Abstract
While the exact health risks associated with nanoplastics are currently the focus of intense research, there is no doubt that humans are exposed to nanoplastics and that food could be a major source of exposure. Nanoplastics are released from plastic materials and articles used during food production, processing, storage, preparation, and serving. They are also likely to enter the food chain via contaminated water, air, and soil. However, very limited exposure data for risk assessment exists so far due to the lack of suitable analytical methods. Nanoplastic detection in food poses a great analytical challenge due to the complexity of plastics and food matrices as well as the small size and expectedly low concentration of the plastic particles. Multidetector field flow fractionation has emerged as a valuable analytical technique for nanoparticle separation over the last decades, and the first studies using the technique for analyzing nanoplastics in complex matrices are emerging. In combination with online detectors and offline analysis, multidetector field flow fractionation is a powerful platform for advanced characterization of nanoplastics in food by reducing sample complexity, which otherwise hampers the full potential of most analytical techniques. The focus of this article is to present the current state of the art of multidetector field flow fractionation for nanoplastic analysis and to discuss future trends and needs aiming at the analysis of nanoplastics in food.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoplastics were flagged together with microplastics as a potential future food safety issue by European Food Safety Authority’s Panel on Contaminants in the Food Chain already in 2016 [1]. However, the impact of a contaminated food chain on humans and the resulting health hazards are unclear due to the limited number of studies on the topic [2]. Evidence for gastrointestinal uptake of nanoplastics is emerging [3]. Human exposure is further evidenced by detection of plastic particles in human blood and stool [4, 5]. Currently, various definitions for nanoplastics exist. Nanoplastics could, e.g., be defined as nanomaterials as specified in the European Commission Recommendation on the definition of nanomaterials [6]. Here, a material is considered a nanomaterial if it consists of 50% or more solid particles with one or more external dimensions in the size range of 1 to 100 nm in the number-based size distribution. In the context of this article, we will follow the proposal by Hartmann et al. [7] that nanoplastics are objects in the size range of 1 to < 1000 nm consisting of synthetic or heavily modified natural polymers as an essential ingredient that, when present in natural environments without fulfilling an intended function, are solid and water insoluble at 20 °C. They can have various shapes and structures and might be either intentionally produced (primary nanoplastics) or formed by fragmentation in the environment or during use (secondary nanoplastics).
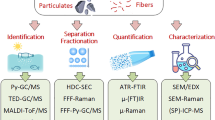
To date, several analytical methods have been reported for the identification and quantification of microplastics (1 µm to 1 or 5 mm depending on the definition) in different food matrices, including fruit, vegetables, table salt, bottled water, milk, and seafood [8, 9]. At the same time, there is only a limited number of studies reporting on the development of analytical methods for nanoplastics in food. To gain a better insight into the presence of nanoplastics in food and understand actual human exposure to nanoplastics (in qualitative and quantitative terms) and potential risk for human health, it is of utmost importance to develop appropriate analytical methods with high accuracy, selectivity, and sensitivity. Ideally, the analytical method should allow detection (presence yes/no), identification (which type of plastics), characterization (size, shape, etc.), and quantification (mass and/or number concentration) of nanoplastics.
Analytical challenges related to nanoplastic analysis are described in several reviews and also apply to nanoplastic analysis in food [10,11,12,13]. Briefly summarized, potential challenges are:
-
The similarity in composition, and consequently also density, of nanoplastics and food matrix constituents makes chemical/physical separation challenging.
-
The small size is expected to make nanoplastics more vulnerable toward chemical degradation during sample preparation (the procedures working for microplastics cannot be applied) [14].
-
The small size prevents separation of nanoplastics from the matrix by conventional sieves and filters as used for microplastics [13].
-
Most techniques for the identification of microplastics are not directly applicable to nanoplastics.
-
Most analytical techniques are not sensitive enough to cope with the expectedly low number and mass concentrations in food [10].
-
Analytical techniques are impacted by interferences caused by matrix residues [10].
-
Contamination is expected to occur during sampling, sample preparation, and analysis (as it is for microplastics) [10], but the levels and main sources are so far unknown.
-
Suitable reference materials that are required for method validation, harmonization, and standardization are lacking [10, 11].
To overcome these challenges, work is continuously ongoing to improve sample preparation procedures, to adapt existing and to develop entirely new analytical techniques. Field flow fractionation (FFF) is a very promising technique for reducing sample complexity by separating nanoplastics from ions, molecules, and organic and inorganic colloids/particulate matter including residues from matrix degradation during sample preparation, which could otherwise interfere with the analysis. FFF can further be coupled to an array of online detectors (multidetector (MD)-FFF), which can provide direct information on the nanoplastics during the separation like size or carbon concentration.
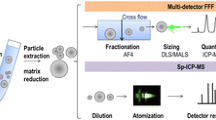
FFF is a separation technique, where retention is caused by balancing the diffusional movement of particles and an externally generated field force, which acts on the particles perpendicular to the laminar flow of a carrier liquid through the FFF channel (Fig. 1) [15]. The channel is typically a few hundred micrometers high and contains no stationary phase. The external field pushes the particles toward the accumulation wall from where the particles diffuse back. Depending on the resulting position of the particles in the channel, they will be transported at different velocities toward the detectors due to the parabolic flow rate profile in the channel. Different field forces can be applied, e.g., a symmetric or asymmetric flow (AF4), a centrifugal force (centrifugal/sedimentation FFF), or an electrical field (electrical FFF). The type of field force determines which property of the particles is responsible for the separation.
The most commonly used type of field flow fractionation for nano-sized particles is AF4. Here, a flow perpendicular to the wall, the so-called cross flow, is split off from the main channel flow (parallel to the wall) and leaving the channel through a semipermeable membrane (Fig. 1) [16]. The separation depends on the diffusion coefficient of the particles, which is related to their hydrodynamic diameter [15]. Particles in the size range of approximately 1 to 1000 nm, which is the relevant size range for nanoplastics, are typically separated in normal mode (principle shown in Fig. 1). Here, smaller particles elute first due to their higher diffusion coefficient and the resulting larger distance from the accumulation wall where the flow velocity is higher. At larger particle sizes (> 1 µm), steric inversion occurs where the normal-mode separation begins to convert to steric-mode separation (largest particles elute first). The presence of particles with sizes below and above the steric inversion allows no meaningful separation, and pre-fractionation of the sample, e.g., by filtration and centrifugation, is required [15]. The asymmetric principle is easier to operate and has increased separation efficiency in comparison to symmetric FFF [16]. The major advantage of AF4 is the flexibility and versatility made possible by applying an optimized cross flow profile, varying the retention power in the course of elution. Hollow-fiber flow FFF (HF5), which works by a similar principle but in a fiber instead of a channel, currently plays a minor role, but could become more important for routine applications that benefit from a disposable separation device [16]. Another type of FFF for nano-sized particles is centrifugal (or sedimentation) FFF that separates according to buoyant mass, which is related to particle size and density [17]. Its lower size limit depends on the density difference between the carrier liquid and the particles and the maximum speed of the centrifuge. The dependence of the separation on density makes sedimentation FFF interesting for the separation of polymer types with different densities and when differences between matrix and nanoplastics exist.
FFF uses many components of the classical liquid chromatography system including autosampler, pumps, and detectors (Fig. 1), and is often called a chromatography-like technique. The main advantage over chromatography-based separation techniques like size-exclusion (SEC) and hydrodynamic chromatography (HDC) is the already-mentioned absence of a stationary phase which avoids the interaction with particles and mechanical stress. For a more detailed comparison of AF4 with other separation techniques, the reader is referred to [12].
After a short summary of the current knowledge regarding the occurrence of nanoplastics in food, we will give an overview on the application of FFF for pure nanoplastics and for nanoplastics in complex matrices, suitable online detectors, and techniques for offline analysis. Finally, we will provide an outlook on the future of nanoplastic analysis in food using FFF.
What do we know about the occurrence of nanoplastics in food?
According to a review by Toussaint et al. from 2019 [6], only a minimal number of studies have evaluated the presence of nanoplastics in species that are part of the human food chain. The review lists one study that describes the capacity of nanoplastic adsorption onto algae and five studies that demonstrate the uptake of nanoplastics in marine species such as mussels, oysters, and fish. However, it also mentions that no peer-reviewed study has so far clearly demonstrated the presence of nanoplastics in the related food products.
Very few studies have proved the presence of nanoplastics in environmental samples so far. The first of these studies by Ter Halle et al. detected nanoplastics (polyvinyl chloride/PVC, polyethylene terephthalate/PET, polystyrene/PS, and polyethylene/PE) in the North Atlantic Subtropical Gyre in 2017 [18]. Another study by the same research group detected nanoplastics (PVC, PS, PE) in soil amended with plastic debris [19]. Nanoplastics have further been detected, e.g., in alpine snow [20] and in water sampled from a Greenland firn core as well as an Antarctic Sea ice core [21].
The limited number of published studies, reporting the presence of nanoplastics in food, environmental samples, human tissues, and other complex matrices, is most likely related to the analytical challenges that we address here in this paper. These analytical challenges are reduced in controlled release studies due to the absence of a complex matrix and the potentially higher concentrations of nanoplastics (in comparison to environmental samples). Looking at release studies of nanoplastics with relevance to food, one major source of nanoplastics appears to be plastic food contact materials (FCMs), i.e., materials and articles used during food production, processing, storage, preparation, and serving. This source would most likely affect all foods. A worst-case scenario appears to be the heating of food in single-use plastics. The release of nanoplastics from FCMs under relevant use conditions has been demonstrated for, e.g., nylon and PET teabags (steeping at 95 °C) [22], polypropylene (PP) infant feeding bottles (standard formula-preparation steps including cleaning, sterilizing, and mixing) [23], and food-grade nylon bags (exposure to ultrapure water at 22 °C or 90 °C for 1 h) as well as hot beverage cups lined with low-density PE (exposure to ultrapure water at 22 °C or 100 °C for 20 min allowed to cool naturally during exposure) [24]. Temperature was demonstrated to increase the release of plastic particles [23, 24]. Released nanoplastic concentrations in all studies were in the range of approximately 1011 to 1012 particles/L. However, more detailed experiments are needed to confirm with certainty a potential release of plastic particles [25]. It cannot be excluded that, for example, crystallization of oligomers plays a role in some of these findings [25].
Besides the direct release into food from plastic FCMs, nanoplastics will also enter the food chain via contaminated soil, water, and air. As mentioned previously, nanoplastics were already demonstrated to exist in (plastic debris–amended) soil and ocean water. Most focus in relation to microplastics has been on seafood due to the obvious link to marine pollution with plastics. A recent study provided the first quantitative assessment of nanoplastic uptake by the fish gut using palladium-labeled nanoplastics in an ex vivo gut sac exposure [26]. Out of 1.03 × 1010 particles, 700,000 particles (i.e., 0.007%) passed across the gut epithelium.
Fruits and vegetables might contain nanoplastics originating from nanoplastic-contaminated soil or water, including contamination via the use of agricultural film, sludge and organic fertilizer application, sewage irrigation, and atmospheric deposition [9]. Current studies show that nano- and microplastics are mainly concentrated in the roots of fruits and vegetables, but also transferred to other parts of the plants [9]. It was recently demonstrated that < 3% of the administrated dose (5000 µg/L) of 244-nm europium-doped PS particles was transported to the shoots of wheat and lettuce [27]. Root vegetables might be particularly interesting to study, as their edible parts might contain a higher concentration of nanoplastics than other fruits and vegetables.
In many cases, it will not be possible to say whether nanoplastics were already in the food before processing or not. Examples are drinking water and beverages where nanoplastics could originate from freshwater sources or be released from plastic pipes, processing equipment, or the bottle. Similarly, microplastics detected in 23 milk samples from Mexico were likely released from damaged filtration membranes used in the dairy industry rather than being already present in the cow’s milk [28].
How can FFF help with the analysis of nanoplastics in food?
FFF for analysis of pure nanoplastics
The FFF community has typically used PS (latex) nanospheres for channel calibration, and separation of this type of “nanoplastics” can be easily achieved. While most work of recent years (related to analysis of particles) has focused on metal and metal oxide nanoparticles, a few studies on plastic particles do exist. Contado et al. used symmetric flow and sedimentation field flow fractionation combined with online UV absorption detection for size characterization of biodegradable polylactic acid (PLA) nanospheres with sizes of 90, 220, 300, and 390 nm [17]. Gigault et al. investigated PS-based nanoplastics by AF4 coupled to UV–vis spectroscopy and multi-angle light scattering (MALS) [29]. In a study by Paul et al., AF4 in combination with online MALS and dynamic light scattering (DLS) was used to characterize 25-nm poly(methyl methacrylate) (PMMA), 250-nm PLA, and 366-nm melamine formaldehyde nanoplastics [30].
One general challenge of FFF are particle-membrane interactions, which might be overcome by carefully adjusting the pH and ionic strength of the carrier liquid as well as by the addition of surfactants [15]. Typical surfactants for FFF are sodium dodecyl sulfate and the commercial detergent mixes FL-70 (Fisher Scientific) and NovaChem (Postnova). Exemplifying this, polydisperse PE nanoplastics (< 500 nm) could not be separated in 0.47 mM NaHCO3 buffer as used for PS spheres, most likely due to their hydrophobic character [31]. Changing the carrier liquid to 0.025% (v/v) FL-70 allowed separation of the PE nanoplastics. The most commonly used membrane materials are polyethersulfone and regenerated cellulose.
Another parameter of FFF that needs to be controlled is the mass of sample (ng to µg) that can be injected before “overloading” phenomena are observed. In combination with the sample dilution that occurs during fractionation, a low injected mass might result in nanoplastic concentrations too low for the online detectors and/or potential offline analysis. Semi-preparative channels allow the injection of larger sample amounts. In addition, instrumental solutions exist where the sample dilution is reduced about tenfold via an additional outlet flow at the end of the channel that is actively regulated [16].
The principal challenges in method development for FFF are described, e.g., in [15].
FFF for analysis of nanoplastics in complex matrices including food
Drinking water and some beverages might be injected directly into FFF. For semi-solid and solid foods, some form of sample preparation will be required to convert the sample into a suspension (Fig. 2). The sample preparation method will depend on the composition of the food and needs to assure that the nanoplastics are not significantly altered. Protocols for microplastics, using strong acids/bases or oxidants, are most likely not directly applicable to nanoplastics as these are expected to be more vulnerable to chemical degradation due to their high surface area to volume ratio [14]. Enzymatic digestion, as a relatively mild procedure, appears to be a promising approach [31], but is matrix-specific and leaves some of the matrix in the form of particulates in the nanosize range [32].
So far, very few studies exist that attempted to apply FFF for analyzing nanoplastics in a complex matrix. Wahl et al. made use of AF4 when analyzing nanoplastics (concentrations not reported) in soil amended with plastic debris [19]: AF4 was used prior to analysis by pyrolysis gas chromatography-mass spectrometry (pyrGC-MS) (for nanoplastic identification) to remove soil organic matter that remained after the soil–water extraction. Nanoplastic sizes were determined by online MALS detection. Hydrodynamic sizes were further calculated from AF4 retention times. Valsesia et al. made use of MD-AF4 mainly as a preparative technique for analyzing 100-nm PS spheres in tunicates after enzymatic digestion [32]. No proper separation of the PS spheres was achieved, most likely due to channel overloading. Online UV and MALS were used to determine the size of the eluting fractions. Approximate particle number concentrations were determined by SEM after spotting of the fractions on a surface-functionalized chip (limit of detection/LOD 106 particles/organism, corresponding to 1 ng/g) and nanoplastics chemically identified by confocal Raman microscopy.
Correia et al. tested the applicability of MD-AF4 to analyze either 100 nm PS spheres or polydisperse PE nanoplastics (< 500 nm) spiked to European seabass followed by enzymatic digestion [31]. Separation and online size determination by MALS were possible for the PS spheres in the enzymatically digested fish but not for the PE nanoplastics due to a high background light scattering signal of the spiked and non-spiked fish. It was suggested that one or more of the components present in FL-70, which was used in the carrier liquid, interacted with organic residues from the enzymatic digestion. The LOD for the 100-nm PS spheres in fish based on the 90° light scattering signal was 52 μg/g fish. Combining this with an analytical method for identification of the nanoplastics’ chemical composition was not attempted in the study, but offline analysis by spectroscopy or spectrometry techniques was suggested.
These studies demonstrate the potential of FFF when used in combination with online detectors and offline analysis. In the following section, we will discuss suitable online detectors in more detail.
Suitable online detectors for FFF for nanoplastic analysis
Classical online detection techniques for FFF are UV–vis spectroscopy, DLS, MALS, and inductively coupled plasma-mass spectrometry (ICP-MS) (Table 1). DLS and MALS allow, besides detection, an online determination of particle size (characterization). In principle, AF4 theory permits the determination of hydrodynamic diameter from the retention time, but higher accuracy and significantly more information are obtained by direct determination with MALS and DLS [16]. MALS is typically applicable for particles with diameters > 20 nm (non-isotropic scatterers) and online DLS for particles between 1 and 100 nm. Both techniques are applicable for nanoplastics but require sufficiently high concentrations. Coupling of FFF and nanoparticle tracking analysis (NTA) is possible if a flow splitter is applied [33] and has been successfully demonstrated for a mixture of 50-, 100-, and 200-nm PS beads. NTA allows size determination and more importantly particle counting down to particle sizes of ~ 30 nm.
AF4-ICP-MS has primarily been used for metal and metal oxide nanoparticles. In general, ICP-MS is rarely used for carbon determination due to the high ionization potential of carbon, which results in low ionization efficiency leading to high limits of detection (LOD), and due to high carbon background levels [34]. Nischwitz et al. used AF4-ICP-MS to successfully detect and quantify 21-, 100-, 250-, and 740-nm PS spheres (injected mass 100 µg) based on the 12C-signal [35]. The same researchers coupled quadrupole ICP-MS or an organic carbon detector (OCD) to AF4 for analyzing particulate carbon in freshwater samples and obtained LODs for carbon of 1.4 mg/L for AF4-ICP-MS and 0.08 mg/L for AF4-OCD [34]. Mowla et al. quantified mixtures of 50-, 100-, 200-, and 500-nm PS spheres by AF4 coupled to a total organic carbon (TOC) detector [36]. The limit of quantification (LOQ) was estimated to be < 0.3 µg injected mass for all particle sizes. In a mixture with clay (inorganic colloid) and humic acid (dissolved organic matter), the TOC detector maintained the sensitivity to the nanoplastics without interference from the co-eluting clay. Further, online fluorescence detection was applied after Nile Red staining of the nanoplastics but a highly variable response with particle size and an anomalously low dye uptake by the 200-nm particles was observed. It must be kept in mind that ICP-MS, TOC analyzer, and OCD allow distinguishing carbon-based from inorganic particles/colloids, but they do not provide information about the nature of the measured carbon. If the presence of any other carbon-based particle can be excluded (e.g., by suitable sample preparation), detection and quantification of nanoplastics would be possible.
Schwaferts et al. coupled AF4 and centrifugal FFF not only to UV and MALS, but also Raman micro-spectroscopy, to analyze the size and composition of monodisperse 200–600-nm PS and 500-nm PMMA nanoplastics [37]. Low Raman scattering was overcome by trapping the nanoplastics with 2D optical tweezers. The setup enabled identification of 200-nm PS particles at number concentrations of 4.5 × 1013 particles/L (200 mg/L). The authors conclude that it will be necessary to validate the application range of the technique to preprocessed samples and demonstrate its applicability for real-world samples and that in many cases a concentration step will be vital to provide a sufficiently high particle number.
Considering the limitations of the existing online detectors, there appears to be a need for finding additional techniques that are suitable for online coupling to FFF. These techniques should be compatible with the carrier liquids used for AF4 (water containing surfactants or aqueous buffers). Their sensitivities need to be sufficient for the expected mass/number concentrations of nanoplastics, and they need to work at a certain flow rate. The typical flow rates of AF4 are around 0.5–1 mL/min but could be further reduced by a flow split or a solution that retains the nanoplastics, like the previously described optical tweezers.
There are various potential online techniques based on mass spectrometry. ICP-MS has already been mentioned as a technique used for coupling to FFF. A modification of “classical” ICP-MS is single-particle ICP-MS (spICP-MS), which has been widely applied to the analysis of inorganic nanoparticles due to its high sensitivity, selectivity, and fast analysis and which has also shown potential for the detection, size characterization, and mass/number quantification of larger nanoplastics (depending on the definition) and microplastics in the size range of 0.62 to 5 µm with limits of detection down to 105 particles/L when monitoring the 12C [38] or 13C signal [39, 40]. spICP-MS has, e.g., been applied for the detection of 1- to 5-µm PS particles in ultrapure water [39, 40] and seawater [38] as well as for the screening of 1- to 5-µm microplastics released from plastic teabags [40]. The detection of smaller nanoplastics by spICP-MS via monitoring of the carbon signal can be limited by the high carbon background typically present in food matrices and surfactants used in a carrier liquid and/or by the interferences from the co-existing carbon-containing particles. As an alternative, labeling of nanoplastics with metal probes (e.g., nanoparticles, ions, and organometallic compounds), followed by spICP-MS detection of the corresponding metal, could be a more sensitive and selective approach [41]. Using this approach, nanoplastics that were separated from samples and in situ labeled with gold nanoparticles were counted in environmental waters with sizes down to 50 nm [42]. Barber et al. demonstrated the coupling of asymmetric flow and centrifugal FFF with spICP-MS for characterizing gold nanoparticles contained in nanoplastic colloids (polystyrene-block-poly(acrylic acid)) [43].
Since the high-energy plasma of the ICP-MS atomizes plastic particles, information regarding their chemical identity is lost. Milder ionization techniques would allow for the detection of marker molecules and therewith the determination of a plastic particle’s chemical identity. Regarding this, interesting results have been achieved in the field of aerosol analysis. Costa Vera et al. used commercial PS latex spheres with mean diameters of 196, 496, 806, 966, and 1495 nm as size calibration standards for their single aerosol particle bipolar time-of-flight instrument (LAMPAS 2) [44]. They were able to receive mass spectra for both polarities featuring smaller hydrocarbon ions; however, due to the ionization technique (UV-laser), no intact styrene monomers were generated. The mass spectra received from this setup did not, for various reasons, allow for a size correlation via signal intensity. However, this work proves that even sub-micrometer particles of relevant plastic types (here PS) can be characterized by advanced mass spectrometers. Given a successful size separation via an FFF-related technique and a suitable ionization source, particle size, chemical identity, and particle numbers could be determined in a single run. A promising design for a suitable ion source might be “laser spray,” which might be seen as a hybrid of electrospray and matrix-assisted laser desorption/ionization (MALDI) [45].
Potential offline techniques for analysis of nanoplastic-containing fractions collected from FFF
In addition to online analysis, fractions from FFF can be collected, either manually or automatically, using a fraction collector. The typical sample volumes can be expected to be in the milliliter range, depending on the flow rate used for the separation and the fraction of interest. Any of the techniques listed in Table 1 and many more are applicable. The listed techniques require a varying degree of resources, and not all will be suitable for screening large numbers of samples.
Most techniques will benefit from the reduced sample complexity, as potential interferences are removed or at least reduced. As an example, pyrGC-MS was demonstrated to be applicable for detecting PS and PMMA in tissues of aquatic animals (i.e., potential seafood) after alkaline digestion and protein precipitation [46]. However, the authors of the study highlight that remaining organic matters may affect the pyrGC-MS signal of micro- and nanoplastics, in particular when the polymers only produce one indicator compound.
Potential issues with surfactants used in the carrier liquid have to be considered in relation to the offline techniques. As examples, low degradation temperatures (lower than for the plastics, i.e., < 500/600 °C) would be favorable for pyrGC-MS whereas formation of micelles should be avoided for light scattering–based techniques. A washing step after the FFF separation could be useful to remove the surfactants. A concentration step, like solvent evaporation, centrifugation, or centrifugal ultrafiltration, might be required if the nanoplastic concentration is too low.
Whereas size information and carbon concentration might be retrieved from MD-FFF, the currently only commercially available online detection system, an optical trapping-based Raman flow cell, for the identification of nanoplastics has still to demonstrate its applicability to real-world samples [37]. Potential offline techniques that could be applied for the purpose of nanoplastic identification are the aforementioned pyrGC-MS, liquid chromatography coupled to high-resolution-mass spectrometry (LC-HRMS), nanostructured laser desorption/ionization time-of-flight mass spectrometry (NALDI-TOF–MS), and thermal desorption-proton transfer reaction-mass spectrometry (TD-PTR-MS). Schirinzi et al. separated PS particles from aqueous media by filtration and dissolved/extracted them in toluene [47]. After concentrating the solution, detection limits of around 30 pg/L in LC-HRMS were achieved. Replacing the filtration or rather introducing a size separation via FFF might create a powerful tool without significantly changing the sample preparation and analysis time. Wang et al. used NALDI-TOF–MS to analyze trace amounts of plastics in snow with reported LODs of 100 pg for PE down to 5 pg for PEG [48]. TD-PTR-MS was applied for the analysis of nanoplastics in water sampled from a Greenland firn core and an Antarctic Sea ice core after filtration of the samples through a 200-nm filter [21]. The calculated LOD for PS was 0.34 ng.
For further characterization of the nanoplastics besides size, like shape and porosity, electron microscopy appears to be particularly suitable.
Outlook: How could the future of nanoplastics analysis in food involving FFF look like?
As mentioned before, the ideal method should allow detection, characterization, identification, and quantification of nanoplastics in the food. It should be quick (i.e., allow high sample throughput), cheap, and sensitive. The sample preparation protocol would ideally be generic and independent of the type of food matrix and nanoplastic type. The separation system would allow injection of large sample volumes, online size determination followed by online identification, and quantification of the nanoplastics on a single-particle basis.
Although generic sample preparation protocols are favorable, they have not yet been either successfully developed or applied for engineered nanomaterials in complex matrices. Acid and alkaline approaches are more generic regarding the type of food matrix but might not be applicable for all types of nanoplastics. If the aim is to have a generic method for all types of nanoplastics, a certain specificity toward the food matrix probably has to be accepted. Sample amounts that can be processed might be limited, and therefore, pre-concentration and sample pooling is required. The perfect online identification/quantification system for nanoplastics, ideally also working on a single-nanoplastic basis, still needs to be developed.
An example of a more feasible and tiered approach for now could look like this:
(1) Dilution or, if necessary, enzymatic digestion of the food using proteases, lipases, and amylases depending on the composition of the food.
(2) Detection and size determination of nano-sized particulates in the samples by AF4 coupled to UV, MALS, DLS and OCD, or ICP-MS.
(3) Fraction collection (flow splitter before ICP-MS required), concentration by ultrafiltration, washing, and subsequent:
-
Characterization (size and shape) by electron or atomic force microscopy
-
Identification and quantification by a suitable MS method (e.g., pyrGC-MS, NALDI-TOF–MS, TD-PTR-MS)
With the increasing application in the field of nanoparticles and nanoplastics, AF4 could become a mainstream technique. This would further enhance instrumental and software developments and finally make AF4 accessible to a wider user base [16]. The analyst would benefit from novel channels that allow, e.g., easier membrane replacement, larger variety and/or better quality of FFF membranes (e.g., with reduced roughness), and intelligent software that provides the optimal separation parameters [16].
Finally, it should be mentioned that advanced analytical developments are not the only line of action to achieve a reliable assessment of risk related to nanoplastics in our food. Hazard identification and characterization studies can make use of labeled and, consequently, more easily analyzable nanoplastics. Here, it is key that the studied materials match the “real” nanoplastics in terms of polymer types, sizes, shapes, and surface properties. Exposure assessment can be supported by mechanistic studies with labeled particles as well. Finally, release studies for food contact materials could as a starting point make use of food simulants to reduce sample complexity.
References
EFSA Contam Panel (EFSA Panel on Contaminants in the Food Chain). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14:4501–31. https://doi.org/10.2903/j.efsa.2016.4501.
Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol. 2019;53:1748–65. https://doi.org/10.1021/acs.est.8b05512.
Paul MB, Fahrenson CC, Givelet L, Herrmann T, Loeschner K, Böhmert L, Thünemann AF, Braeuning A, Sieg H. Beyond microplastics - investigation on health impacts of submicro- and nanoplastic particles after oral uptake in vitro. Microplastics and Nanoplastics. 2022;2:16. https://doi.org/10.1186/s43591-022-00036-0.
Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of various microplastics in human stool. Ann Intern Med. 2019;171:453–7. https://doi.org/10.7326/M19-0618.
Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. https://doi.org/10.1016/j.envint.2022.107199.
European Commission (2022) Commission Recommendation of 10.6.2022 on the Definition of Nanomaterial. https://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm. Accessed 14 Jun 2022.
Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, Hess MC, Ivleva NP, Lusher AL, Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53:1039–47. https://doi.org/10.1021/acs.est.8b05297.
Toussaint B, Raffael B, Angers-Loustau A, Gilliland D, Kestens V, Petrillo M, Rio-Echevarria IM, Van den Eede G. Review of micro- and nanoplastic contamination in the food chain. Food Addit Contam Part A. 2019;36:639–73. https://doi.org/10.1080/19440049.2019.1583381.
Liu Q, Chen Z, Chen Y, Yang F, Yao W, Xie Y. Microplastics and nanoplastics: emerging contaminants in food. J Agric Food Chem. 2021;69:10450–68. https://doi.org/10.1021/acs.jafc.1c04199.
Ivleva NP. Chemical analysis of microplastics and nanoplastics: challenges, advanced methods, and perspectives. Chem Rev. 2021;121:11886–936. https://doi.org/10.1021/acs.chemrev.1c00178.
Alexy P, Anklam E, Emans T, Furfari A, Galgani F, Hanke G, Koelmans A, Pant R, Saveyn H, Sokull Kluettgen B. Managing the analytical challenges related to micro- and nanoplastics in the environment and food: filling the knowledge gaps. Food Addit Contam Part A. 2020;37:1–10. https://doi.org/10.1080/19440049.2019.1673905.
Schwaferts C, Niessner R, Elsner M, Ivleva NP. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. TrAC Trends Anal Chem. 2019;112:52–65. https://doi.org/10.1016/j.trac.2018.12.014.
Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, van Wezel AP. Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ Sci Nano. 2018;5:1640–9. https://doi.org/10.1039/C8EN00186C.
Chang Y-S, Chou S-H, Jhang Y-J, Wu T-S, Lin L-X, Soo Y-L, Hsiao I-L. Extraction method development for nanoplastics from oyster and fish tissues. Sci Total Environ. 2022;814:152675. https://doi.org/10.1016/j.scitotenv.2021.152675.
von der Kammer F, Legros S, Hofmann T, Larsen EH, Loeschner K. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. TrAC Trends Anal Chem. 2011;30:425–36. https://doi.org/10.1016/j.trac.2010.11.012.
Podzimek S, Johann C. Asymmetric flow field-flow fractionation: current status, possibilities, analytical limitations and future trends. Chromatographia. 2021;84:531–4. https://doi.org/10.1007/s10337-021-04035-w.
Contado C, Dalpiaz A, Leo E, Zborowski M, Williams PS. Complementary use of flow and sedimentation field-flow fractionation techniques for size characterizing biodegradable poly(lactic acid) nanospheres. J Chromatogr A. 2007;1157:321–35. https://doi.org/10.1016/j.chroma.2007.04.038.
Ter Halle A, Jeanneau L, Martignac M, Jardé E, Pedrono B, Brach L, Gigault J. Nanoplastic in the North Atlantic subtropical gyre. Environ Sci Technol. 2017;51:13689–97. https://doi.org/10.1021/acs.est.7b03667.
Wahl A, Le Juge C, Davranche M, El Hadri H, Grassl B, Reynaud S, Gigault J. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere. 2021;262:127784. https://doi.org/10.1016/j.chemosphere.2020.127784.
Materić D, Kasper-Giebl A, Kau D, Anten M, Greilinger M, Ludewig E, van Sebille E, Röckmann T, Holzinger R. Micro- and nanoplastics in Alpine snow: a new method for chemical identification and (semi)quantification in the nanogram range. Environ Sci Technol. 2020;54:2353–9. https://doi.org/10.1021/acs.est.9b07540.
Materić D, Kjær HA, Vallelonga P, Tison J-L, Röckmann T, Holzinger R. Nanoplastics measurements in northern and southern polar ice. Environ Res. 2022;208:112741. https://doi.org/10.1016/j.envres.2022.112741.
Hernandez LM, Xu EG, Larsson HCE, Tahara R, Maisuria VB, Tufenkji N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ Sci Technol. 2019;53:12300–10. https://doi.org/10.1021/acs.est.9b02540.
Li D, Shi Y, Yang L, Xiao L, Kehoe DK, Gun’ko YK, Boland JJ, Wang JJ,. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat Food. 2020;1:746–54. https://doi.org/10.1038/s43016-020-00171-y.
Zangmeister CD, Radney JG, Benkstein KD, Kalanyan B. Common single-use consumer plastic products release trillions of sub-100 nm nanoparticles per liter into water during normal use. Environ Sci Technol. 2022;56:5448–55. https://doi.org/10.1021/acs.est.1c06768.
Busse K, Ebner I, Humpf H-U, Ivleva N, Kaeppler A, Oßmann BE, Schymanski D. Comment on “Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea.” Environ Sci Technol. 2020;54:14134–5. https://doi.org/10.1021/acs.est.0c03182.
Clark NJ, Khan FR, Mitrano DM, Boyle D, Thompson RC (2022) Demonstrating the translocation of nanoplastics across the fish intestine using palladium-doped polystyrene in a salmon gut-sac. Environ Int 159: .https://doi.org/10.1016/j.envint.2021.106994
Luo Y, Li L, Feng Y, Li R, Yang J, Peijnenburg WJGM, Tu C. Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer. Nat Nanotechnol. 2022;17:424–31. https://doi.org/10.1038/s41565-021-01063-3.
Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC. Branded milks – are they immune from microplastics contamination? Sci Total Environ. 2020;714:136823. https://doi.org/10.1016/j.scitotenv.2020.136823.
Gigault J, El Hadri H, Reynaud S, Deniau E, Grassl B. Asymmetrical flow field flow fractionation methods to characterize submicron particles: application to carbon-based aggregates and nanoplastics. Anal Bioanal Chem. 2017;409:6761–9. https://doi.org/10.1007/s00216-017-0629-7.
Paul MB, Fahrenson C, Givelet L, Herrmann T, Loeschner K, Böhmert L, Thünemann AF, Braeuning A, Sieg H. Beyond microplastics - investigation on health impacts of submicron and nanoplastic particles after oral uptake in vitro. Microplastics and Nanoplastics. 2022;2:16. https://doi.org/10.1186/s43591-022-00036-0.
Correia M, Loeschner K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: possibilities, challenges and analytical limitations. Anal Bioanal Chem. 2018;410:5603–15. https://doi.org/10.1007/s00216-018-0919-8.
Valsesia A, Parot J, Ponti J, Mehn D, Marino R, Melillo D, Muramoto S, Verkouteren M, Hackley VA, Colpo P. Detection, counting and characterization of nanoplastics in marine bioindicators: a proof of principle study. Microplastics and Nanoplastics. 2021;1:5. https://doi.org/10.1186/s43591-021-00005-z.
Adkins GB, Sun E, Coreas R, Zhong W. Asymmetrical flow field flow fractionation coupled to nanoparticle tracking analysis for rapid online characterization of nanomaterials. Anal Chem. 2020;92:7071–8. https://doi.org/10.1021/acs.analchem.0c00406.
Nischwitz V, Gottselig N, Missong A, Meyn T, Klumpp E. Field flow fractionation online with ICP-MS as novel approach for the quantification of fine particulate carbon in stream water samples and soil extracts. J Anal At Spectrom. 2016;31:1858–68. https://doi.org/10.1039/C6JA00027D.
Nischwitz V, Gottselig N, Missong A, Klumpp E, Braun M. Extending the capabilities of field flow fractionation online with ICP-MS for the determination of particulate carbon in latex and charcoal. J Anal At Spectrom. 2018;33:1363–71. https://doi.org/10.1039/C8JA00101D.
Mowla M, Shakiba S, Louie SM. Selective quantification of nanoplastics in environmental matrices by asymmetric flow field-flow fractionation with total organic carbon detection. Chem Commun. 2021;57:12940–3. https://doi.org/10.1039/D1CC04852J.
Schwaferts C, Sogne V, Welz R, Meier F, Klein T, Niessner R, Elsner M, Ivleva NP. Nanoplastic analysis by online coupling of Raman microscopy and field-flow fractionation enabled by optical tweezers. Anal Chem. 2020;92:5813–20. https://doi.org/10.1021/acs.analchem.9b05336.
Gonzalez de Vega R, Goyen S, Lockwood TE, Doble PA, Camp EF, Clases D. Characterisation of microplastics and unicellular algae in seawater by targeting carbon via single particle and single cell ICP-MS. Anal Chim Acta. 2021;1174:338737. https://doi.org/10.1016/j.aca.2021.338737.
Bolea-Fernandez E, Rua-Ibarz A, Velimirovic M, Tirez K, Vanhaecke F. Detection of microplastics using inductively coupled plasma-mass spectrometry (ICP-MS) operated in single-event mode. J Anal At Spectrom. 2020;35:455–60. https://doi.org/10.1039/C9JA00379G.
Laborda F, Trujillo C, Lobinski R. Analysis of microplastics in consumer products by single particle-inductively coupled plasma mass spectrometry using the carbon-13 isotope. Talanta. 2021;221:121486. https://doi.org/10.1016/j.talanta.2020.121486.
Marigliano L, Grassl B, Szpunar J, Reynaud S, Jiménez-Lamana J. Nanoplastic labelling with metal probes: analytical strategies for their sensitive detection and quantification by ICP mass spectrometry. Molecules. 2021;26:7093. https://doi.org/10.3390/molecules26237093.
Lai Y, Dong L, Li Q, Li P, Hao Z, Yu S, Liu J. Counting nanoplastics in environmental waters by single particle inductively coupled plasma mass spectroscopy after cloud-point extraction and in situ labeling of gold nanoparticles. Environ Sci Technol. 2021;55:4783–91. https://doi.org/10.1021/acs.est.0c06839.
Barber A, Kly S, Moffitt MG, Rand L, Ranville JF. Coupling single particle ICP-MS with field-flow fractionation for characterizing metal nanoparticles contained in nanoplastic colloids. Environ Sci Nano. 2020;7:514–24. https://doi.org/10.1039/C9EN00637K.
Vera CC, Trimborn A, Hinz K-P, Spengler B. Initial velocity distributions of ions generated by in-flight laser desorption/ionization of individual polystyrene latex microparticles as studied by the delayed ion extraction method. Rapid Commun Mass Spectrom. 2005;19:133–46. https://doi.org/10.1002/rcm.1753.
Hiraoka K. Laser spray: electric field-assisted matrix-assisted laser desorption/ionization. J Mass Spectrom. 2004;39:341–50. https://doi.org/10.1002/jms.621.
Zhou X, Hao L, Wang H, Li Y, Liu J. Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography–mass spectrometry. Anal Chem. 2019;91:1785–90. https://doi.org/10.1021/acs.analchem.8b04729.
Schirinzi GF, Llorca M, Seró R, Moyano E, Barceló D, Abad E, Farré M. Trace analysis of polystyrene microplastics in natural waters. Chemosphere. 2019;236:124321. https://doi.org/10.1016/j.chemosphere.2019.07.052.
Wang Z, Saadé NK, Ariya PA. Advances in ultra-trace analytical capability for micro/nanoplastics and water-soluble polymers in the environment: fresh falling urban snow. Environ Pollut. 2021;276:116698. https://doi.org/10.1016/j.envpol.2021.116698.
Tian L, Skoczynska E, Siddhanti D, van Putten R-J, Leslie HA, Gruter G-JM. Quantification of polyethylene terephthalate microplastics and nanoplastics in sands, indoor dust and sludge using a simplified in-matrix depolymerization method. Mar Pollut Bull. 2022;175:113403. https://doi.org/10.1016/j.marpolbul.2022.113403.
Acknowledgements
M. Velimirovic is a senior postdoctoral fellow of the Research Foundation – Flanders (FWO 12ZD120N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loeschner, K., Vidmar, J., Hartmann, N.B. et al. Finding the tiny plastic needle in the haystack: how field flow fractionation can help to analyze nanoplastics in food. Anal Bioanal Chem 415, 7–16 (2023). https://doi.org/10.1007/s00216-022-04321-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04321-y