morphology of MPs, as we do here [31]. Comparison between analysis methods for MP quantification and identification has been done and mostly compare FTIR, Raman spectroscopy, and pyr-GCMS [22, 32]. No direct comparisons between LDIR and other instrumental methods have been made.
Here, we focus on surface freshwater samples from an urban watershed. Natural water samples are notoriously heterogenous, and grab samples are only able to take a “snapshot” of the water column. We aim to obtain a greater representation of the complex watershed by collecting a larger volume over a longer time window. Recently, an American Society for Testing and Materials (ASTM) pump and sieve method for MP sampling in water systems was published [33]. This method samples a larger volume of the water column, obtaining a more representative sample than a “snapshot.” This paper presents a sampling and measurement methodology via IR with LDIR for MPs in surface waters. Due to the automation of the LDIR, complete removal of natural particles is not required because misidentifications of natural particles for MPs will not occur as they do in other analysis methods, such as Nile Red staining organic material [34, 35]. Larger amounts of natural particles can lead to longer analysis times. However, LDIR’s ability to quickly identify and characterize particles allows for analysis times quicker than those of other IR identification methods. LDIR required a 1″ × 3″ glass slide to hold the sample, which can be difficult for liquid samples to be analyzed on. Here, a gold-coated polyester filter was implemented to dry, concentrate, and collect the liquid sample. This technique allowed for the contents of the liquid sample to be placed on the glass slide for LDIR analysis, something that other researchers see as beneficial but have not yet implemented for LDIR analysis. While others have published with LDIR analysis on surface water samples [25], there has been no use of LDIR on urban streams and rivers. Others have published with LDIR as a technique for MP detection in sediment samples and in alluvial aquifers [24, 36], proving that LDIR is capable of identifying and characterizing MPs in other environmental matrices. Our retrieval and processing method is unique and allows for a high-throughput analysis of MPs in surface waters.
The objective of this study was to investigate the feasibility of LDIR to identify, quantify, and characterize MPs in natural surface water samples. Here, we provide an analytical method for sampling, sample preparation, and analysis of MPs in surface waters, such that it can be standard practice among researchers with access to similar instrumentation.
Materials and methods
Chemicals and polymer materials
Sulfuric acid (CAS: 7664–93-9), hydrochloric acid (CAS: 7647–01-0), iron (II) sulfate heptahydrate (CAS: 7782–63-0), and 30% hydrogen peroxide (CAS: 7722–84-1) were purchased from Fisher Scientific. Polyester gold-coated 5-μm membrane filters (Cat #: 1,300,037) were purchased from Sterlitech Corporation. Super-Q 18 (MΩ•cm) water was produced from a Thermo Scientific GenPure UV/UF with xCAD water filtration system. Lab-made MPs were created by grinding plastic cutlery in a stainless-steel coffee grinder and sieved through a stack sieve to separate based on size; 125- to 250-µm-sized MPs were used for analysis. The Center for Marine Debris Research (CMDR) PolyKit 1.0 was used for polymer standards and comparisons between FTIR and LDIR spectra. Polyethylmethacrylate (PEMA) spheres were purchased from Polysciences Inc.
Field sampling
Surface water samples were collected at Mill Creek (site 1) and the East Fork of Mill Creek (site 2), located in Cincinnati, OH, USA; see Figure S1. An additional sample was collected from the East Fork of the Little Miami River in Cincinnati, OH. Samples were collected using the D8332 ASTM method. This method suggests a minimum total sample volume of 1500 L. However, this was determined for collecting a 24-h interval sample to capture the change in the diurnal flow. Briefly, a cast-iron submersible pump and stainless-steel tubing were used to pump the river water through a stainless-steel sieve stack with mesh sizes of 1 mm and 500, 250, 125, 63, 38, and 20 µm. If overflow of the sieves occurred due to clogging from high particle counts, a vacuum pump was attached to pull the water through the sieve stack. The flow rate was adjusted to 1 gpm with a needle valve and measured by a flow meter attached to the end of the stainless-steel tubing. All materials were constructed of non-plastic material. The pumping of water through the sieve stack was timed to obtain total sample volumes. At the Mill Creek site and the Little Miami site, 25 gal and 15 gal were sampled, respectively. After the sample volume was collected, the top 1-mm-size sieve was removed, and a cover was placed on the sieve stack before it was placed in a metal 5-gal bucket for transport. A field blank consisting of Super-Q water in a 1-L stainless-steel jar was taken into the field and opened for 1 min. Each sieve stack was dried overnight under a laminar-flow hood with high efficiency particulate air (HEPA) filters. Material was removed from each sieve using Super-Q water and funneled into ashed glass jars. The Mill Creek samples were diluted to a final volume of 1 L, and the Little Miami samples were diluted to a final volume of 100 mL with Super-Q water for ease of sample transfer for further processing steps.
Biomass removal
Oxidation reactions were conducted to remove organic matter interference from MP analysis. The Fenton oxidation reaction was used as it oxidizes organic matter while maintaining the integrity of the MPs [37,38,39,40]. For the Mill Creek samples, 300 mL was poured into a glass Erlenmeyer flask with the pH adjusted to 3 using sulfuric acid. The iron (II) catalyst was added to a final concentration of 1 mM, and 30% hydrogen peroxide was poured into the flask to a final concentration of 3 M. The reaction was left to sit under a laminar-flow hood until the reaction stopped, between 6 and 8 h. After the reaction stopped, sulfuric acid was used to remove any carbonates and dissolve any remaining iron (III). Aliquot samples were taken in triplicate from the single Erlenmeyer flask.
Variations of this oxidation method are widely used for MP extraction from natural waters and sediments and allows for the removal of organic matter while keeping the integrity of the MP [24, 37,38,39,40,41]. The Little Miami samples used a variation of the Fenton reaction. After both stirring and pipetting multiple times to homogenize the sample, an aliquot sample volume of 2.5 mL from the total 100-mL sample was transferred to a clean Erlenmeyer flask, and 20 mL of 0.05 M Fe (II) in 0.5% HCl and 20 mL 30% H2O2 were added. The reaction was left until completion, about 30 min. The entire oxidized sample was filtered onto gold-coated filters for LDIR analysis using a vacuum filtration apparatus. Triplicate oxidation reactions were performed in separate, solvent-rinsed and ashed Erlenmeyer flasks. The Little Miami samples used a different Fenton reaction than the Mill Creek samples for a quicker throughput of sample analysis.
LDIR sample preparation and analysis
In preliminary experiments, 100-µL aliquots of environmental samples were taken post-oxidation and pipetted directly onto 1″ × 3″ low-emissivity glass (Kevley) slides and allowed to dry under a laminar-flow hood. Additionally, 100-µL aliquots of preliminary unprocessed environmental samples were dried onto a Kevley slide and compared to a 100-mL filtered sample post-oxidation; see Figure S2.
Each Mill Creek and Little Miami sample was filtered onto a 5.0-μm pore size 25-mm diameter gold-coated polyester filter after oxidation. From the same oxidation batch, triplicate samples of 25 mL and 10 mL of Mill Creek site 1 and site 2 were filtered onto gold filters for LDIR analysis, respectively, while the entire volume of the triplicate samples from the Little Miami site was filtered through the gold-coated filters. The filters were left to dry in small tin boxes under the laminar-flow hood. After drying, the filters were taped flat to a Kevley slide such that they could be inserted into the LDIR with the sample holder.
Particles were analyzed using an Agilent 8700 LDIR. Particle spectra were collected and matched to an IR database using Clarity version 1.4.10 software. The default IR library created by Primpke et al. [42] was augmented with IR spectra of polymers and natural particles from CMDR Polymer Kit 1.0 and OpenSpecy [43]. OpenSpecy contains IR spectra from the default IR library; duplicate IR spectra were deleted. The cause of false negatives would be for polymer types or formulations that are not in our spectral library. In the Clarity software, scan and sweep speeds were set to “fast” while focus offset and polarization were set to zero, with the attenuation set at 90%. The particle analysis was set to autoscan mode with the default particle sensitivity, and particle diameters were set to 20 μm and auto for the lower and upper limits, respectively. LDIR can theoretically detect particles as small as 10 μm; however, per the recommendation of Agilent, and other published research, we set the minimum detection limit to 20 μm [26]. The scan area for analysis was drawn around the entire area where filtration occurred. The spectral range of the LDIR is 975–1800 cm−1, and final spectra were acquired in transflection mode with a spectral resolution of 8 cm−1.
Matches for IR spectra to the library use a hit quality index (HQI) as a parameter for confidence in polymer identity. This parameter was set such that any spectra that had a HQI less than 0.7 were classified as “Unidentified” based on visual inspection of overlaid spectra with their library matches. Other LDIR MP research on sediments accepted an HQI value of 0.65 [24]; here, we increase the threshold value to 0.70 based on comparison of standard polymer material matches to the IR library spectra. Due to the HQI dependency to accept spectral matches to the IR library spectra, additions of unique, environmentally degraded, and additive-containing polymers are desired and ongoing. If multiple matches were above the 0.7 threshold, the highest HQI match was used to determine the identity of the MP. Typically, multiple matches of the same polymer would be above the HQI threshold for each MP, due to many of the same polymer types being in the reference library. The LDIR matches spectra with no preprocessing.
The aspect ratio of each MP was calculated and used to determine the shape of each MP. Threshold values were determined using shape standard MPs. PEMA sphere standards (n = 41), turf rubber (n = 1885), and dryer lint (n = 460) were used for sphere, fragment, and fiber shape standards, respectively. Each standard was placed onto a Kevley slide, and particle analysis was conducted. Particles were visually verified as spheres, fragments, or fibers using the Clarity software and can be seen in Figure S3. The aspect ratio of each particle was found, and the average of the total particles of each shape standard was calculated. If agglomeration occurred, the combined particles would not be used to determine the cut-off values for the aspect ratio of each shape type. Upper and lower quartile range values were found for the average fiber, fragment, and sphere aspect ratios and were used for the cut-off values, Fig. 1. Values that lay in-between the upper and lower quartiles of fiber and fragment, as well as sphere and fragment, were classified as “fibrous fragment” and “spherical fragment,” respectively. These aspect ratio cut-off values were used in a shape-classifying algorithm, where if the measured aspect ratio of a MP was in a certain range that corresponds to the experimentally determined cut-off values for a certain shape, it would be classified as that shape.
Final MP concentrations (count/L) are reported after field blanks are subtracted out and the error is propagated. Field blanks were subject to the Fenton reaction and were also done in triplicate to obtain a representative sample of the pervasiveness of MPs in the environment [44].
LDIR compared to FTIR
Plastic samples with FTIR spectra were obtained from the CMDR and run on the LDIR for comparison. A small section (< 250 µm) was cut from the nurdles such that the particle analysis on the LDIR could detect and analyze them. Particle analysis was run on polyethylene (PE), polyamide (PA), polypropylene (PP), polystyrene (PS), and polyvinylchloride (PVC) standards. IR absorbance spectra from the LDIR for each polymer were obtained. This was done for a total of 10 times for each particle, and the average spectra were calculated in the Clarity software with the “Average Spectra” function; this averaged spectrum was exported for analysis. Additionally, an averaged spectrum of 10 MPs found within environmental surface water samples was obtained for each polymer type and exported for comparison to the LDIR spectra of the CMDR standards. The given CMDR FTIR spectra were imported into the Clarity software then exported for analysis. This was done to obtain the same interval for the wavelengths (x-axis) for each spectrum. Obtaining an accurate HQI requires that the two spectra being compared have the same wavelength values that correspond to their absorbance (y-axis) values. The HQI between the given FTIR spectra and the LDIR spectra of the CMDR samples was used to compare the two analysis methods. The HQI between the environmental spectra and the LDIR spectra of the CMDR samples was calculated and used to determine if LDIR is able to identify polymers in environmental matrices.
Calculations
All statistical analyses were done in R version 4.0.3 [45] using the “Hmisc” statistical package version 4.1–1 [46]. The calculation to obtain the HQI is shown in Eq. 1. To account for differences in absorption magnitudes between spectra, the cosine similarity was used to normalize the spectra based on their magnitudes, Eq. 2. Magnitudes are calculated as the Euclidean norms of the spectra interpreted as high-dimensional vectors, i.e., the square root of the sum of squares of the spectrum values. The dot product of the absorbances of the particle spectrum and the absorbances of the library spectrum is obtained in the numerator and divided by the product of the magnitudes to account for differences in absorbance intensity.
The aspect ratio calculated by the LDIR Clarity software divides the horizontal dimension by the vertical dimension, which causes the aspect ratio of elongated particles (fibers) to be either high or low depending on particle orientation. The determination of the shape was calculated from the aspect ratio of the smaller dimension divided by the larger to account for particle orientation. The raw data export from the Clarity software was processed in R to obtain a calculated aspect ratio of the smallest linear side by the largest, as shown in Eq. 3, where “lengthshort” is the measured length of the shorter side and the “lengthlong” is the measured length of the longer side. All lengths were measured in micrometers (µm).
Quality assurance
All experiments were conducted in a MP-specific laboratory with granite countertops, metal or glass material substituting plastic where possible, and 100% cotton lab coats to prevent contamination from sluffing of microfibers from clothing. All experiments were conducted under a laminar-flow hood with HEPA filters, and aluminum foil was used to cover if the sample needed to be moved outside of the hood. A field blank consisting of 1 L of Super-Q water was taken to the field sites and opened for 1 min. The field blank was then subjected to the same Fenton reaction as the samples; triplicate 100-mL aliquots were filtered for analysis. A lab blank was performed using 100 mL of pure Super-Q water filtered through a gold-coated filter to determine contamination due to the Super-Q water system; triplicate analysis was done on the Super-Q blank. Three Kevley slides were placed underneath the laminar-flow hood for 24 h to determine air deposition of particles within the work area.
Experiments to determine recoveries from the sieve and oxidation method were conducted. Lab-made MP particles (125–250 µm) with no uniform shape were used to better represent environmental samples. One hundred MPs were counted and added to a sieve stack and wet sieved; see Method S1 for a detailed process on MP formation. The particles were then rinsed from the sieve stack and filtered onto a gold-coated filter for analysis on the LDIR. In order to account for loss during the Fenton reaction, another group of 100 lab-made MPs were counted and processed in a solvent-rinsed and ashed beaker using the Fenton reaction as described previously and filtered onto a gold-coated filter for LDIR analysis. Using Eq. 4, percent recoveries for sieving and for oxidation were calculated.
Particle count was conducted visually using the Clarity software. All recovery experiments were performed in triplicate.
Results and discussion
Blanks and recoveries
Contamination of MPs in laboratory and field settings can account for a large portion of identified MPs [47]. Due to the large number of plastics in normal laboratory settings (e.g., clothing, beakers, tubing, pipettes, etc.), quality control best practices were implemented to minimize potential MP contamination [23]. MP counts from Super-Q blanks (n = 10 ± 3), lab air blanks (n = 1 ± 1), and field blanks (n = 42 ± 11 and n = 32 ± 6) were found, and all had a low particle count compared to the environmental samples which ranged from 162 to 747 counts/L. Non-plastic particles were found in each blank sample but are not reported here; see Table S1–S5 for detailed tables. The sieve recovery was run in triplicate and determined to be 77.7 ± 3.3%. Recoveries of the Fenton reaction were also conducted in triplicate, and the recovery was 88.3 ± 1.2%, and were similar to those of previous studies [40].
Comparison of analytical techniques
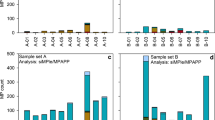
As shown in Fig. 2, the provided FTIR and the acquired LDIR spectra of CMDR Polymer Kit 1.0 match well with values greater than the 0.7 HQI cut-off value. The LDIR spectra of the CMDR Polymer Kit 1.0 standards and the environmental MP spectra have similar absorbance magnitudes as they were both obtained from the LDIR. The provided FTIR spectra have much lower absorbance intensities but still match the IR peaks measured by the LDIR, shown by the HQILDIR. The FTIR spectra were obtained given with the CMDR Polymer Kit 1.0; no FTIR analysis was performed herein. Polyvinylchloride (PVC) has the lowest HQI between LDIR and FTIR spectra at 0.821, which is significantly greater than the 0.7 threshold for spectra to be accepted. The other polymer HQILDIR values were 0.900 for polyamide (PA), 0.930 for polystyrene (PS), 0.943 for polypropylene (PP), and 0.949 for polyethylene (PE). These significant matches between spectra support the accuracy of polymer identification using the automated LDIR technique, with average analysis times of 8 s per particle. Additionally, similar information can be obtained about the size and shape of each particle by the LDIR as would be by FTIR techniques. The HQIENVR that compared the LDIR of plastics standards to polymers identified from environmental samples is also high for each polymer (ranging from 0.882 to 0.977), further suggesting that the LDIR is capable of identifying polymers in environmental matrices. One primary disadvantage is that LDIR only detects spectra between 975 and 1800 cm−1, commonly referred to as the “fingerprint region.” FTIR has a broader IR range and obtains absorbances outside of the fingerprint region, which can yield more information about oxidation exposure (e.g., –OH absorbing at 3400 cm−1) and should be taken into account when constructing MP characterization studies. Previous MP detections have relied heavily on IR identification performed by FTIR analysis [30, 32], but this technique is extremely time-consuming, especially for environmental samples that can have magnitudes of particles, as it requires manual identification for the particles to be identified. A previous study performed a test of different MP analytical methods from 18 different laboratories spanning 8 countries and found FTIR to have questionable results when it came to the identification of polymer type [22]. However, the automation of the LDIR allows samples to be run without direct manual inspection of every single particle, thus saving on time with a much greater output of results. The automated analysis also reduces the subjectivity that the user may introduce to particle identification and reduces subjectivity of optical techniques. For example, Nile Red is known to dye both the targeted MP particles and untargeted natural particles causing the user to more subjectively determine which stained particles are real MPs and what particles are artifacts [34, 35]. This makes the LDIR desirable for high-throughput analysis with proven accuracy in various polymer identifications of both natural polymers and MPs.
Comparison of LDIR to FTIR spectra and polymer standards to environmental particles’ spectra. Polystyrene (PS), polypropylene (PP), polyethylene (PE), polyamide (PA), and polyvinylchloride (PVC) spectra are compared in plots a–e. FTIR (red) is the Center for Marine Debris Research (CMDR) Polymer Kit 1.0 spectra, LDIR (green) is the spectra measured of the CMDR polymers by the LDIR, and Environmental (blue) is a polymer identified within an environmental sample. HQILDIR is the hit quality index of the LDIR spectra to the FTIR spectra of the same CMDR polymer. HQIENVR is the hit quality index of the LDIR spectra of the CMDR polymer to a polymer of the same identification found in a surface water sample
Shape assignment of microplastics
The shape and quality of MPs are crucial in understanding how they can affect the health of their fate and transport in a watershed system. As mentioned previously, we utilized standard materials of lint fibers, rubber pieces, and laboratory-made spheres in order to obtain threshold values of the measured aspect ratio for MP shapes using the LDIR. From our results, fibers were determined to have an aspect ratio of 0.3632 or lower, fibrous fragments ranged from 0.3632 to 0.5106, fragments ranged from 0.5106 to 0.8011, spherical fragments ranged from 0.8011 to 0.8923, and a value above 0.8923 was classified as a sphere, Fig. 1. Previous MP research has used the aspect ratio to determine particle shape. The aspect ratio for fibers was set to 0.3333 in other studies, corroborating the experimentally found cut-off value of 0.3632 here [24, 48,49,50]. The LDIR obtains other shape parameters such as circularity, solidity, and eccentricity. These parameters also use height and width in their calculations, and to prevent redundancy, we only used the aspect ratio for shape determination. Circularity, solidity, and eccentricity were also unable to differentiate between two or more of the shape fractions, and thus, were not used to determine the shape of particles. The aspect ratio of each MP is a parameter that other instruments can measure. By using the aspect ratio for shape determination, a comparison across instruments and laboratories can be made.
Comparison of media for sample loading
Typically, LDIR uses IR-reflective Kevley slides or similar 1″ × 3″ slides that fit into the sample holder for analysis. Kevley slides are not fully resistant to acid and peroxide and are susceptible to corrosion of the IR-reflective coating which can cause the misidentification of particles. This was seen in preliminary samples of post-Fenton aliquots on the Kevley slide that corroded the IR-reflective surface, leading to poor IR identifications and artifacts which the LDIR misidentified as particles, Fig. 3. Additionally, small aliquots that are required to fit on these slides are not fully representative of a surface water sample and can lead to an overestimation of MP content. A larger volume must be analyzed to obtain a more representative sample of the surface water’s MP content, Table 1. Filtration of a sample onto a gold-coated filter allows for a representative volume of sample to be collected. Additionally, agglomeration of particles along the outer edge of the aliquot on the Kevley slide, referred to as the “coffee-ring effect,” can cause loss of accuracy in results. The gold filter method allows for a more even distribution of the samples onto the filter and does not have the same coffee-ring effect that the Keyley slide aliquot sample is prone to. Figure 3c and 3d show the highlighted particles from an aliquot and a filter preparation method from the same environmental sample, respectively. The filter has an even distribution of particles over the analysis area while the aliquot has the majority of particles along the outer edge. This coffee-ring effect increases the chances of particle agglomeration, leading to a miscount of total particles and possibly misidentification of polymer types.
Corrosion of Kevley slide’s reflective surface from the acid post-Fenton reaction and with pure acid shown in (a). Example of a 100-µL aliquot and a 100-mL sample filtered through a gold-coated polyethylene filter taped to the Kevley slide shown in (b). Total particles from the same sample location found by LDIR are highlighted from an aliquot (c) and from a filtered (d) sample preparation method. For scale, the slide is 25 mm wide
Unlike the Kevley slide surface, gold is resistant to acid and oxidation and, because of the short timescale which the post-Fenton solution is in contact with the filter, it did not affect the gold coating. Gold is also IR reflective in the range that the LDIR detects (975–1800 cm−1), allowing for correct identification of particles with no interference. Other researchers have implemented a gold filter for FTIR and Raman spectroscopy, but none have been implemented for LDIR use [51]. Initial experiments compared the aliquot on the Kevley slide to the gold filtration technique and found an overestimation of MP content when using the aliquot to represent the sample. This was due to the small volume of the aliquot, making the concentration factor a 10,000 times multiplier. Meaning if one MP was found in the aliquot, the concentration of MPs in the water sample would be 10,000 particles/L. Filtration of 100 mL uses a concentration factor of 10 and provides a better representation of the MP content, not only in the total particle count but also in the distribution of identified polymers. Table 1 shows a comparison of particle counts of the initial experiments between the aliquot and the filter techniques. These findings support the use of gold filters to obtain the greatest representation of MPs in surface waters compared to the previously used Kevley slide technique Fig. 4.
Environmental sample analysis
The ASTM D8332 MP sampling method was used to collect a representative sample from an urban watershed at three different locations. The ASTM method uses a submersible pump to filter river water through a stack of sieves in the field; it can sample a larger volume of water but takes more time, effort, and planning than a grab sample. While grab samples are logistically simple, they may not represent the MP content in the water column as well as the ASTM method due to lack of volume size and temporal inclusion [52]. Triplicate extractions and filtrations were conducted from each sieved sample, and the averages of the MP counts are reported in Tables S6–S8. This is contradictory to other research that do not have triplicate averages and only perform one duplicate at a sample site to account for heterogeneity within the water column, or just a single sample with no replicate [4]. Two triplicate methods were compared: for Mill Creek site 1 and site 2, a larger, single oxidation and the pouring out of three samples after the Fenton reaction subsided, and for the Little Miami site, three smaller, separate oxidations where the entire sample was filtered after the Fenton reaction subsided. Larger variations in particle count were found in the Mill Creek samples, which we attributed to the heterogeneity within the natural water system [21, 23, 44], as well as a lack of mixing before filtration. The first triplicate of the Mill Creek samples always had more MPs than the next two triplicate samples; see Figure S4 for further details. This is possibly due to MPs floating and the majority being removed from the flask during the first pour. A sample from the Little Miami site was used to determine if triplicate samples should be oxidized separately. These triplicates were more homogenous than the triplicate samples obtained from a single, larger oxidation. From these results, running samples in triplicate are recommended to obtain a robust MP count, and they should be oxidized and filtered separately to obtain an accurate average. Even though oxidation in a single, larger batch obtains a representative sample, the errors are larger due to variations in pouring the sample into thirds.
In addition to MPs, natural particles are a common and expected result from environmental samples and include natural polyamide, polyamide, chitin, cellulosic, cellulosic acetate, cellulosic chemically modified, polyurethane, silica, silicone, coal, and algae. Natural polyamide and polyamide are both considered natural particles due to the HQI algorithm’s inability to distinguish the two types of IR spectra. All other particles were identified as MPs; Table 2 shows the abbreviations for each identified MP and their corresponding polymer name. While rubber has been reported as a natural particle in other LDIR research [24], we report rubber as a synthetic MP due to commonly identified anthropogenic sources or rubber material in the environment [4]. With the LDIR being able to identify, quantify, and characterize both anthropogenic and naturally occurring particles, conclusions of the water column based on the abundance and type of natural particles can be made. In this research, we do not use the information from the naturally occurring particles. However, their abundance in each sample can be found in Tables S6–S8 and further research is suggested to obtain more accurate results. The total abundance of all particles was directly proportional to the total analysis time. Time ranged from 3 to 14 h for complete analysis of a filter and was solely dependent on the total number of particles present. Typically, in the environmental samples, the total number of particles ranged from 1500 to 6000.
Mill Creek MP counts varied between site 1 (n = 162/L ± 53) and site 2 (n = 747/L ± 165), both of which are an order of magnitude higher than the blank sample. Both total counts and the unique identities of MPs were higher in site 2 than site 1. Although total counts varied between sites, the size and shape distribution were found to be similar among MPs, with the majority of particles having < 100-µm diameters and being fragments; see Figure S5. Although both sample locations were near each other, and sampling volumes and techniques were the same between both sample sites, site 1 and site 2 were from different rivers and likely have different sources of MP pollution, such as the wastewater treatment plant located upstream of site 2. Wastewater treatment effluents are known to be a point source of MPs within urban watersheds, even though overall removal efficiencies of MPs in wastewater treatment facilities can be high [19, 20]. Both sites were located in urban industrial areas which provide multiple and complex MP sources [10, 11, 53]. The Little Miami site MP count was between the two Mill Creek samples (n = 303/L ± 20) and roughly 10 times larger than the field blank (n = 32 ± 6). Even though the Little Miami site was less urbanized and may have different MP sources, similar trends in size and shape to the Mill Creek samples are observed. Most MPs are < 100 µm in diameter and are fragments, Fig. 5. A common pattern of exponentially increasing particle count as particle diameter decreases was found in each environmental sample, Fig. 5. This pattern has also been found in other MP research [24, 54, 55]. However, determining MP sources within freshwater systems needs further exploring.
Distribution of MP counts per liter by polymer type (a) after field blanks were subtracted. Error bars represent standard deviations of triplicate samples. The total size distribution of each polymer type (b), the red dashed line represents the size limit of detection of the LDIR at 20 µm. Overall MP shapes were calculated (c) with fragments being the majority of identified MPs being fragments
Relationship between total particle counts to the corresponding diameters of every particle found of the Little Miami (LM) site (a), Mill Creek (MC) site 1, (b) and MC site 2 (c). The exponential increase in particle count as the diameter decreases is a common pattern in MP particle analysis in environmental matrices. The red dashed line in the LDIR particle size limit of detection of 20 µm
Conclusions
This study provides the first implementation of LDIR on urban surface freshwater samples for MP detection, quantification, and characterization. Additionally, this is believed to be the first publication using the ASTM D8332 method for MP sampling in a natural water system. Based on our results, it is recommended that triplicate samples be taken for a representative count of MPs. Oxidation via the Fenton reaction should be done separately on each triplicate sample in separate ashed flasks. Large numbers of particles were detected in the environmental samples with only a portion identified as MPs (8–14%). The ability of the LDIR to identify every particle does not only allow for analysis of plastic particles but also yields information about the distribution of natural particles in the water column (i.e., high suspended sediment load). Filtration of liquid samples on gold-coated filters is used herein and recommended for further analysis of MPs in liquid samples by LDIR. Particle characterization with LDIR is more automated than FTIR characterization methods and allows for more non-plastic particles to be present without exponentially increasing analysis times, due to the average analysis time of 8 s per particle. The LDIR obtained accurate spectra of polymer types and had a high confidence match to the given CMDR FTIR library, suggesting that the LDIR is capable of obtaining the same information as FTIR methods. Based on the observations from the LDIR compared to other identification techniques for MPs, we suggest the use of LDIR for MP identification, quantification, and characterization in environmental matrices. However, standardization of the instrument among various matrices needs to be further explored.
References
Qin F, Du J, Gao J, Liu G, Song Y, Yang A, Wang H, Ding Y, Wang Q (2020) Bibliometric profile of global microplastics research from 2004 to 2019. Int J Environ Res Public Health 17 (16). :https://doi.org/10.3390/ijerph17165639.
Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S. Plastic rain in protected areas of the United States. Science. 2020;368(6496):1257–60. https://doi.org/10.1126/science.aaz5819.
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GL, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC. The deep sea is a major sink for microplastic debris. R Soc Open Sci. 2014;1(4):140317. https://doi.org/10.1098/rsos.140317.
Werbowski LM, Gilbreath AN, Munno K, Zhu X, Grbic J, Wu T, Sutton R, Sedlak MD, Deshpande AD, Rochman CM. Urban stormwater runoff: a major pathway for anthropogenic particles, black rubbery fragments, and other types of microplastics to urban receiving waters. ACS ES&T Water. 2021;1(6):1420–8. https://doi.org/10.1021/acsestwater.1c00017.
Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, Mintenig SM, De France J. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–22. https://doi.org/10.1016/j.watres.2019.02.054.
Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY (2020) A global perspective on microplastics. J Geophys Res Oceans 125 (1). :https://doi.org/10.1029/2018jc014719
Ling SD, Sinclair M, Levi CJ, Reeves SE, Edgar GJ. Ubiquity of microplastics in coastal seafloor sediments. Mar Pollut Bull. 2017;121(1–2):104–10. https://doi.org/10.1016/j.marpolbul.2017.05.038.
Klockner P, Seiwert B, Eisentraut P, Braun U, Reemtsma T, Wagner S. Characterization of tire and road wear particles from road runoff indicates highly dynamic particle properties. Water Res. 2020;185:116262. https://doi.org/10.1016/j.watres.2020.116262.
Kirstein IV, Hensel F, Gomiero A, Iordachescu L, Vianello A, Wittgren HB, Vollertsen J. Drinking plastics? - quantification and qualification of microplastics in drinking water distribution systems by microFTIR and Py-GCMS. Water Res. 2021;188:116519. https://doi.org/10.1016/j.watres.2020.116519.
Luo W, Su L, Craig NJ, Du F, Wu C, Shi H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ Pollut. 2019;246:174–82. https://doi.org/10.1016/j.envpol.2018.11.081.
Jarlskog I, Stromvall AM, Magnusson K, Galfi H, Bjorklund K, Polukarova M, Garcao R, Markiewicz A, Aronsson M, Gustafsson M, Norin M, Blom L, Andersson-Skold Y. Traffic-related microplastic particles, metals, and organic pollutants in an urban area under reconstruction. Sci Total Environ. 2021;774:145503. https://doi.org/10.1016/j.scitotenv.2021.145503.
Yuan Q, Sun R, Yu P, Cheng Y, Wu W, Bao J, Alvarez PJJ (2021) UV-aging of microplastics increases proximal ARG donor-recipient adsorption and leaching of chemicals that synergistically enhance antibiotic resistance propagation. J Hazard Mater:127895. :https://doi.org/10.1016/j.jhazmat.2021.127895
Ziccardi LM, Edgington A, Hentz K, Kulacki KJ, Kane Driscoll S. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: a state-of-the-science review. Environ Toxicol Chem. 2016;35(7):1667–76. https://doi.org/10.1002/etc.3461.
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–99. https://doi.org/10.1016/j.jhazmat.2017.10.014.
Lin J, Yan D, Fu J, Chen Y, Ou H. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020;186:116360. https://doi.org/10.1016/j.watres.2020.116360.
McDougall L, Thomson L, Brand S, Wagstaff A, Lawton LA, Petrie B. Adsorption of a diverse range of pharmaceuticals to polyethylene microplastics in wastewater and their desorption in environmental matrices. Sci Total Environ. 2021;808:152071. https://doi.org/10.1016/j.scitotenv.2021.152071.
Miller ME, Hamann M, Kroon FJ. Bioaccumulation and biomagnification of microplastics in marine organisms: a review and meta-analysis of current data. PLoS One. 2020;15(10):e0240792. https://doi.org/10.1371/journal.pone.0240792.
Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF (2020) A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 17 (4). :https://doi.org/10.3390/ijerph17041212
Blair RM, Waldron S, Gauchotte-Lindsay C. Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period. Water Res. 2019;163:114909. https://doi.org/10.1016/j.watres.2019.114909.
Ben-David EA, Habibi M, Haddad E, Hasanin M, Angel DL, Booth AM, Sabbah I. Microplastic distributions in a domestic wastewater treatment plant: removal efficiency, seasonal variation and influence of sampling technique. Sci Total Environ. 2021;752:141880. https://doi.org/10.1016/j.scitotenv.2020.141880.
Stanton T, Johnson M, Nathanail P, MacNaughtan W, Gomes RL. Freshwater microplastic concentrations vary through both space and time. Environ Pollut. 2020;263(Pt B):114481. https://doi.org/10.1016/j.envpol.2020.114481.
Müller YK, Wernicke T, Pittroff M, Witzig CS, Storck FR, Klinger J, Zumbulte N. Microplastic analysis-are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample. Anal Bioanal Chem. 2020;412(3):555–60. https://doi.org/10.1007/s00216-019-02311-1.
Miller E, Sedlak M, Lin D, Box C, Holleman C, Rochman CM, Sutton R. Recommended best practices for collecting, analyzing, and reporting microplastics in environmental media: lessons learned from comprehensive monitoring of San Francisco Bay. J Hazard Mater. 2021;409:124770. https://doi.org/10.1016/j.jhazmat.2020.124770.
Cheng Y-L, Zhang R, Tisinger L, Cali S, Yu Z, Chen HY, Li A. Characterization of microplastics in sediment using stereomicroscopy and laser direct infrared (LDIR) spectroscopy. Gondwana Res. 2021. https://doi.org/10.1016/j.gr.2021.10.002.
Scircle A, Cizdziel JV, Tisinger L, Anumol T, Robey D (2020) Occurrence of microplastic pollution at oyster reefs and other coastal sites in the Mississippi sound, USA: impacts of freshwater inflows from flooding. Toxics 8 (35). :https://doi.org/10.3390/TOXICS8020035
Bao M, Huang Q, Lu Z, Collard F, Cai M, Huang P, Yu Y, Cheng S, An L, Wold A, Gabrielsen GW. Investigation of microplastic pollution in Arctic fjord water: a case study of Rijpfjorden, Northern Svalbard. Environmental Science and Pollution Research. 2022;29(37):56525–34. https://doi.org/10.1007/s11356-022-19826-3.
Hildebrandt L, El Gareb F, Zimmermann T, Klein O, Kerstan A, Emeis KC, Pröfrock D (2022) Spatial distribution of microplastics in the tropical Indian Ocean based on laser direct infrared imaging and microwave-assisted matrix digestion. Environmental Pollution 307. :https://doi.org/10.1016/j.envpol.2022.119547
López-Rosales A, Andrade J, Fernández-González V, López-Mahía P, Muniategui-Lorenzo S (2022) A reliable method for the isolation and characterization of microplastics in fish gastrointestinal tracts using an infrared tunable quantum cascade laser system. Marine Pollution Bulletin 178. :https://doi.org/10.1016/j.marpolbul.2022.113591.
Zarfl C. Promising techniques and open challenges for microplastic identification and quantification in environmental matrices. Anal Bioanal Chem. 2019;411(17):3743–56. https://doi.org/10.1007/s00216-019-01763-9.
Huppertsberg S, Knepper TP. Instrumental analysis of microplastics-benefits and challenges. Anal Bioanal Chem. 2018;410(25):6343–52. https://doi.org/10.1007/s00216-018-1210-8.
Zhang Y, Peng Y, Peng C, Wang P, Lu Y, He X, Wang L. Comparison of detection methods of microplastics in landfill mineralized refuse and selection of degradation degree indexes. Environ Sci Technol. 2021;55(20):13802–11. https://doi.org/10.1021/acs.est.1c02772.
Kappler A, Fischer D, Oberbeckmann S, Schernewski G, Labrenz M, Eichhorn KJ, Voit B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem. 2016;408(29):8377–91. https://doi.org/10.1007/s00216-016-9956-3.
ASTM (2020) ASTM D8332–20: Standard practice for collection of water samples with high, medium, or low suspended solids for identification and quantification of microplastic particles and fibers. Annual Book of ASTM Standards 11.02. :https://doi.org/10.1520/d8332-20.
Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA. Lost, but found with Nile Red: a novel method for detecting and quantifying small microplastics (1 mm to 20 mum) in environmental samples. Environ Sci Technol. 2017;51(23):13641–8. https://doi.org/10.1021/acs.est.7b04512.
Stanton T, Johnson M, Nathanail P, Gomes RL, Needham T, Burson A. Exploring the efficacy of Nile Red in microplastic quantification: a costaining approach. Environmental Science & Technology Letters. 2019;6(10):606–11. https://doi.org/10.1021/acs.estlett.9b00499.
Samandra S, Johnston JM, Jaeger JE, Symons B, Xie S, Currell M, Ellis AV, Clarke BO (2022) Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci Total Environ 802. :https://doi.org/10.1016/j.scitotenv.2021.149727.
Hurley RR, Lusher AL, Olsen M, Nizzetto L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol. 2018;52(13):7409–17. https://doi.org/10.1021/acs.est.8b01517.
Chen Y-J, Fan T-Y, Wang L-P, Cheng T-W, Chen S-S, Yuan M-H, Cheng S (2020) Application of Fenton method for the removal of organic matter in sewage sludge at room temperature. Sustain 12 (4). :https://doi.org/10.3390/su12041518.
Pfohl P, Roth C, Meyer L, Heinemeyer U, Gruendling T, Lang C, Nestle N, Hofmann T, Wohlleben W, Jessl S (2021) Microplastic extraction protocols can impact the polymer structure. Microplastics Nanoplastics 1 (1). :https://doi.org/10.1186/s43591-021-00009-9.
Cunsolo S, Williams J, Hale M, Read DS, Couceiro F. Optimising sample preparation for FTIR-based microplastic analysis in wastewater and sludge samples: multiple digestions. Anal Bioanal Chem. 2021;413(14):3789–99. https://doi.org/10.1007/s00216-021-03331-6.
Masura J, Baker J, Foster G, Arthur C, Herring C (2015) Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Technical Memeorandum NOS-OR&R-48.
Primpke S, Wirth M, Lorenz C, Gerdts G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem. 2018;410(21):5131–41. https://doi.org/10.1007/s00216-018-1156-x.
Cowger W, Steinmetz Z, Gray A, Munno K, Lynch J, Hapich H, Primpke S, De Frond H, Rochman C, Herodoto O. Microplastic spectral classification needs an open source community: open Specy to the rescue! Analytical Chemistry. 2021;93(21):7543–8. https://doi.org/10.1021/acs.analchem.1c00123.
Bruge A, Dhamelincourt M, Lanceleur L, Monperrus M, Gasperi J, Tassin B. A first estimation of uncertainties related to microplastic sampling in rivers. Sci Total Environ. 2020;718:137319. https://doi.org/10.1016/j.scitotenv.2020.137319.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
Harrel F. Hmisc: Harrell Miscellaneous. R package Version 4.1–1; 2018.
Gwinnett C, Miller RZ. Are we contaminating our samples? A preliminary study to investigate procedural contamination during field sampling and processing for microplastic and anthropogenic microparticles. Mar Pollut Bull. 2021;173(Pt B):113095. https://doi.org/10.1016/j.marpolbul.2021.113095.
Cole M. A novel method for preparing microplastic fibers. Sci Rep. 2016;6:34519. https://doi.org/10.1038/srep34519.
Roy N, Wijaya KP, Götz T, Sundar S (2022) Transport of ellipsoidal microplastic particles in a 3D lid-driven cavity under size and aspect ratio variation. Appl Math Comput 413. :https://doi.org/10.1016/j.amc.2021.126646.
Koelmans AA, Redondo-Hasselerharm PE, Nor NHM, de Ruijter VN, Mintenig SM, Kooi M. Risk assessment of microplastic particles. Nature Rev Mater. 2022;7(2):138–52. https://doi.org/10.1038/s41578-021-00411-y.
Cabernard L, Roscher L, Lorenz C, Gerdts G, Primpke S. Comparison of Raman and Fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ Sci Technol. 2018;52(22):13279–88. https://doi.org/10.1021/acs.est.8b03438.
Hung C, Klasios N, Zhu X, Sedlak M, Sutton R, Rochman CM. Methods matter: methods for sampling microplastic and other anthropogenic particles and their implications for monitoring and ecological risk assessment. Integr Environ Assess Manag. 2021;17(1):282–91. https://doi.org/10.1002/ieam.4325.
Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136:105411. https://doi.org/10.1016/j.envint.2019.105411.
Uurasjarvi E, Hartikainen S, Setala O, Lehtiniemi M, Koistinen A. Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environ Res. 2020;92(1):149–56. https://doi.org/10.1002/wer.1229.
Enders K, Lenz R, Stedmon CA, Nielsen TG. Abundance, size and polymer composition of marine microplastics >/=10mum in the Atlantic Ocean and their modelled vertical distribution. Mar Pollut Bull. 2015;100(1):70–81. https://doi.org/10.1016/j.marpolbul.2015.09.027.
Acknowledgements
This research was funded and conducted by the Center for Environmental Solutions and Emergency Response (CESER) of the U.S. Environmental Protection Agency (EPA), Cincinnati, OH. Additional support was provided by appointments in the Research Participation Program at the Office of Research and Development (ORD), EPA, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the Department of Energy and EPA (DW089925247). We thank Bruce Smith for his help with the sampling, and we extend further thanks to the lab technician who helped make this research possible, Dilip K. Duvvuru, as well as the internal reviewers at the EPA who provided input. The research results presented in this paper do not necessarily reflect the views of the agency or its policy. This document has been reviewed by the U.S. Environmental Protection Agency, Office of Research and Development, and approved for publication. Mention of trade names or products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Whiting, Q.T., O’Connor, K.F., Potter, P.M. et al. A high-throughput, automated technique for microplastics detection, quantification, and characterization in surface waters using laser direct infrared spectroscopy. Anal Bioanal Chem 414, 8353–8364 (2022). https://doi.org/10.1007/s00216-022-04371-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04371-2