Abstract
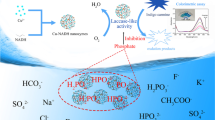
The rapid development of nanozymes for ultrasensitive detection of contaminate has resulted in considerable attention. Herein, a carboxyl- and aminopropyl-functionalized copper organophyllosilicate (Cu-CAP) was synthesized by a facile, one-pot sol–gel method. The bifunctional groups endow it with superior catalytic activity than that of natural enzyme. Besides, it possesses outstanding catalytic stability under harsh conditions such as high temperature, extremely high or low pH, and high salinity. Apart from laccase-mimetic activity, Cu-CAP also shows oxidation of the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) to the blue-colored TMBox in the presence of H2O2, which is similar to natural horseradish peroxidase (HRP). Interestingly, this colorimetric system was suppressed by hydroquinone (HQ) specifically. Inspired by this, Cu-CAP was used to develop a highly sensitive and selective colorimetric method for the determination of HQ. This assay displayed an extremely low detection limit of 23 nM and was applied for the detection of HQ in environmental water with high accuracy. This approach offers a new route for the rational design of high performance nanozymes for environmental and biosensing applications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroquinone (HQ) is a diphenol compound that commonly serves as a chemical raw material, by-product, or synthetic intermediate. However, HQ can seriously pollute the environment and even threaten human health because of its high toxicity and low degradation. Therefore, developing a facile technique for the detection of HQ is of great importance for environmental protection. A variety of methods, including electroanalysis [1], fluorometry [2], chromatography [3, 4], and colorimetry [5] have been used for the detection of HQ to date. Among them, colorimetric analysis is the most promising because of its easy operation, fast response, and low cost [6,7,8,9]. Colorimetric analysis can be classified into two categories: direct and indirect measurement. The oxidation of HQ to p-benzoquinone (BQ) has been used as the basis for direct colorimetric detection, in which KMnO4 [6], K2Cr2O7 [7], or NH4VO3 [8] are typically employed in the redox reaction. However, these approaches suffered from limited sensitivity and selectivity. Recently, indirect measurement via the reducing action of HQ on an oxidant-containing colorimetric system has been receiving increased attention for the high sensitivity and accuracy [10,11,12]. However, the key challenge is to construct a high-performance catalyst to improve the color change effectively and achieve a lower detection limit. Therefore, the facile construct of a catalyst with outstanding efficiency is of great importance in a detection system.
Since the discovery of ferromagnetic nanoparticles with intrinsic peroxidase-like activity by Yan’s group in 2007 [13], an increasing number of nanomaterials have been identified that possess enzyme-mimicking properties [14,15,16,17,18,19]. These artificial enzymes, called nanozymes, have attracted sustained attention owing to their low cost, high stability, facile preparation, and tunable activity properties when compared with natural enzymes [20]. Up to now, nanozymes have been used for extensive applications in the fields of biosensing [21], environmental treatment [22], disease diagnosis, and therapy [23]. Notably, nanozymes involved in detecting environmental contaminants have been attracting increasing attention as an emerging application [22, 24, 25]. The target molecule can be easily monitored via direct interactions between the target and nanozyme or by the effects of the target on nanozyme-catalyzed systems via a colorimetric assay [25]. However, some environmental samples possess complex components or extremely low concentrations of the target molecule, presenting significant challenges related to the limited catalytic activity and low target recognition ability of the nanozyme compared with the natural analogs that result in low sensitivity and specificity. Therefore, constructing a high-performance nanozyme with good specificity is urgently needed.
To date, numerous transition-metal-based nanozymes have been synthesized and exhibit diverse enzyme-like activities. For example, transition-metal-doped MnO2 nanocoatings (Zn-MnO2 and Cu-MnO2) mimic catalase and superoxide dismutase activities, which is primarily attributed to the multivalent states of Mn and the oxygen vacancies after Cu2+/Zn2+ doping [26]. In addition, a carbon-shielded 3D Co–Mn nanowire array on Ni foam (CoMn NW/NF@C) displayed peroxidase/catalase dual enzyme-like activity, the multi-active site with uniform alignment contributed to the enhancement of electron transfer and improved the catalytic performance comparing with the monometallic ones [27]. Some mixed metal oxides, such as Co1.5Mn1.5O4 [28], Co-V MMO [17], and CeVO4 [29], also possess dual- or multi-enzyme-like activity. However, these nanozymes often require a complex synthesis procedure or high temperature, which restricts their industrial applications. Therefore, a facile and efficient strategy for constructing a high-performance dual- or multi-functional nanozyme is of significant interest for practical applications.
Natural phyllosilicates are known to possess a lamellar structure that offers high specific surface area for adsorption and catalysis, but they suffer from poor swelling ability in water that often impedes their application. As a result, organophyllosilicates have received considerable attentions for their inherent ordered 2D structures and can furnish interesting surface properties to circumvent this problem. In this work, a carboxyl- and aminopropyl-functionalized copper-based organophyllosilicate (Cu-CAP) was synthesized by a facile sol–gel method. The bifunctional groups endow it with enhanced dispersibility and substrate affinity that result in outstanding dual-enzyme mimetic catalytic performance. Finally, it was employed in a colorimetric system for the detection of HQ in water.
Materials and methods
Chemicals
Laccase (≥ 1 U/mg) from Trametes versicolor was purchased from Bomei Biotechnology Co., Ltd. (Hefei, China). Copper dichloride, sodium dihydrogen phosphate, disodium hydrogen phosphate, ethanol, succinic anhydride, and HQ were obtained from Chengdu Kelong Chemical Reagent Company (Chengdu, China). 3-Aminopropyltriethoxysilane (APTES), 2,4-dichlorophenol (2,4-DP), 4-aminoantipyrine (4-AP), and 3,3’,5,5’-tetramethylbenzidine (TMB) were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). The lake water is taken from Mianyang district local rivers. All the chemical reagents were of analytical grade unless otherwise specified. Milli-Q water was used to prepare all the buffers and solutions.
Synthesis of Cu-CAP
Bifunctional Cu-CAP was prepared by one-pot sol–gel method (Scheme 1). Generally, 8.4 g CuCl2·2H2O and 0.1835 g succinic anhydride were dissolved in 200 mL ethanol solution under ultrasonication. Afterward, 13 mL of APTES was added drop-wise into prepared solution at room temperature; after 5 min mixing, a dark solution was immediately produced. For a further 12 h of stirring, the precipitated material was centrifugated and washed with ethanol (50 mL) three times to remove the excess reactants, and dried at 40 °C.
Characterization of Cu-CAP
The morphologies and microstructures of the as-prepared samples were observed by scanning electron microscopy (SEM; Ultra 55, Zeiss, Berlin, Germany) and transmission electron microscopy (TEM; Libra 200, Zeiss, Berlin, Germany). X-ray diffraction (XRD) patterns were collected using an X’pert Pro diffractometer (PANalytical, Eindhoven, the Netherlands) with Cu-Kα radiation (λ = 1.5178 Å, 40 kV × 40 mA) in the 2θ range of 3–70° at a scan rate of 0.02° s−1. The Fourier transform infrared (FTIR) spectra were recorded using a Spectrum One spectrometer (PerkinElmer, Waltham, MA, USA). X-ray photoelectron spectroscopy (XPS) was carried out using an XPS spectrometer (Escalab 250Xi, Thermo Scientific, Waltham, MA, USA) with a monochromatic Al Kα source. The absorption spectra of the samples were collected using an UV–Vis spectrophotometer (UV-2800A, Unico, Franksville, WI, USA). The particle size distribution of Cu-CAP was determined using a particle size analyzer (Plus 90, Brookhaven, Holtsville, NY, USA). The zeta potential of Cu-CAP was measured with a zeta potential analyzer (Zeta PAL, Brookhaven, NY, USA).
The dual-enzyme mimetic activity of Cu-CAP
The laccase-like catalytic activity of Cu-CAP was investigated in phosphate buffer solution (3.5 mL, 0.01 M, pH 7.4), 2,4-DP (500 μL, 1 mg/mL) was selected as the substrate, with assistant of 4-AP (500 μL, 1 mg/mL), and it could be oxidized into a colored product catalyzed by Cu-CAP (500 μL, 1 mg/mL) within 1 h. The UV–Vis spectrophotometer was employed to detect the colored product at 510 nm.
To evaluate the peroxidase-like catalytic activity, TMB was served as the chromogenic substrate, in the acetate buffer (0.1 M, pH 4.0) it could be oxidized by H2O2 in the presence of Cu-CAP. The produced TMBox can be detected at 652 nm by UV–Vis spectrophotometer.
Steady-state kinetic analysis of Cu-CAP
Kinetic analysis of laccase-like activity was performed by monitoring absorbance change at 510 nm. Laccase or Cu-CAP (500 μL 1 mg/mL), 4-AP (500 μL 1 mg/mL), and various concentration of 2,4-DP were added to the phosphate buffer (3.5 mL, 0.01 M, pH 7.4); after reacting for 10 min at 25℃, the absorbance of product were recorded, and the apparent kinetic parameters were calculated by Lineweaver–Burk plot:
where v is the apparent initial catalytic rate, Km is the apparent Michaelis–Menten constant, vmax is the maximum apparent initial reaction rate, and [S] is the substrate concentration.
Catalytic stability of Cu-CAP
To evaluate the thermal stability, Cu-CAP was stored at different temperature (30–90 °C) for 1 h separately and then performs the catalytic reaction at room temperature. Laccase was processing the same reaction for comparison. The pH stability was measured by incubating Cu-CAP or laccase at variety of pH (3.0–9.0) for 8 h separately, and the catalytic activity was tested and compared with that at pH 7. To study the effect of ionic strength, diverse concentrations of NaCl (50, 100, 200, 300, 400, 500 mM) were incubated with Cu-CAP or laccase for 1 h and then added the substrates to perform the catalytic reaction for 5 min. All the catalytic reactions were tested for 1 h unless otherwise stated, and the products were detected at 510 nm by UV–Vis spectrophotometer.
Colorimetric detection of hydroquinone
Detection of HQ was based on the reduction of HQ on the TMB-H2O2-Cu-CAP platform. Briefly, acetate buffer (0.1 M, pH 4.0), TMB (0.4 mM), Cu-CAP (0.04 mg/mL), and H2O2 (350 mM) were mixed in a cuvette; after reacting for 20 min at room temperature, TMB was oxidized to the blue TMBox which present a maximum absorbance at 652 nm of UV–Vis spectrum. Then, 20 μL HQ with different concentration were added into the above solution, the color would fade, and the absorbance decreased. Finally, the absorbance was monitored at 652 nm and the concentration of HQ could be determined. The real sample was filtered and adjusted the pH to 4.0 with buffer before reaction and then determined at the same optimal conditions.
Results and discussion
Characterization
Organophyllosilicates are regarded as promising surrogates of natural clays for their inherent ordered 2D structures and regulatable surface properties. In this work, a copper-containing organophyllosilicate, Cu-CAP, was synthesized. The nanostructure of Cu-CAP was determined by SEM and TEM. As shown in Fig. 1, Cu-CAP presented a regular layered structure similar to that of natural phyllosilicate. Interestingly, unlike natural phyllosilicate, Cu-CAP can freely exfoliate in water to form nanosized sheets having a clear dispersion with an average size of approximately 137 nm (See Electronic Supplementary Material Fig. S1). Moreover, the ζ potential of 52.3 mV indicated Cu-CAP had good dispersibility in water, which is beneficial for aqueous catalytic reactions. XRD patterns confirmed the amorphous structure of Cu-CAP. The diffraction peaks at 2θ = 5.5° indicated the interlayer spacing was 1.6 nm, which suggested the presence of a bilayer arrangement of functional groups. The broad in-plane reflections at 2θ = 10.2° and 23.0° were consistent with spacing values of d002 = 0.87 nm and d020,110 = 0.38 nm, respectively, indicating Cu-CAP belongs to the phyllosilicate family. FTIR analysis showed that Cu-CAP contained organofunctional entities such as -OH (3341 cm−1), -CH2 (2921 cm−1), -NH3+ (1967 cm−1), -NH2 (1604 cm−1), -Si–C- (1121 cm−1), -Si–O-Si- (1023 cm−1), and -C-N- (930 cm−1). In addition, -C = O (1719 cm−1) peaks confirmed a carboxyl group was present in the structure.
XPS was applied to identify the surface chemical states of Cu-CAP (Fig. 2). The XPS survey spectrum indicated the presence of C, N, O, Si, and Cu that originate from the precursor compounds (Fig. 2a). Subsequently, the high-resolution of C1s spectrum was examined. The peaks at 284.1, 284.8, 285.8, and 288.5 eV were assigned to CH2, C–C, C-N, and C = O, respectively; the appearance of C = O indicated the carboxyl group was successfully grafted on the framework. In addition, the N 1 s spectrum was utilized to examine whether the carboxyl group was connected with amidogen through amide bond. A comparison of the N 1 s spectrum before and after functionalization with carboxyl was performed (Fig. 2c, d). It was suggested that there were two groups in the structure before carboxyl-functionalization, one at 399.4 eV is attribute to the free amino group, and the other at 401.25 eV is regarded as some H-bonding interactions between amino groups or with substrate hydroxyl groups [30]. After functionalized with -COOH, the free amidogen peak decreased sharply and deconvoluted into two peaks, amide group at 399.6 eV and free amino group at 399.1 eV. Since the amide group was derived from the reaction of the carboxyl group with the amidogen on the organophyllosilicate structure, it can be further calculated the –COOH functional rate was 21.8%.
Laccase-like activity of Cu-CAP
Laccase is an oxidase enzyme that contains copper in its active center and can catalyze the reaction of numerous substrates, including phenols, polyphenols, and polyamines, into the related quinones while reducing the oxygen to water [31]. As the by-products are environmentally benign, laccase is regarded as a green catalyst that is environmentally friendly. To investigate the laccase-like activity of copper-containing Cu-CAP, laccase substrate 2,4-DP and 4-AP were selected as the substrate and chromogenic agent, respectively. As shown in Fig. 3, the product absorbance at 510 nm significantly increased when both reactants were present with Cu-CAP. However, this absorbance was barely detected for each compound separately with Cu-CAP or for both compounds in the absence of Cu-CAP, which is in accordance with the performance of natural laccase. Thus, Cu-CAP presented intrinsic laccase-mimetic activity.
Kinetic analysis of Cu-CAP as a laccase-like nanozyme
To better understand the catalytic mechanism of Cu-CAP as a laccase-like nanozyme, the steady-state kinetics of the nanozyme activities was evaluated. Various concentrations of 2,4-DP were reacted with 4-AP and Cu-CAP, while natural laccase was employed for the comparison. As a result, a typical Lineweaver–Burk curve was obtained, and the kinetic parameters were calculated using the Michaelis–Menten equation (Eq. 1). The Km of Cu-CAP was calculated of 0.08 mM, which is lower than that of natural laccase (0.23 mM), indicating Cu-CAP possessed superior substrate affinity than the natural analogue. According to the Lewis acid–base theory, phenols was regarded as a Lewis base, which is prefer to combine with carboxyl group, while the gaseous oxygen was identified as a Lewis acid that exhibit high affinity with amidogen. As a consequence, enhanced affinity would be achieved between Cu-CAP and substrates benefited from the synergistic effect of the bifunctional groups.
Catalytic stability of Cu-CAP
Natural enzymes are known to be easy to denature in non-physiological environments, which impedes their applications. Therefore, the effect of temperature, pH, and ionic strength were investigated to determine the stability of Cu-CAP under harsh conditions (Fig. 4).
Firstly, Cu-CAP and laccase were each stored at various temperatures (30–90 °C) before the catalytic activity assay to verify thermal stability. As shown in Fig. 4a, Cu-CAP displayed a superior thermal stability to that of laccase. When the temperature was higher than 70 °C, laccase was completely deactivated, whereas Cu-CAP retained approximately 95% of its activity.
Then, pH stability was studied using a wide pH range for the storage conditions. As indicated in Fig. 4b, the catalytic ability of Cu-CAP was not significantly affected by pH. For laccase, however, either strongly acidic or alkaline conditions led to a significant drop in activity. Notably, laccase completely lost activity at pH 3 owing to the deformation of laccase in the unfavorable pH environment. Therefore, Cu-CAP can be served as an ideal nanozyme for applications under various pH conditions.
Additionally, the effect of salinity was also investigated (Fig. 4c). As the concentration of NaCl increased, the catalytic activity of Cu-CAP increased significantly, but laccase was affected very little by ionic strength.
Finally, to examine long-term storage stability, Cu-CAP and laccase were each dispersed in ultrapure water and stored for 12 days. As shown in Fig. 4d, laccase gradually lost its activity over time, reaching approximately 10% of its original activity on day 12, whereas Cu-CAP retained over 85% of its original activity on the 12th day. The excellent long-term storage ability of Cu-CAP could facilitate its substitution for natural laccase in practical applications.
Peroxidase-like activity of Cu-CAP
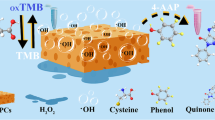
Peroxidase was regarded as a widespread catalyst for the oxidation of diverse substrate in the presence of H2O2. To evaluate the peroxidase mimetic activity of Cu-CAP, TMB was selected as the chromogenic substrate, and it was observed that in the acetate buffer, TMB could be oxidized to a blue-colored TMBox by H2O2 in the presence of Cu-CAP and can be detected at 652 nm by UV–Vis spectrophotometer (See Electronic Supplementary Material Fig. S2). Additional experiments revealed that neither TMB nor H2O2 are colored and no blue colored product was observed in the absence of Cu-CAP. These results demonstrated Cu-CAP has peroxidase-mimetic activity.
The inherent mechanism of this peroxidase-like activity was evaluated by the radical scavengers, in which isopropanol (IPA) was selected as.OH scavenger and p-benzoquinone (BQ) was used for trapping O2·−. As shown in Fig. S3, there was an evident decrease in the absorbance at 652 nm upon the addition of IPA to the catalytic system, indicating the presence of.OH radicals during the catalytic process. Moreover, when BQ was introduced to the reaction system, the absorbance at 652 nm was also significantly reduced, demonstrating the generation of O2·− in the catalytic process. Additionally, according to the Lewis acid–base theory, TMB containing amino group with one lone pair of electrons can be regarded as Lewis base, which possessed high affinity to carboxyl group on the surface of Cu-CAP, and will lead to the promotion of the electron transfer between substrates and intermediates. As a result, the mechanism was deduced as follows: Firstly, H2O2 and TMB are adsorbed on the surface of Cu-CAP. Then, one electron transfer takes place for the production of.OH and O2·−[32]. Subsequently, O2·− converted to ·OH with the assistance of H2O2 via the Haber–Weiss reaction [33]. Finally, the high redox potential of the.OH radical result in the oxidation of TMB to the related blue-colored product. The presumable reaction equations are illustrated below.
Determination of HQ
As shown in Fig. 5a, the addition of HQ to the Cu-CAP/TMB/H2O2 catalytic system caused a decrease in the amount of the blue-colored TMBox formed, as seen by the decrease in the absorbance at 652 nm as HQ concentration increased. However, this phenomenon was not observed when employing other phenols or amines as the catalyst, including catechol, resorcinol, phenol, 2,4-dichlorophenol, dopamine, and bisphenol A (Fig. 5c). These suggest HQ performed a reduction effect to the Cu-CAP/TMB/H2O2 catalytic system with high selectivity and thus inspired us to develop a colorimetric strategy for the detection of HQ.
Determination of HQ by the TMB/H2O2/Cu-CAP system. a The UV–Vis spectra of Cu-CAP-based colorimetric system upon the addition of 0–140 μM HQ (inset: the related photograph of colorimetric system, from left to right corresponed to 0 μM-140 μM). b The impact of HQ concentration on the colorimetric system (inset: the linear calibration plot for HQ). c The effect of various phenols and amines to the chromogenic system. d The interference of ions to the chromogenic system
Optimization of the colorimetric system
The effects of reaction time, pH, temperature, Cu-CAP dosage, TMB concentration, and H2O2 concentration were investigated and optimized to construct a highly sensitive and accurate colorimetric assay (See Electronic Supplementary Material Fig. S4). The catalytic activity was strongly affected by temperature, when the temperature increased, the catalytic activity decreased, and when the temperature was higher than 40 °C, the absorbance of the product decreased sharply. As a result, 25 °C was selected as the optimal temperature. The pH was regarded as a vital factor in the catalytic process because pH may change the surface charge of the catalyst and affect the interaction between the catalyst and substrate. Therefore, a pH range of 2 to 8 was investigated, and pH 4 was found to be optimal. The concentrations of Cu-CAP, TMB, and H2O2 were also evaluated. As shown in Fig. S4, the catalytic activity increased as the concentration of the reactants increased. After considering the accuracy of the quantitative determination in the practical analysis, the optimal concentrations of Cu-CAP, TMB, and H2O2 were chosen as 0.04 mg/mL, 0.4 mM, and 350 mM, respectively. The time-dependent absorbance response of the colorimetric system is also presented in Fig. S4b. The absorbance increased as the reaction time increased, but the trend slowed after reacting for more than 20 min. Therefore, 20 min was selected in the following experiments.
Colorimetric detection of HQ
A facile and sensitive colorimetric assay for the detection of HQ was constructed using the optimal catalytic conditions. As shown in Fig. 5a, as the concentration of HQ increased, the colored solution gradually faded, and the related UV–Vis absorption decreased. This suggested HQ had a concentration-dependent reducing action on the colorimetric system (Fig. 5b). The relationship of the absorbance to the HQ concentration was △Abs = 0.0196CHQ + 0.0475 (R2 = 0.9996), with a linear range from 0 to 100 μM. The limit of detection (LOD) was 23 nM(3σ/S). To the best of our knowledge, this is the lowest LOD that has been reported (Table 1). This method could be applied to the detection of trace amounts of HQ in real samples.
Selectivity of the colorimetric detection system
Specificity is important for practical applications to avoid false positive results. To evaluate the selectivity of the colorimetry assay for the determination of HQ, various possible coexisting compounds, including metal ions, polyphenols, polyamines, and phenols, were investigated (Fig. 5c, d). When adding HQ into the colorimetric system, the absorbance of TMBox decreased significantly (Fig. 5c). However, when 1 mM metal ions or anions which were relatively tenfold to HQ were introduced, or 0.1 mM phenolic or amine compounds that equal to the amount of HQ were added, there was negligible change to the expected absorbance. Furthermore, T test was employed to evaluate the influence of the interference, indicating the signal response showed statistically different from that of the aforementioned ions or phenols with a significance level of 0.05. In conclusion, the Cu-CAP/TMB/H2O2 system perform an extremely high selectivity for HQ when the samples coexisted with 1 mM interfering ions and 0.1 mM phenolic compounds, which showed potential applications for the HQ detection in such clean water sample.
Detection of HQ in real sample
The colorimetric system was applied to the testing of HQ in lake water and simulated water. Different concentrations of HQ were added to a water sample that had been adjusted to pH 4, and the recovery values were calculated by the equation Recovery (%) = [(Found −Before)/Added] × 100%. As displayed in Table 2, the recovery of the tested samples ranged from 94.97 to 99.50%, and the RSDs were all below 3%. This demonstrated that the Cu-CAP/TMB/H2O2 colorimetric system can be applied to detect trace HQ in real samples with acceptable accuracy and precision.
Conclusion
Cu-CAP was synthesized by a facile, one-pot sol–gel method. It displayed excellent water dispersibility and regulatable dual-enzyme mimetic activities (laccase-like and peroxidase-like). Kinetic analysis revealed Cu-CAP had superior substrate affinity to natural laccase owing to the synergetic Lewis acid–base interactions between the bifunctional groups and substrates. Moreover, Cu-CAP also presented outstanding catalytic stability under harsh conditions and long-term storage process. Besides, on the basis of HQ with specific reduction on the Cu-CAP-based peroxidase-like catalytic system with a concentration-dependent manner, a colorimetric platform for the detection of HQ was established. A good liner range from 0 to 100 μM and an extremely low LOD of 23 nM were achieved. Finally, Cu-CAP was used to detect HQ in a real sample with good accuracy. Therefore, the as-prepared dual enzyme-like Cu-CAP nanozyme can be served as an ideal candidate for the detection of trace HQ in water.
References
Promsuwan K, Kaewjunlakan C, Saichanapan J, Soleh A, Limbut W. Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim Acta. 2021;377: 138072. https://doi.org/10.1016/j.electacta.2021.138072.
Wang, Yu-Min, Jiang, Jian-Hui, Zhang, Chong-Hua, et al. Conjugated polymer nanoparticles-based fluorescent biosensor for ultrasensitive detection of hydroquinone. Anal Chim Acta. 2018:60–5.https://doi.org/10.1016/j.aca.2018.01.027
Wittig J, Wittemer S, Veit M. Validated method for the determination of hydroquinone in human urine by high-performance liquid chromatography-coulometric-array detection. J Chromatogr B. 2001;761(1):125–32. https://doi.org/10.1016/S0378-4347(01)00321-8.
Sakodinskaya IK, esiderio CD, Nardi A, Fanali S. Micellar electrokinetic chromatographic study of hydroquinone and some of its ethers. Determination of hydroquinone in skin-toning cream. J Chromatogr. 1992;596(1):95–100. https://doi.org/10.1016/0021-9673(92)80208-C
Zhao XL, HY. Yao, XX. Xu, C. Liu, QY. Liu, ZX. Zhang, XX. Zhang, X. . Hydroquinone colorimetric sensing based on platinum deposited on CdS nanorods as peroxidase mimics. Microchimica Acta. 2020. https://doi.org/10.1007/s00604-020-04451-z
Sirajuddin, Bhanger MI, Niaz A, Shah A, Rauf A. Ultra-trace level determination of hydroquinone in waste photographic solutions by UV–vis spectrophotometry. Talanta. 2007;72(2):546–53. https://doi.org/10.1016/j.talanta.2006.11.021
Zenovia Moldovan DEP, Iulia Gabriela David, Mihaela Buleandra, Irinel Adriana Badea. A derivative spectrometric method for hydroquinone determination in the presence of kojic acid, glycolic acid, and ascorbic acid. J Spectro. 2017;2017:1–9. https://doi.org/10.1155/2017/6929520
Uddin S, Rauf A, Kazi TG, Afridi HI, Lutfullah G. Highly sensitive spectrometric method for determination of hydroquinone in skin lightening creams: application in cosmetics. Int J Cosmetic Sci. 2011;33(2):132–7. https://doi.org/10.1111/j.1468-2494.2010.00599.x.
García P, Santoro M, Kedor-Hackman E, Singh AK. Development and validation of a HPLC and a UV derivative spectrophotometric methods for determination of hydroquinone in gel and cream preparations. J Pharm Biomed Anal. 2005;39(3–4):764. https://doi.org/10.1016/j.jpba.2005.04.016.
Wang X, Zhao M, Song Y, Liu Q, Chen S. Synthesis of ZnFe2O4/ZnO heterostructures decorated three-dimensional graphene foam as peroxidase mimetics for colorimetric assay of hydroquinone. Sensor Actuat B-Chem. 2018;283:130–7. https://doi.org/10.1016/j.snb.2018.11.079.
Song, Yawen, Zhao, Minggang, Li, Hui, et al. Facile preparation of urchin-like NiCo2O4 microspheres as oxidase mimetic for colormetric assay of hydroquinone. Sensor Actuat B-Chem. 2018;255:1927–36. https://doi.org/10.1016/j.snb.2017.08.204
Ma Z, Li P, Lu Q, Liu M, Li H, Zhang Y, et al. Bifunctional colorimetric biosensors via regulation of the dual nanoenzyme activity of carbonized FeCo-ZIF. Sensor Actuat B-Chem. 2019;290:357–63. https://doi.org/10.1016/j.snb.2019.03.130.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotech. 2007;2(9):577–83. https://doi.org/10.1038/nnano.2007.260.
Sharifi M, Faryabi K, Talaei AJ, Shekha MS, Falahati M. Antioxidant properties of gold nanozyme: a review. J Mol Liq. 2020;297: 112004. https://doi.org/10.1016/j.molliq.2019.112004.
He W, Ying L, Yuan J, Yin JJ, Wu X, Hu X, et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials. 2011;32(4):1139–47. https://doi.org/10.1016/j.biomaterials.2010.09.040.
He L, Lu Y, Gao X, Song P, Huang Z, Liu S, et al. Self-cascade system based on cupric oxide nanoparticles as dual-functional enzyme mimics for ultrasensitive detection of silver ions. ACS Sustain Chem Eng. 2018;6(9):12132–9. https://doi.org/10.1021/acssuschemeng.8b02476.
Wang Y, Chen C, Zhang D, Wang J. Bifunctionalized Novel Co-V MMO Nanowires: intrinsic oxidase and peroxidase like catalytic activities for antibacterial application. Appl Catal B- Environ. 2019;261: 118256. https://doi.org/10.1016/j.apcatb.2019.118256.
Zhang P, Sun D, Cho A, Weon S, Choi W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nature Comm. 2019;10(1):940. https://doi.org/10.1038/s41467-019-08731-y.
Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22(19):2206–10. https://doi.org/10.1002/adma.200903783.
Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. ChSRv. 2019;48:3683–704. https://doi.org/10.1039/C8CS00718G.
Liu Y, Wang X, Wei H. Light-responsive nanozymes for biosensing. Analyst. 2020;145:1–19. https://doi.org/10.1039/D0AN00389A.
Meng Y, Li W, Pan X, Gadd GM. Applications of nanozymes in the environment. Environ Sci Nano. 2020;7:1–41. https://doi.org/10.1039/C9EN01089K.
Khan S, Sharifi M, Bloukh SH, Edis Z, Falahati M. In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta. 2021;224: 121805. https://doi.org/10.1016/j.talanta.2020.121805.
Gomaa EZ. Nanozymes: a promising horizon for medical and environmental applications. JCS. 2021:1–23. https://doi.org/10.1007/s10876-021-02079-4
Xin LA, Lw A, Dan DB, Liang NA, Jp A, Xn A. Emerging applications of nanozymes in environmental analysis: opportunities and trends. TrAC, Trends Anal Chem. 2019;120:115653–65. https://doi.org/10.1016/j.trac.2019.115653.
Liu S, Li K, Shao D, Shen Q, Zheng X. Dual enzyme-like activities of transition metal-doped MnO2 nanocoatings and their dependence on the electronic band structure and ionic dissolution. ApSS. 2020;534(3): 147649. https://doi.org/10.1016/j.apsusc.2020.147649.
Lu M, Li B, Guan L, Li K, Lin Y. Carbon-shielded three-dimensional Co–Mn nanowire array anchored on Ni foam with dual-enzyme mimic performance for selective detection of ascorbic acid. ACS Sustain Chem Eng. 2019;7(18):15471–8. https://doi.org/10.1021/acssuschemeng.9b03095.
Liu X, Yang J, Cheng J, Xu Y, Chen W, Li Y. Facile preparation of four-in-one nanozyme catalytic platform and the application in selective detection of catechol and hydroquinone. Sensor Actuat B-Chem. 2021;337: 129763. https://doi.org/10.1016/j.snb.2021.129763.
Zha J, Zhang P, Qin Y, Chen T, Ye] F. Fabrication of CeVO4 as nanozyme for facile colorimetric discrimination of hydroquinone from resorcinol and catechol. Sensor Actuat B-Chem. 2017;247:469–78. https://doi.org/10.1016/j.snb.2017.03.042
An Y, Chen M, Xue Q, Liu W. Preparation and self-assembly of carboxylic acid-functionalized silica. JCIS. 2007;311(2):507–13. https://doi.org/10.1016/j.jcis.2007.02.084.
Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551–65. https://doi.org/10.1016/S0031-9422(02)00171-1.
Li Wang RP, Xue Liu, Chendi Heng, Yanni Miao, Wei Wang, Andrew Carrier, Ken Oakes and Xu Zhang. Nitrite-enhanced copper-based Fenton reactions for biofilm removal. Chem. Comm. 2021;57:5514–7. https://doi.org/10.1039/D1CC00374G
Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50. https://doi.org/10.1016/S0300-483X(00)00231-6.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, grant numbers 42061134018, 42011530085, and 41877323), the Russian Science Foundation (RSF, grant number 21–47-00019), the Sichuan Science and Technology Program (grant number 2019JDJQ0056), and the Postgraduate Innovation Fund Project by Southwest University of Science and Technology (grant number 20YCX0022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, R., Sun, S., Liu, J. et al. Bifunctional nanozyme of copper organophyllosilicate for the ultrasensitive detection of hydroquinone. Anal Bioanal Chem 414, 1039–1048 (2022). https://doi.org/10.1007/s00216-021-03728-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03728-3