Abstract
The presence of benzothiazoles (BTHs) and organic ultraviolet filters (UV filters) in aquatic ecosystems has emerged as a significant environmental issue, requiring urgent and efficient determination methods. A new, rapid, and sensitive determination method using gas chromatography triple quadrupole mass spectrometer (GC–MS/MS) was developed for the simultaneous extraction and analysis of 10 commonly used BTHs and 10 organic UV filters in surface water, wastewater, sediment, and sludge. For aqueous samples, solid-phase extraction (SPE) method was employed with optimizing of SPE cartridge type, pH, and elution solvent. For solid samples, ultrasonic extraction-solid-phase extraction purification (UE-SPE) and pressurized liquid extraction (PLE) methods were compared. And extraction conditions for ultrasonic extraction method (extraction solvents and extraction times) and PLE method (extraction temperatures and extraction cycles) were optimized. The limits of quantification for the 20 target compounds in surface water and wastewater were 0.01–2.12 ng/L and 0.05–6.14 ng/L, while those for sediment and sludge with UE-SPE method were 0.04–5.88 ng/g and 0.22–6.61 ng/g, respectively. Among the 20 target compounds, the recoveries ranged from 70 to 130% were obtained for 16, 15, 15, and 15 analytes in the matrix-spiked samples of surface water, wastewater, sediment, and sludge with three levels, respectively. And the precision was also acceptable with relative standard deviation (RSD) below 20% for all analytes. The developed methods were applied for the determination and quantification of target compounds in surface water, sediment, wastewater, and sludge samples collected from two wastewater treatment plants (WWTPs) and the Pearl River in Guangzhou, China. BTHs were frequently detected in surface water and wastewater, while UV filters were mainly found in sediment and sludge. Benzotriazole (BT) and 2-hydroxybenzothiazole (2-OH-BTH) were the two major BTHs in influent wastewater and surface water, respectively, with concentrations up to 966 and 189 ng/L. As for sediment and sludge, 2-(2′-hydroxy-5′-octylphenyl)-benzotriazole (UV-329) was a predominant chemical, detected at concentrations of 111 and 151 ng/g, respectively.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benzothiazoles (BTHs) and organic ultraviolet filters (UV filters) are groups of high-production-volume chemicals with widespread applications in domestic and industrial activities [1,2,3]. Generally, BTHs are commonly used as biocides in paper and leather manufacturing, as corrosion inhibitors in antifreeze formulations, and as vulcanization accelerators in rubber production [1, 3]. Organic UV filters are composed of one or more benzene rings and/or carbonyl groups with high electron delocalization, exhibiting a high molar absorptivity in both UVA (320–400 nm) and UVB (280–320 nm) ranges [4]. They are primarily used in personal care products (i.e., sunscreens and cosmetics) to prevent skin damage, as well as in plastics, adhesives, and rubber to protect products against photodegradation and discoloring [2, 4]. Considering their massive and continuous application, BTHs and organic UV filters have directly entered the environment through human activities or uncontrolled spillages from industries, and indirectly through wastewater treatment plant (WWTP) discharges, which is the major pollution source of BTHs and UV filters into receiving environments [3, 5, 6]. The residues of BTHs and UV filters have been frequently detected in various environmental matrices, including wastewater [7, 8], surface water [9, 10], seawater [11, 12], sediment, and sewage sludge [13,14,15], with concentrations ranging from sub-ng/L to tens μg/L. Moreover, these two types of compounds can further accumulate in aquatic biota [16,17,18,19], humans [20,21,22], and are bio-transferred in food chains, posing endocrine-disrupting effects in aquatic fauna [2] and even carcinogenic effects in humans [3].
Due to their widespread presence and persistence in the environment, as well as potential adverse effects on human and ecosystem, BTHs and organic UV filters are considered as emerging contaminants [1,2,3, 18, 21]. Hence, there is a growing need to develop reliable and efficient methods for their determination. And different extraction methods have been developed for the analysis of BTHs and organic UV filters in a variety of environmental matrices separately [4, 7, 11,12,13,14,15,16,17,18,19,20]. For aqueous samples, solid-phase extraction (SPE) is the most frequently used method, due to its simple and easy to be performed [4, 8, 9], while for solid samples, the most frequently employed extraction method is pressurized liquid extraction (PLE), and microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UE) are also suitable options [4, 10, 19]. Given their relatively low concentrations in the environmental samples, extraction methods are of great importance in the sample preparation.
Additionally, sensitive determination techniques are also required due to the complexity of environmental matrices, and therefore, chromatographic techniques are undoubtedly the most appropriate tools for the determination of BTHs and organic UV filters. Generally, UV filters are analyzed using gas chromatography (GC) coupled to mass spectrometry (MS) or triple quadruple mass spectrometry (MS/MS), whereas the analyses of BTHs are mostly conducted via liquid chromatography (LC) or ultra-high performance liquid chromatography (UHPLC) coupled with different detectors [4, 11, 14, 19]. Compared with GC–MS and LC–MS/MS, GC–MS/MS has been widely applied for the analysis of trace organic contaminants, due to its high selectivity and sensitivity of multiple reaction monitoring (MRM) techniques in tandem mass spectrometry, and reduced matrix effects and interferences [9, 23, 24]. Based on their different chemical properties, most previous studies preferred to develop methods for separate analysis of BTHs and organic UV filters [3,4,5]. Although these methods have been demonstrated to exhibit acceptable performance, separate analysis needs a large number of samples and long periods of time, leading to high costs. However, BTHs are generally co-occurrence with organic UV filters in the environment, due to their commonly using in personal care products. To our knowledge, the simultaneous determination of BTHs and organic UV filters in a variety of matrices using GC–MS/MS has not been reported. To better investigate the occurrence of various BTHs and organic UV filters in the environment, it is necessary to further optimize and improve our previous method [25], and broad its application for more types of target analytes.
The main objective of this study was to develop an analytical method with GC–MS/MS for the simultaneous determination of 10 BTHs and 10 organic UV filters in various environmental matrices, including surface water, wastewater, sediment, and sludge. To obtain optimum recoveries for all target compounds, various SPE operating parameters (SPE cartridge type, pH, and elution solvent) for aqueous samples were optimized, whereas UE-SPE operating conditions (extraction solvent and extraction times) and PLE operating conditions (extraction solvent, extraction temperature, and extraction cycles) for solid samples were tested. The performance of the proposed method was evaluated in terms of its accuracy and precision, limits of detection (LODs), limits of quantification (LOQs), and matrix effects. Finally, to evaluate the applicability of the developed method, the pollution levels of target compound were determined in samples collected from two WWTPs and the Pearl River in Guangzhou, China.
Methods and materials
Chemicals and materials
High-purity standards of 10 BTHs (BTH (97%), 2-methylbenzothiazole (2-MeBTH, ≥ 98%), 2-aminobenzothiazole (2-ABTH, 98%), 2-methylthio-benzothiazole (2-Me-S-BTH, 97%), 2-hydroxybenzothiazole (2-OH-BTH, ≥ 98%), N-tert-butylbenzothiazole-2-sulphenamide (TBBS, 97%), 2-mercaptobenzothiazole (2-SH-BTH, 97%), benzothiazide (BTHZ, 93.6%), N,N-dicyclohexyl-2-benzothiazolesulfonamide (DCBS, ≥ 95%), and bezotriazole (BT, 98%)), and 10 organic UV filters (2-ethylhexyl salicylate (EHS, 99.4%), homosalate (HMS, 98.8%), benzophenone-3 (BP-3, ≥ 98%), 3-(4-methylbenzylidene) camphor (4-MBC, 99%), 2-(2′-hydroxy-5′-methylphenyl) benzotriazole (UV-P, ≥ 99%), 2-ethylhexyl 4-methoxycinnamate (EHMC, 99.4%), 2-(3-t-butyl-2-hydroxy-5-methylphenyl)-5-chlorobenzotriazole (UV-326, ≥ 98%), 2-(2′-hydroxy-5′-octylphenyl)-benzotriazole (UV-329, 99%), 2-(2-hydroxy-3,5-dipenryl-phenyl) benzotriazole (UV-328, ≥ 98%), and 2,4-di-tert-butyl-6-(5-chloro-2H-benzotriazol-2-yl) phenol (UV-327, ≥ 98%)) were purchased from Sigma Aldrich (St. Louis, MO, USA). Benzotriazole-d4 (purity > 98%), benzyl cinnamate (purity > 98%), chrysene-d12 (purity > 98%), and 13C-2-hydroxy-4-methoxybenzophenone (purity > 98%) served as internal standards (ISs) for the quantification of BTHs and organic UV filters, and were purchased from Toronto Research Chemicals (North York, Canada), CNW Technologies (Düsseldorf, Germany), Dr. Ehrenstorfer GmbH (Augsburg, Germany), and Accustandard Inc. (New Haven, USA), respectively. The chemical structures and basic physicochemical properties of the 20 target compounds and four isotope-labeled ISs are summarized in Table S1.
The HPLC-grade reagents acetonitrile (ACN), dichloromethane (DCM), ethyl acetate (EtAC), methanol (MeOH), and n-hexane (n-Hex) were purchased from Merck (Düsseldorf, Germany). Formic acid (HPLC grade, purity ≥ 95%) was acquired from ANPEL Laboratory Technologies (Shanghai, China). The glass fiber filters (GF/F, 0.7 μm) were purchased from Whatman (Maidstone, UK). The C18 (500 mg, 6 mL) cartridges were purchased from Supelco Inc. (Bellefonte, USA), while Oasis HLB (500 mg, 6 mL) cartridges were purchased from Waters (Milford, USA).
Sample collection
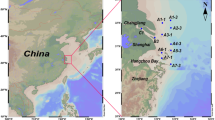
For method development and validation, surface water and sediment samples were collected from the Liuxi Reservoir, Conghua District of Guangzhou, whereas wastewater and sludge samples were collected from the Liede WWTP in Guangzhou, China. Additionally, for the method application, surface water and sediment samples were collected from the Pearl River in Guangzhou, China, while wastewater and sludge samples were collected from two WWTPs in Guangzhou, China. Wastewater were collected as 24 h flow integrated composite samples, while the surface water and sediment were collected as one-time grabbed samples. Three replicates were collected for each type of sample at each sampling site. Water samples were collected in pre-cleaned brown glass bottles, and 50 mL of methanol (v/v = 5%) was separately added to inhibit microbial activity. Sediment and dewatered sludge samples were collected in glass jars, and approximately 2 g of sodium azide was added to suppress microbial activity [26]. All collected samples were transported to the laboratory in cooler as quickly as possible and stored at 4 °C, then processed within 48 h. The sediment and sludge samples were freeze-dried, and sieved through a 100-mesh standard sieve, and then kept at − 20 °C in the dark prior to extraction. The workflow chart for the established sample extraction method is presented in Fig. S1.
Extraction for aqueous samples
The collected surface water and wastewater samples were filtered through glass fiber filters (0.7 μm, Whatman, GF/F, UK) to remove suspended particles. Subsequently, 1 L of water samples were extracted by SPE with cartridges. Due to the wide range of log Kow of target compounds and based on the previous studies [5, 11, 25, 27], the SPE method for water samples was optimized by testing two SPE cartridges (Oasis HLB 6 mL, 500 mg and Supelco C18 6 mL, 500 mg), two pH values (3 and 7), and six combined elution solvents (5 mL MeOH + 5 mL DCM; 5 mL MeOH + 5 mL EtAC; 5 mL DCM + 5 mL ACN; 5 mL DCM + 5 mL n-Hex; 5 mL MeOH + 5 mL n-Hex; 5 mL EtAC + 5 mL n-Hex) using Milli-Q water.
The optimized SPE method was described as follows. Prior to use, the Oasis HLB cartridges were preconditioned with 10 mL of methanol and 10 mL of Milli-Q water in sequence. The filtered water samples (1 L) were adjusted to pH 3 with 4 M H2SO4 and spiked with 100 ng of mixed ISs, and then loaded into the HLB cartridges at a flow rate of 5–10 mL/min. After loading the water samples, each sample bottle was consecutively washed twice with 50 mL of MeOH:water (v/v = 5:95), which also passed through the HLB cartridges.
Subsequently, the cartridges were dried under vacuum for 2 h, and target compounds retained on the cartridges were eluted with 5 mL of MeOH and 5 mL of DCM. The combined eluents were evaporated to dryness with a gentle stream of nitrogen, reconstructed in 1 mL of DCM, and filtered through a 0.22-μm nylon membrane into a 2-mL amber glass vial, then stored at − 20 °C for further analysis.
For recovery studies, surface water collected from Liuxi Reservoir and wastewater collected from Liede WWTP were spiked with standards at three levels of 20, 50, and 100 ng/L, respectively. The spiked samples were treated with the same method for water samples as described above.
Extraction for solid samples
Ultrasonic extraction-solid-phase extraction purification (UE-SPE) and PLE methods are two commonly used techniques for the extraction of emerging contaminants from solid samples; so these two extraction methods were optimized and compared in the present study.
Based on the previous studies [27, 28], extraction solvents (MeOH/DCM (v/v, 50:50), MeOH/EtAC (v/v, 50:50), DCM/ACN (v/v, 50:50), DCM/n-Hex (v/v, 50:50), MeOH/n-Hex (v/v, 50:50), and EtAC/n-Hex (v/v, 50:50)) and extraction times (2 and 3 times) were further optimized for the development of the UE-SPE method in this study. The optimized UE-SPE method was given as follows. Freeze-dried sediment (2 g) and sludge samples (1 g) were separately weighed into 30-mL glass tubes, followed by spiking 100 ng of mixed ISs, and then mixed and kept at 4 °C in the dark overnight. Fifteen milliliters of DCM/ACN (v/v, 50:50) were added into each glass tube, mixed on a vortex for 30 s, and ultrasonicated for 15 min, then centrifuged at 4,000 rpm for 10 min. The extracted procedure was repeated twice, and all supernatants were combined into a pre-cleaned round bottom flask. Subsequently, the extracts were evaporated at 50 °C to remove organic solvents and diluted to 1,000 mL with Milli-Q water (add citric acid to adjust the pH to 3). The eluted extracts were transferred into HLB cartridges (6 mL, 500 mg) for enrichment and purification of target compounds. The elution and reconstitution procedures were the same as those used for water samples.
For the development of the PLE method, a Speed Extractor E-916 (BUCHI Labortechnik AG, Flawil, Switzerland) was used. Three parallel freeze-dried samples (2.0 g of sediment samples or 1.0 g of sludge samples) were weighed into a 40-mL stainless steel extraction cell, and quartz sand was added as dispersing agent. Referring to the previous study [25], 1.0 g of sodium sulfate, 1.0 g of silica, and 1.0 g of copper powder were used as sorbents for in-cell cleanup. Prior to extraction, 100 ng of mixed ISs was added to the extraction cells to monitor the recoveries of the target compounds. Based on the results of the UE extraction, DCM/ACN (v/v, 50:50) was employed as extraction solvent for the development of the PLE method. Extraction temperatures (80 and 120 °C) and extraction cycles (2 and 3 cycles) were considered optimal. The extracts were evaporated by a BUCHI Multivapor Syncore Polyvap for 5 min, transferred into glass tubes, and concentrated to near dryness under a gentle stream of nitrogen; subsequently, they were reconstructed in 1 mL of DCM, filtered through a 0.22-μm nylon membrane into a 2-mL amber glass vial, then stored at − 20 °C for further analysis.
Recovery of target compounds in sediment collected from Liuxi Reservoir were examined at 20, 50, and 100 ng/g, while those for sludge from WWTP were examined at 10, 50, and 100 ng/g, respectively.
GC–MS/MS identification and quantification
In the present study, the target BTHs and organic UV filters were analyzed by GC–MS/MS (Agilent 7890BGC–7000D series triple quadrupole mass spectrometer) equipped with electron impact ionization (EI) source. Chromatographic separation was performed with an Agilent DB-5MS (30 m × 0.25 mm, 0.25 μm) capillary column with the injection volume of 1 µL in splitless mode. Helium (purity 99.999%) was used as carrier gas at a flow rate of 1.0 mL/min. The GC oven temperature was set as follows: 60 °C held for 1 min, followed by increases to 170 °C at 40 °C/min and to 300 °C at 10 °C/min (held for 11.25 min). The temperatures of the MS quadrupole, ion source, and injection port were set to 150, 280, and 280 °C, respectively. The collision gas was nitrogen at a flow rate of 1.5 mL/min, and the quenching gas was helium at a flow rate of 2.25 mL/min. Quantitative analysis of target compounds was conducted in the MRM mode. The optimized MRM conditions of the 20 target compounds are presented in Table S2. Data acquisition was performed using the Agilent MassHunter (Ver. B.09.00) software.
The identification of each target compound was conducted by comparing the retention times (within ± 2%) and the ion ratios (within ± 20%) between samples and calibration standards. The internal standard method was employed for quantitative analysis, using the calibration standards containing serial concentrations (1.00, 5.00, 10.0, 50.0, 100, 200, 500, and 1,000 μg/L) and a uniform concentration (100 μg/L) of four isotope-labeled ISs. Quality assurance and control assessments (QA/QC) were strictly performed during the identification and quantification processes of target compounds in this study.
Method validation
The method validation was performed by evaluating its accuracy and precision, LODs, LOQs, and matrix effect (ME), based on the US-FDA guideline [29]. The accuracy of the method was estimated as the mean recovery of each target compound in the native spiked samples. As shown in Eq. (1), the accuracy (R, %) was calculated as the percentage of the measured concentration (corrected by those in procedure blank samples) relative to the spiked concentration for each target compound [30]. The precision of the method, referred as the repeatability (intra-day variability), was calculated as the relative standard deviation (RSD) of three replicate analyses at three levels, respectively. According to the US-FDA guideline [29], the extent of recovery of an analyte and of the IS should be consistent, precise, and reproducible. Additionally, the RSD should be within 20%. The equation was as follows:
where \({C}_{\mathrm{ss}}\) and \({C}_{\mathrm{b}}\) were the measured concentrations of each target compound in the spiked samples and procedure blank samples, respectively, and \({C}_{\mathrm{s}}\) was the spiking concentration of each target compound.
Generally, LODs and LOQs were calculated to check the sensitivity of the developed method for target compounds. LODs and LOQs were defined as 3 and 10 times of the signal-to-noise ratio (S/N) under the lowest spiked concentration of calibration curve, respectively. The S/N ratios were obtained from data of the recovery experiment with the software MassHunter B.09.00 (Agilent, USA).
The ME expressed as the ratio of the analyte concentration obtained in matrix sample to the concentration in solvent, which was tested with spiking concentration of 100 µg/L in this study. The value of ME was calculated using Eq. (2) [23]:
where \({S}_{\mathrm{spiked}}\) and \({S}_{\mathrm{non}-\mathrm{spiked}}\) were the measured concentrations (with IS correction) of an analyte in the spiked final extract and the corresponding non-spiked samples, and \({S}_{\mathrm{solvent}}\) was the spiking concentration of each analyte; ME > 100% or ME < 100% indicated signal enhancement or suppression, respectively.
Considering background contamination commonly occurred in the determination of BTHs and organic UV filters at environmental levels, several measures were taken to minimize potential contamination [27]. Personnel conducting the samplings and experiments were not allowed to wear cosmetics and body lotions possibly containing target analytes. Meanwhile, nitrile surgical gloves were used throughout the whole experiment. All glassware was previously washed up, and baked at 450 °C for 4 h, then further sequentially rinsed with HPLC grade methanol and ultrapure water before use. Furthermore, procedural blanks and instrumental blanks were employed to track the potential background contamination, then the values of the target compounds in the blank samples were subtracted from environmental samples.
Results and discussion
Method development
Optimization of instrumental conditions
The instrumental conditions including mass spectrometry parameters and GC oven temperature program were optimized referring to the methodologies reported in the previous studies [18, 24, 25], to improve the separation of target compounds, sharpen the target peak, shorten the analytical time, and enhance their signal responses. Individual standards of analytes were applied to set optimal values of mass spectrometry parameters (i.e., ion transition and collision energy) for the detection of BTHs and organic UV filters in GC–MS/MS with MRM mode (Table S2). Two MRM transitions were required for each target compound, of which the more prominent ion transition was used for quantitation and the other one for qualitative analysis. Based on above results, mixed standards were injected to further modified GC oven temperature program. The total ion chromatograms (TIC) and the extracted ion chromatograms (EIC) for the 20 target compounds in standard solution at the concentration of 200 µg/L are presented in Fig. S2 and Fig. 1, respectively. Compared to the previous study [25], the 20 target compounds could be well separated with good peak shapes in a shorter period of 18 min.
Optimization of sample extraction
Aqueous samples
The SPE method is the most commonly employed approach for separately determining BTHs and organic UV filters in aqueous samples [4, 25]. In this study, various parameters such as water pH, SPE cartridges, and elution solvents were employed to optimize the simultaneous extraction of target BTHs and organic UV filters with the spiked concentration of 100 ng/L in Milli-Q water. As shown in Fig. 2a, the recoveries of target compounds could be affected by water pH value, using the same HLB cartridge and eluting with 5 mL of MeOH and 5 mL of DCM. Due to their specific functional groups, BTHs and organic UV filters were observed with obviously different ionization constant (pKa) values, exhibiting different adsorption behaviors in cartridges at pH 3 and pH 7. The recoveries for the 10 BTHs were between 50 and 150% at pH 3, whereas those for 2-Me-S-BTH and EHS were 0% at pH 7. However, the recoveries for the targeted organic UV filters were not obviously affected by pH, with exception of BTHZ, which had a recovery of < 40% at pH 7 and > 65% at pH 3. Consistent with the results of a previous study [25], water pH exhibited more obvious effects on the extraction of BTHs than organic UV filters, and acidic condition was more appropriate. Hence, water pH was adjusted to 3 prior to the extraction of target compounds with the SPE method.
In addition, previous studies have demonstrated that the extraction efficiency and reproducibility of target compounds can be improved by the selection of appropriate SPE cartridges [11, 17]. Hence, two commonly used SPE cartridges, namely C18 and HLB, were examined in this study. As shown in Fig. 2b, the HLB cartridge exhibited a good extraction efficiency for the 20 target compounds, when eluting with 5 mL of MeOH and 5 mL of DCM. However, a relatively low extraction efficiency (< 40%) for BTHs was observed with the C18 cartridge, especially for 2-MeBTH, BT, 2-Me-S-BTH, and EHS, with efficiencies of 0%. Due to their polar functional groups, BTHs exhibited better interactions with hydrophilic-lipophilic balanced reversed-phase sorbents in HLB cartridges than those in C18. Moreover, HLB cartridges exhibited stronger ability to present both hydrophobic and hydrophilic intermolecular forces to target analytes than C18 cartridges, proving to be suitable for the extraction of various types of compounds [31, 32]. Additionally, elution solvents also significantly influenced the recoveries of target compounds (Fig. 3). Among different elution solvents, only 5 mL of MeOH, combined with 5 mL of DCM, achieved satisfactory recoveries (50–136%) for all target compounds. The recoveries of BTHs were lower than 50% by the elution of DCM, MeOH, and EA combined with Hex, except for 2-SH-BTH; for 2-MeBTH, 2-Me-S-BTH, BT, and EHS, the recovery was 0%. Based on these results, the optimized SPE method for the detection of BTHs and UV filters from water samples is as follows: adjusting the pH to 3, then extracting the samples with HLB cartridge, and eluting with 5 mL of MeOH and 5 mL of DCM.
Solid samples
To maximize the recoveries of target compounds, two extraction methods were compared: UE-SPE and PLE. For UE-SPE, the SPE purification conditions were the same with those discussed for aqueous samples, whereas extraction solvents and extraction times for UE needed to be optimized. The influences of extraction solvents on the recoveries of target compounds extracted with three times are shown in Fig. 4a–f, suggesting that DCM/ACN (50/50, v/v) was more suitable than the other five groups. The recoveries of target compounds ranged between 50 and 150% using DCM/ACN (50/50, v/v) as extraction solvent, whereas those of at least 10 compounds were below 50% using other solvents. Additionally, extraction efficiency with different times (two and three times) was further conducted to evaluate whether comparable recoveries could be achieved with less volume of solvents. As shown in Fig. S4, the extraction efficiency of target compounds in solid samples significantly increased with increasing extraction times. The recoveries of 20 target compounds were 50–150% when extraction was performed three times, whereas those of 8 compounds were below 50 or even 0% when extraction was only performed two times. Therefore, the optimized UE method for solid samples is as follows: DCM/ACN (50/50, v/v) as extraction solvent and extraction for three times.
For PLE, the impacts of extraction solvents, extraction temperatures (80 and 120 °C), and extraction cycles (two and three cycles) were evaluated for the target compounds. As shown in Fig. S3, the recoveries of most target BTHs were unsatisfactory (below 50%) which were not markedly improved by changing extraction temperatures and extraction cycles, except for 2-SH-BTH. For organic UV filters, better performance (68–116%) was achieved under extraction temperatures of 120 °C and extraction two cycles, which was consistent with our previous study [25]. In some previous studies [25, 33, 34], PLE has been identified as a good alternative method for the extraction of BTHs and UV filters from environmental solid samples, with higher recoveries and lower LODs. However, PLE exhibited to be not suitable for the simultaneous determination of various BTHs and organic UV filters in this study, especially for BTHs. Compared with PLE, the recoveries of 20 target compounds extracted with UE-SPE method fell in the acceptable range. Therefore, optimized UE-SPE was selected as the most suitable extraction method for solid samples.
Method validation
Based on the quantitation of the internal standard method, calibration curves exhibited satisfactory linearity with correlation coefficients (R2) ≥ 0.993 (Table S2) for all target compounds, indicating that the acceptance criteria were met. The precisions of the method were also evaluated as intra-day repeatability (RSD) of target compounds in surface water, wastewater, sediment, and sludge, ranging from 0.57 to 18.6%, 0.30 to 16.7%, 0.66 to 14.6%, and 0.02 to 15.6%, respectively (Table S3).
Within the optimized extraction methods, the recoveries, LODs, LOQs, and matrix effects of aqueous samples and solid samples are summarized in Tables 1 and 2. Recoveries could be influenced by the loss of extraction procedure and effects of signal noises from various matrices, which were corrected by matrix effect for target analytes in Tables 1 and 2. Among the 20 target compounds, the recoveries ranged from 70 to 130% were obtained for 16, 15, 15, and 15 in the matrix-spiked samples of surface water, wastewater, sediment, and sludge with three levels, respectively. Most of the analytes were observed with recoveries ranging from 70 to 130% and RSD within 20%. The LODs of the 20 target compounds in surface water, wastewater, sediment, and sludge were 0.01–0.64 ng/L, 0.08–1.84 ng/L, 0.01–1.77 ng/g, and 0.07–1.91 ng/g, respectively. The LOQs of the 20 target compounds in surface water, wastewater, sediment, and sludge were 0.03–2.12 ng/L, 0.16–6.14 ng/L, 0.04–5.88 ng/g, and 0.22–6.61 ng/g, respectively. Hence, the developed analytical methods were highly sensitive for most of target BTHs and organic UV filters in various environmental matrices, with LODs below 2 ng/L. In comparison, the LODs of 2-MeBTH, 2-SH-BTH, 2-OH-BTH, and BT in surface water and wastewater samples were lower than those previously reported with the SPME method [23]. Moreover, the LODs of 4-MBC and EHMC in surface water samples were more than 10 times lower than those reported in the previous studies using dispersive liquid–liquid microextraction [24]. Upon using the optimized UE-SPE method in this work, the LODs of BT, BP-3, 4-MPC, EHMC, UV-326, and UV-329 in solid samples were lower compared with those obtained with PLE method [25]. Compared to our previous study [25], the optimized extraction methods were more sensitive for the simultaneous analysis of various BTHs and organic UV filters in a shorter time, providing acceptable recoveries. Thus, the proposed methods can be promoted and applied for the determination of BTHs and organic UV filters in different environmental matrices.
As shown in Tables 1 and 2, varied matrix effects were observed among the four matrices. Generally, the matrix effect intensities increased in the following order: surface water < wastewater, and sediment < sludge. MS signal suppressions were observed for most target analytes in aqueous samples, with the exception for 2-OH-BTH, 2-SH-BTH, BT, BP-3, and UV-P (ME > 120%). In contrast, MS signal enhancements were observed for most target analytes in solid samples, with the exception for TBBS, BTHZ, UV326, and UV329 (ME < 70%). Overall, the matrix effects of BTHs were lower than those of organic UV filters (Tables 1 and 2). Due to the complex composition in various matrices, MS signal suppressions or enhancements of BTHs and organic UV filters were also widely reported in the previous studies [25, 27]. However, it was difficult to take further cleanup steps to effectively eliminate matrix effects of the complex co-extracted components for these two groups of chemicals with significantly different physicochemical properties. To compensate for the matrix effects, the recoveries presented in Tables 1 and 2 were corrected with matrix effects.
Method application
To ensure the suitability of the validated method for monitoring target compounds in different environmental matrices, the proposed method was applied to simultaneously detect residual BTHs and organic UV filters in surface water and sediment collected from the Pearl River, influent wastewater, effluent wastewater, and sludge samples collected from two WWTPs in Guangzhou, China (Table 3). The detection rates for BTHs were high (> 80%) in wastewater samples, of which BT and 2-OH-BTH were detected in all collected samples, with concentrations up to 966 and 372 ng/L in influent wastewater samples, respectively. Due to their high-water solubility, remarkably, concentrations of BTHs have been reported from WWTPs in many countries, with the frequent detection of BT, 2-SH-BTH, and 2-OH-BTH in both influent and effluent [3]. Compared to BTHs, organic UV filters have been frequently detected in sludge samples, with concentrations of < LOQs — 151 ng/g, due to their high lipophilicity and stability against biotic degradation [11]. In particular, UV-326, UV-327, UV-328, and UV-329 (log Kow > 5) [5] were only detected in sludge samples, with concentrations ranging from 10.1 to 151 ng/g. In general, the concentrations of the 20 target compounds in the effluent wastewater were lower than those in the influent wastewater, indicating that part of them might be removed from water phase in WWTPs. However, multiple BTHs and organic UV filters were observed in the effluent and sludge, among which 2-OH-BTH and UV329 were detected with concentration levels up to 114 ng/L and 151 ng/g, respectively. Consistent with previous studies, effluents from WWTPs and also sewage sludge were the major sources of BTHs and organic UV filters in the environment due to the incomplete removal and eventual release, either through directly reusing for irrigation or as fertilizer in agriculture [2, 3, 5].
Also, most of the 20 target compounds were detected in samples collected from the Pearl River, except for 2-ABTH, 4-MBC, and DCBS, with the concentrations of < LOQs — 189 ng/L in surface water samples and < LOQs — 111 ng/g in sediment samples (Table 3). In this study, 2-OH-BTH and 2-SH-BTH were frequently detected in surface water samples, with concentrations up to 189 and 145 ng/L, respectively. The occurrence of 2-OH-BTH was reported in surface water samples from Catalonia, Spain, with a concentration of 45 ng/L; in urban runoff, Rhode Island, USA, the level was up to 691 ng/L [3]. As shown in Table 3, 2-OH-BTH has also frequently been detected in sediment, wastewater, and sludge samples, which might be related to its formation from other BTHs during photodegradation [3] or biodegradation [35]. Also, 2-SH-BTH, as the most widely used compound of vulcanization accelerators in rubber manufacturing, has frequently been detected in the environmental samples due to its extensive annual production and recalcitrance [3]. Regarding organic UV filters, the ones most frequently detected in sediment were BP-3 and EHMC, which was consistent with previous data from China [36, 37]. Compared to a previous study performed along the Pearl River [38], similar concentrations of BP-3 and EHMC and relatively lower concentrations of EHS and HMS were detected in this study. Additionally, UV-329 was also one of the predominant organic UV filters in Pearl River sediments, with concentrations higher than those reported from the West River and the North River [9]. Most likely, this can be explained by the extensive usage of BTHs and organic UV filters and their incomplete removal in WWTPs [1, 3, 6, 11]. Based on these results, it is crucial to use sensitive and timely detection methods for long-term investigations of BTHs and organic UV filters in aquatic environments.
In the present study, target BTHs and organic UV filters were frequently detected in surface water and sediment from Pearl River. Compare to the predicted no–effect concentration (PNEC) data showed in Table S1, higher levels of several organic UV filters such as UV-326, UV-328, and UV-329 in sediments were found and indicated that might lead to potential ecological risks. Although most of target analytes were found with concentrations lower than those of PNEC, their ubiquitous occurrence in the environment might lead to chronic exposure with unknown long-term effects. Hence, the potential ecological risks of target contaminants in the environment might not be ignored, and more ecotoxicological studies are needed for a better understanding of their environmental risks in the future.
Conclusions
In this study, we developed a series of sensitive methods which enabled simultaneously extract and detect 10 BTHs and 10 organic UV filters from a variety of environmental matrices including surface water, wastewater, sediment, and sludge. The optimized extraction methods involved SPE coupled with HLB cartridges for aqueous samples, and ultrasonic extraction combined with SPE purification for solid samples. All 20 target compounds could be simultaneously detected using GC–MS/MS, with a shorter analysis period of 18 min and lower LODs compared with previously published methods. The feasibility and robustness of the developed methods were confirmed by application in water and solid samples collected from the Pearl River and two WWTPs in Guangzhou, China. The proposed analytical methods are effective and practical for the detection of multiple target BTHs and organic UV filter trace residues in different environmental matrices, with concentrations up to several hundred ng/L or ng/g.
References
Shi ZQ, Liu YS, Xiong Q, Cai WW, Ying GG. Occurrence, toxicity and transformation of six typical benzotriazoles in the environment: a review. Sci Total Environ. 2019;661:407–21.
Carve M, Nugegoda D, Allinson G, Shimeta J. A systematic review and ecological risk assessment for organic ultraviolet filters in aquatic environments. Environ Pollut. 2021;268: 115894.
Liao C, Kim UJ, Kannan K. A review of environmental occurrence, fate, exposure, and toxicity of benzothiazoles. Environ Sci Technol. 2018;52:5007–26.
Chisvert A, Benedé JL, Salvador A. Current trends on the determination of organic UV filters in environmental water samples based on microextraction techniques—a review. Anal Chim Acta. 2018;1034:22–38.
Ramos S, Homem V, Alves A, Santos L. A review of organic UV-filters in wastewater treatment plants. Environ Int. 2016;86:24–44.
Giokas DL, Salvador A, Chisvert A. UV filters: from sunscreens to human body and the environment. Trends Anal Chem. 2007;26:360–74.
Murrell KA, Dorman FL. Characterization and quantification of methyl-benzotriazoles and chloromethyl-benzotriazoles produced from disinfection processes in wastewater treatment. Sci Total Environ. 2020;699: 134310.
Tsui MM, Leung HW, Lam PK, Murphy MB. Seasonal occurrence, removal efficiencies and preliminary risk assessment of multiple classes of organic UV filters in wastewater treatment plants. Water Res. 2014;53:58–67.
Hu LX, Cheng YX, Wu D, Fan L, Zhao JH, Xiong Q, Chen QL, Liu YS, Ying GG. Continuous input of organic ultraviolet filters and benzothiazoles threatens the surface water and sediment of two major rivers in the Pearl River Basin. Sci Total Environ. 2021;798: 149299.
Peng X, Xiong S, Ou W, Wang Z, Tan J, Jin J, Tang C, Liu J, Fan Y. Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J Hazard Mater. 2017;323:139–46.
Cadena-Aizaga MI, Montesdeoca-Esponda S, Torres-Padrón ME, Sosa-Ferrera Z, Santana-Rodríguez JJ. Organic UV filters in marine environments: an update of analytical methodologies, occurrence and distribution. Trends Environ Analy Chem. 2020;25: e00079.
Ku PC, Liu TY, Lee SH, Kung TA, Wang WH. An environmentally friendly strategy for determining organic ultraviolet filters in seawater using liquid-phase microextraction with liquid chromatography-tandem mass spectrometry. Environ Sci Pollut Res Int. 2020;27:9818–25.
Herrero P, Borrull F, Pocurull E, Marce RM. A quick, easy, cheap, effective, rugged and safe extraction method followed by liquid chromatography-(Orbitrap) high resolution mass spectrometry to determine benzotriazole, benzothiazole and benzenesulfonamide derivates in sewage sludge. J Chromatogr A. 2014;1339:34–41.
Herrero P, Borrull F, Marce RM, Pocurull E. A pressurised hot water extraction and liquid chromatography-high resolution mass spectrometry method to determine polar benzotriazole, benzothiazole and benzenesulfonamide derivates in sewage sludge. J Chromatogr A. 2014;1355:53–60.
Ramos S, Homem V, Santos L. Modified dispersive solid-phase extraction and cleanup followed by GC-MS/MS analysis to quantify ultraviolet filters and synthetic musk compounds in soil samples. J Sep Sci. 2021;44:3107–16.
Tsui MMP, Lam JCW, Ng TY, Ang PO, Murphy MB, Lam PKS. Occurrence, distribution, and fate of organic UV filters in coral communities. Environ Sci Technol. 2017;51:4182–90.
Huang YF, Chang JP, Chen HC, Huang YM. Simultaneous trace analysis of 10 benzophenone-type ultraviolet filters in fish through liquid chromatography-tandem mass spectrometry. Environ Pollut. 2021;286: 117306.
Jia J, Zhu Q, Liu N, Liao C, Jiang G. Occurrence of and human exposure to benzothiazoles and benzotriazoles in mollusks in the Bohai Sea, China. Environ Int. 2019;130: 104925.
Chen CH, Chung WH, Ding WH. Determination of benzotriazole and benzothiazole derivatives in marketed fish by double-vortex-ultrasonic assisted matrix solid-phase dispersion and ultrahigh-performance liquid chromatography-high resolution mass spectrometry. Food Chem. 2020;333: 127516.
Martin-Pozo L, Gomez-Regalado MDC, Cantarero-Malagon S, Navalon A, Zafra-Gomez A. Determination of ultraviolet filters in human nails using an acid sample digestion followed by ultra-high performance liquid chromatography-mass spectrometry analysis. Chemosphere. 2021;273: 128603.
Huang Y, Law JC, Lam TK, Leung KS. Risks of organic UV filters: a review of environmental and human health concern studies. Sci Total Environ. 2021;755: 142486.
Chen X, Zhou Y, Hu C, Xia W, Xu S, Cai Z, Li Y. Prenatal exposure to benzotriazoles and benzothiazoles and cord blood mitochondrial DNA copy number: a prospective investigation. Environ Int. 2020;143: 105920.
Naccarato A, Gionfriddo E, Sindona G, Tagarelli A. Simultaneous determination of benzothiazoles, benzotriazoles and benzosulfonamides by solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry in environmental aqueous matrices and human urine. J Chromatogr A. 2014;1338:164–73.
Ramos S, Homem V, Santos L. Simultaneous determination of synthetic musks and UV-filters in water matrices by dispersive liquid-liquid microextraction followed by gas chromatography tandem mass-spectrometry. J Chromatogr A. 2019;1590:47–57.
Liu YS, Ying GG, Shareef A, Kookana RS. Simultaneous determination of benzotriazoles and ultraviolet filters in ground water, effluent and biosolid samples using gas chromatography-tandem mass spectrometry. J Chromatogr A. 2011;1218:5328–35.
Zhou LJ, Ying GG, Liu S, Zhao JL, Chen F, Zhang RQ, Peng FQ, Zhang QQ. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A. 2012;1244:123–38.
Peng XZ, Jin JB, Wang CW, Ou WH, Tang CM. Multi-target determination of organic ultraviolet absorbents in organism tissues by ultrasonic assisted extraction and ultra-high performance liquid chromatography–tandem mass spectrometry. J Chromatogra A. 2015;1384:97–106.
Vila M, Llompart M, Garcia-Jares C, Dagnac T. Different miniaturized extraction methodologies followed by GC-MS/MS analysis for the determination of UV filters in beach sand. J Sep Sci. 2018;41(17):3449–58.
Guidance for Industry. Bioanalytical method validation guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). 2018.
Zhong Y, Chen ZF, Liu SS, Dai X, Zhu X, Zheng G, Liu S, Liu G, Cai Z. Analysis of azole fungicides in fish muscle tissues: multi-factor optimization and application to environmental samples. J Hazard Mater. 2017;324:535–43.
Asimakopoulos AG, Bletsou AA, Wu Q, Thomaidis NS, Kannan K. Determination of benzotriazoles and benzothiazoles in human urine by liquid chromatography-tandem mass spectrometry. Anal Chem. 2013;85:441–8.
Xu WH, Yan W, Licha T. Simultaneous determination of trace benzotriazoles and benzothiazoles in water by large-volume injection/gas chromatography–mass spectrometry. J Chromatogra A. 2015;1422:270–6.
Herrero P, Borrull F, Pocurull E, Marcé RM. An overview of analytical methods and occurrence of benzotriazoles, benzothiazoles and benzenesulfonamides in the environment. Trends Analyt Chem. 2014;62:46–55.
Vila M, Pablo Lamas J, Garcia-Jares G, Dagnac T, Llompart M. Optimization of an analytical methodology for the simultaneous determination of different classes of ultraviolet filters in cosmetics by pressurized liquid extraction–gas chromatography tandem mass spectrometry. J Chromatogra A. 2015;1405:12–22.
El-Bassi L, Iwasaki H, Oku H, Shinzato N, Matsui T. Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere. 2010;81:109–13.
Huang W, Xie Z, Yan W, Mi W, Xu W. Occurrence and distribution of synthetic musks and organic UV filters from riverine and coastal sediments in the Pearl River estuary of China. Mar Pollut Bull. 2016;111:153–9.
Vila M, Llompart M, Garcia-Jares C, Homem V, Dagnac T. Development and optimization of a solid-phase microextraction gas chromatography-tandem mass spectrometry methodology to analyse ultraviolet filters in beach sand. J Chromatogr A. 2018;1564:59–68.
Pintado-Herrera MG, Wang C, Lu J, Chang YP, Chen W, Li X, Lara-Martin PA. Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J Hazard Mater. 2017;323:128–38.
Funding
This study was financially supported by the National Natural Science Foundation of China (NSFC 41877359), Guangdong Natural Science Foundation (2020A1515010448), Guangdong Provincial Key Laboratory of Chemical Pollution and Environmental Safety (2019B030301008), and China Postdoctoral Science Foundation (No. 2021M701272).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, Q., Wu, D., Cheng, YX. et al. Development and validation of a simultaneous method for the analysis of benzothiazoles and organic ultraviolet filters in various environmental matrices by GC–MS/MS. Anal Bioanal Chem 414, 6541–6555 (2022). https://doi.org/10.1007/s00216-022-04212-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04212-2