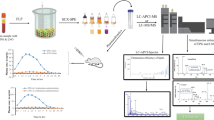
Abstract
Herbal medicines have historically been practiced in combinatorial way, which achieves therapeutic efficacy by integrative effects of multi-components. Thus, the accurate and precise measurement of multi bioactive components in matrices is inalienable to understanding the metabolism and disposition of herbal medicines. In this study, aiming to provide a strategy that improves analyte coverage, evaluation of six protocols employing sample pretreatment methods- protein precipitation (PPT), liquid-liquid extraction (LLE), sugaring-out-assisted liquid-liquid extraction (SULLE), and salting-out-assisted liquid-liquid extraction (SALLE)- was performed by LC-MS/MS using rat plasma and a mixture of alkaloid (evodiamine, rutaecarpine, dehydroevodiamine), terpenoid (limonin, rutaevin, obacunone), and flavonoid (liquiritin, isoliquiritin, liquiritigenin) standards isolated from Tetradium ruticarpum and Glycyrrhiza uralensis. These protocols were as follows: (1) PPT with methanol, (2) PPT with acetonitrile, (3) LLE with methyl tertiary-butyl ether-dichloromethane, (4) LLE with ethyl acetate-n-butanol, (5) SALLE with ammonium acetate, (6) SULLE with glucose. The results suggested that SALLE produced broader analyte coverage with satisfactory reproducibility, acceptable recovery, and low matrix interference. Then, sample preparation procedure of SALLE, chromatographic conditions, and mass spectrometric parameters were optimized, followed by method validation, showing that good sensitivity (LLOQ ≤ 1 ng mL−1), linearity (r ≥ 0.9933), precision (RSD ≤ 14.45%), accuracy (89.54~110.87%), and stability could be achieved. Next, the developed method was applied successfully to determine the pharmacokinetic behavior of the nine compounds in rat plasma after intragastric administration with an extract from Tetradium ruticarpum and Glycyrrhiza uralensis (Wuzhuyu-Gancao pair). Based on an extensive review and experiments, a sample preparation procedure that matches with LC-MS/MS technique and can get wider analyte coverage was outlined. The developed SALLE method is rapid, reliable, and suitable for bioanalysis of analytes with diverse polarity, which was expected to be a promising strategy for the pharmacokinetic studies of herbal medicines.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sample preparation approaches for quantitative bioanalysis primarily include protein precipitation (PPT) [1], liquid-liquid extraction (LLE) [2], and solid-phase extraction (SPE) [3]. PPT is one of the most frequently used sample pretreatment methods which alter the solvation potential of solvent, more specifically, by adding organic solvents such as methanol [4], acetonitrile [5], and 2-propanol [6]. However, the immediate protein precipitation procedures lead to co-extraction of endogenous compounds that result in high matrix interference [7, 8]. LLE is a simple method with excellent extraction performance. It extracts compounds based on their relative solubilities in aqueous phase and organic solvents, which is generally unsuitable for hydrophilic compounds [2, 9, 10]. SPE is one of the predominant techniques for clean-up of complex biofluids, but the multiple steps, low recovery, poor reproducibility, and high cost severely limit its application in high-throughput quantitative bioanalysis [11,12,13].
Recently, phase separation from a homogeneous solution, an alternative to the traditional LLE, has found its increased application in bioanalysis, because of its simplicity, economy, and excellent extraction performance [14, 15]. Reported approaches used for phase separation include cooling at subzero-temperature [16], salting-out [17,18,19], and sugaring-out [15, 20]. Subzero-temperature LLE is a time-consuming method which is difficult to operate and suffers from low throughput [21]. Sugaring-out-assisted liquid-liquid extraction (SULLE) is another phase separation method with good removal ability by commonly adding a monosaccharide (glucose, xylose, arabinose, and fructose) or a disaccharide (sucrose and maltose) [22]. But relatively low extraction recovery might be a challenge encountered by SULLE [20]. Salting-out-assisted liquid-liquid extraction (SALLE) with polar salt solvents can produce high extraction efficiency. However, the use of a high concentration of inorganic salt, such as zinc sulfate [17], magnesium sulfate [23], potassium phosphate [24], and sodium chloride [19], may lead to unwanted reactions and have impact on mass spectrometric ionization. The optimization of SALLE with mass spectrometry-friendly volatile salt solution has become a preferred extraction approach because of its compatibility with reversed-phase chromatography and mass spectrometry [18, 25, 26].
Herbal medicines have been widely applied in medical field for a long time. Due to the multi-components and multi-targets, explanation of efficacy mechanism and theory of traditional Chinese medicine (TCM) has become very challenging. Pharmacokinetic research, which can help to explore the relationship between efficacy and concentration, has become an inalienable part to study TCM in a modern way. It is widely accepted that the metabolism and disposition of TCM and its preparations in vivo can be associated with the pharmacokinetic behavior of one or several bioactive components [27]. The fruit of Tetradium ruticarpum (FTR, Wuzhuyu in Chinese) is an herbal medicine traditionally used with Coptidis Rhizoma, Ginseng Radix, Jujubae Fructus, and Zingiberis Rhizoma to treat digestive diseases [28]. Moreover, processing with licorice (the root and rhizome of Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L., and Glycyrrhiza inflata Batal., Gancao in Chinese) has been recommended for FTR application in Chinese Pharmacopoeia [29]. Although there were a few pharmacological and toxicological studies regarding the influence of licorice on FTR treatment, the metabolism and disposition of this herb pair using LC-MS/MS techniques have been not reported and its compatibility mechanism remains obscure [30,31,32]. Evodiamine (EVO), rutaecarpine (RUT), dehydroevodiamine (DHE), limonin (LIM), rutaevin (RVN), obacunone (OBA), liquiritin (LIQ), isoliquiritin (ILIQ), and liquiritigenin (LIQN), nine of the major components isolated from the two herbs and associated with efficacy, are grouped into three different biosynthetic classes (alkaloids, terpenoids, and flavonoids) and possess significantly different polarity [28, 33, 34]. Therefore, exploration of the dynamic changes of the concentrations of these nine compounds in vivo using a reliable sample preparation workflow which matches with the analytical techniques and can get wider analyte coverage might be crucial to disclose the potential mechanism of the two given herbs on their combined medication and processing technique.
The aim of the present study was to develop a sensitive method for simultaneous determination of alkaloids, terpenoids, and flavonoids in rat plasma using a UHPLC-MS/MS system. In this work, six sample extraction methods were designed as follows: (1) PPT with methanol (method A), (2) PPT with acetonitrile (method B), (3) LLE with tertiary-butyl ether (MTBE)-dichloromethane (method C), (4) LLE with ethyl acetate-n-butanol (method D), (5) SALLE with ammonium acetate (method E), (6) SULLE with glucose (method F). All extracted samples were analyzed by UHPLC-MS/MS. Extraction performance of PPT, LLE, SALLE, and SULLE was compared based on the criteria of matrix effect and extraction recovery [35], and the optimal method for the nine compounds was further optimized and validated. Finally, the developed method was successfully applied in pharmacokinetic study to determine the concentrations of EVO, RUT, DHE, LIM, RVN, OBA, LIQ, ILIQ, and LIQN in rat plasma.
Materials and methods
Chemicals, reagents, and animals
Evodiamine (EVO, 99.96%, Lot: AF9102121), rutaecarpine (RUT, 99.04%, Lot: AF20051651), limonin (LIM, 98.76%, Lot: AF9111721), rutaevin (RVN, 91.06%, Lot: AF9122363), obacunone (OBA, 99.75%, Lot: AF20032301), and liquiritigenin (LIQN, igenin AF200323011721) were purchased from Alfa Biotechnology Co., Ltd. (Chengdu, Sichuan, China); dehydroevodiamine (DHE, 99.82%, Lot: DST191027-136) was purchased from Desite Biotechnology Co., Ltd. (Chengdu, Sichuan, China); liquiritin (LIQ, ≥ 98%, Lot: 070003-201602) and isoliquiritin (ILIQ, ≥ 98%, Lot: 250026-201511) were purchased from Shanghai Natural Biological Co., Ltd. (Shanghai, China); osalmid was used as internal standard (IS, ≥ 99%, Lot: 20100609) and purchased from the National Institute for Food and Drug Control (Beijing, China). The structures of EVO, RUT, DHE, LIM, RVN, OBA, LIQ, ILIQ, LIQN, and IS are shown in Fig. 1.
Acetonitrile and methanol (HPLC grade) were from Fisher Scientific (Waltham, MA, USA). Formic acid (HPLC grade) was from CNW Technologies GmbH (Düsseldorf, Germany). Ammonium formate and ammonium acetate (HPLC grade) were from Honeywell Fluka (Charlotte, NC, USA). Methyl tertiary-butyl ether (MTBE, analytical reagent), dichloromethane (CH2Cl2, analytical reagent), ethyl acetate (analytical reagent), n-butanol (analytical reagent), and glucose (analytical reagent) were ordered from Hushi Chemical Reagent Co., Ltd. (Shanghai, China). Ultra-pure water was prepared through a Milli-Q Academic System (Millipore, Billerica, MA, USA).
Male (n = 3) and female (n = 3) Sprague-Dawley (SD) rats were obtained from the Animal Center of Shanghai University of Traditional Chinese Medicine. The animals were kept in an environmentally controlled breeding room at temperature of 22 ± 2 °C, a relative humidity of 50 ± 10%, and dark-light cycle for 12 h. Food and water are allowed spontaneously for rats during feeding. The animals were allowed to adapt to the experimental environment for 3 days and were fasted overnight prior to the experiment with free access to water. The animal experimental research was approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (Approval Number: PZSHUTCM200731003) and guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Instruments
An LCMS-8050 triple quadrupole mass spectrometer equipped with a UHPLC model 30AD and an electrospray ionization source was from Shimadzu (Kyoto, Japan). Data was acquired and processed with LabSolutions 5.82 SP1 from Shimadzu. A VX-200 vortex mixer from Labnet International, Inc. (Edison, NJ, USA) was used to ensure thorough mixing. Other instrumentations included centrifuge from Himac (Takeda, Japan) and balances from Mettler Toledo (Columbus, OH, USA).
Sample collection
FTR was collected from Zhangshu City (GPS coordinates: 115° 06′ 33″ to 115° 42′ 23″ E, 27° 49′ 07″ to 28° 09′ 15″ N), Jiangxi Province, and identified as the fruit of Euodia rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang. Licorice, identified as Glycyrrhiza uralensis Fisch., was purchased from Shanghai Kangqiao Chinese Medicine Tablet Co., Ltd. (Shanghai, China). The botanical origins of the materials were identified by Lihong Wu, Professor of the Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine. Test specimens were deposited at the Herbarium of Shanghai Research and Development Center for Standardization of Traditional Chinese (Shanghai, China). FTR and licorice were extracted twice times with water (1:10, w/v) for 2 h each, respectively. The extraction solutions were filtered and combined, and then concentrated under vacuum to obtain extract (0.1 g mL−1 of FTR, 0.1 g mL−1 of licorice) for intragastric administration.
Rat blood samples were collected from ophthalmic venous plexus before administration (time zero) and at 0.083, 0.167, 0.333, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after oral administration with the extract (20 mL kg−1). Blood was harvested into heparinized polypropylene tubes and centrifuged at 4000 rpm for 15 min at 4 °C to obtain plasma. The plasma samples were frozen and stored at −20 °C until analysis.
Preparation of working solutions, calibration standards, and quality control samples
Individual stock standard solutions of EVO, RUT, DHE, LIM, RVN, OBA, LIQN, LIQ, ILIQ, and IS were prepared on a weight basis in methanol at 500 μg mL−1. The mixture standard solution was prepared by transferring certain volumes of above individual stock solutions except that of IS, and then mixing with methanol:water (1:1, v/v) in a volumetric flask. Working solutions of analytes interested were prepared by serial dilution of the mixture standard solution to appropriate concentration with methanol:water (1:1, v/v). The working solution of IS was prepared in methanol:water (1:1, v/v) at 100 ng mL−1. All these solutions were kept at 4 °C. The storage time of stock solutions reached at least half a year. Working solutions were renewed in a week.
Calibration standards and quality control (QC) samples were prepared by spiking the working solutions of analytes into blank plasma. A total of 10 levels of calibration standards and three levels of QCs were prepared.
Sample extraction procedures
To assess the extraction performance of different sample preparation approaches, six sample extraction methods were simultaneously compared with six replicates of each method.
Protein precipitation
Plasma sample (50 μL) was added with 5 μL of IS, except for the double blank. Instead, 5 μL of methanol:water (1:1, v/v) was added to the double blank to compensate the composition. The plasma sample was mixed with 500 μL cold methanol and acetonitrile (stored at −20°C), respectively. The mixtures were vortexed for 3 min and then centrifuged at 13,000 rpm for 10 min at 4 °C. Next, the supernatant was collected and dried under a stream of nitrogen gas at room temperature, then reconstituted in 50 μL of methanol:acetonitrile:water (2:1:1, v/v) and centrifuged at 13,000 rpm for 15 min at 4 °C before analysis. A 5-μL aliquot was injected through an LC-MS/MS system.
Liquid-liquid extraction
Plasma sample (50 μL) was added with 5 μL of IS, except for the double blank. Instead, 5 μL of methanol:water (1:1, v/v) was added to the double blank to compensate the composition. The plasma sample was mixed with 500 μL MTBE:CH2Cl2 (4:1, v/v) and ethyl acetate:n-butanol (3:2, v/v), respectively. The mixtures were vortexed for 3 min and then centrifuged at 13,000 rpm for 10 min at 4 °C. The organic layer was withdrawn and dried under a stream of nitrogen gas at room temperature, then reconstituted in 50 μL of methanol:acetonitrile:water (2:1:1, v/v) and centrifuged at 13,000 rpm for 15 min at 4 °C before analysis. A 5-μL aliquot was injected through an LC-MS/MS system.
Salting-out-assisted liquid-liquid extraction
Plasma sample (50 μL) was added with 5 μL of IS, except for the double blank. Instead, 5 μL of methanol:water (1:1, v/v) was added to the double blank to compensate the composition. Then, 200 μL of cold acetonitrile (stored at −20°C) was added into the sample and vortexed for 1 min. Fifty microliters of of 2 M ammonium acetate was added sequentially. The mixture was vortexed for 5 min and centrifuged at 13,000 rpm for 10 min at 4 °C. One hundred microliters of the organic layer was withdrawn and mixed with 100 μL of deionized water. A 5-μL aliquot was injected through an LC-MS/MS system.
Sugaring-out-assisted liquid-liquid extraction
The extraction workflow of this protocol was identical to SALLE, while 2 M ammonium acetate was replaced with 2 M glucose.
Chromatography
A Waters ACQUITY UPLC® HSS T3 column (2.1 × 100 mm, 1.8 μm) was used for separation. The mobile phase consisted of methanol:acetonitrile (2:1, v/v, solvent A) and 0.1% formic acid solution (solvent B). The optimized gradient elution conditions were used as follows: t = 0~0.5 min, gradient linearly from 5 to 5% A; t = 0.5~1 min, gradient linearly from 5 to 40% A; t = 1~8 min, gradient linearly from 40 to 100% A; t = 8~9.5 min, gradient linearly from 100 to 100% A; t = 9.5~10 min, gradient linearly from 100 to 5% A; t = 10~11.5 min, gradient linearly from 5 to 5% A. The flow rate was 0.3 mL min−1. The column temperature was kept at 40 °C. The analytes and the IS were eluted over 0~8 min while the last 3.5 min was used for column cleaning and re-equilibration.
Mass spectrometric detection
The mass spectrometer was operated in both positive and negative ion multiple reaction monitoring (MRM) modes. The interface voltage was set to 4000 V. The source temperature was set to 300 °C. The drying gas flow, heating gas flow, and nebulizing gas flow were 10, 10, and 3 L min−1, respectively. CID gas pressure was 270 kPa.
Results and discussion
Optimization of mass parameters
MRM mode was chosen because of its superior sensitivity and selectivity in quantitative bioanalysis. The full-scan mass spectra were first carried out to select ionization type and precursor ion for the developed MRM method. EVO, RUT, DHE, LIM, RVN, and OBA were detected in positive ion mode, while LIQN, LIQ, and ILIQ were obtained in negative ion mode based on the structures of the compounds and the response in mass spectrometry. In positive mode, protonated ion peaks ([M + H]+) possessed the strongest response, although the ammonium adduct and sodium adduct ions could also be detected. Likewise, deprotonated ion peaks ([M - H]-) were monitored as the base peaks in negative mode. Following searching precursor ion, product ion, collision energy (CE), and Q1 and Q3 pre bias voltage parameters were selected and optimized to yield stronger response and better linearity. The optimized parameters are shown in Table 1. The product ion mass spectra and proposed fragmentation pathways of the analytes and IS are given in Supplementary Information (ESM) Fig. S1.
Optimization of chromatography conditions
The influence of the composition of mobile phase (such as acetonitrile-water, methanol-water, and acetonitrile-methanol-water), the ionic intensity and pH value of the buffer (such as 0.01%, 0.1% formic acid solution, 0.1% formic acid-5 mM ammonium acetate solution, and 2 mM, 5 mM ammonium acetate solution), and the type of column (such as Waters ACQUITY UPLC® BEH C18 and HSS T3 columns) on chromatographic separation and response in the mass spectrometry were investigated.
In fact, although the gradient elution program was optimized, neither acetonitrile nor methanol had a good separation performance for the ten compounds, as LIQ and DHE, as well as EVO and OBA, had similar chromatographic retention behavior in these conditions. The ability to maximize selectivity and improve separation effect with a specific column could be achieved by adjusting the proportion of acetonitrile and methanol. A better separation between the ten components was obtained on the strength of an organic phase with methanol:acetonitrile (2:1, v/v). Additionally, the presence of ammonium acetate could improve response of EVO and RUT in positive ion mode but reduce that of LIQN, LIQ, and ILIQ in negative mode. Besides, the weak-acid-weak-base amine salt was not beneficial to peak shape. Thus, 0.1% formic acid solution was a pretty good compromise. Thirdly, the retention, separation, and symmetry of the peaks eluted from HSS T3 column were better than those of the peaks eluted from BEH C18 column. Therefore, the column packed with high-strength silica (HSS) particles was chosen. As Fig. 2 shown, good resolution and peak shape of the ten compounds had been obtained by this developed separation method. The retention time is listed in Table 1.
Optimization of sample preparation approaches
Extraction performance comparison
When it comes to plasma samples with complicate base and which contain trace components, a pretreatment step will usually be imperative for purifying and enrichment. To assess the extraction performance, six sample preparation procedures were compared. The reproducibility, matrix effect, and recovery with six replicates of each procedure served as main evaluation index for the optimization.
On average, PPT methods, especially with acetonitrile, achieved the highest extraction recovery efficiency among these approaches. Matrix interference of DHE, LIM, and LIQ was the main difficulty encountered by PPT methods (see Fig. 3, as well as ESM Table S1). It is generally accepted that matrix interference can be caused by the endogenous compounds of biological matrix which coelute with analytes during chromatographic separation and then interfere the electrospray ionization efficiency in the mass spectrometer [36, 37]. PPT is regarded as a rapid and simple method but with deficient purification capability of biological samples. For plasma samples, phospholipids are reported to be one of the major endogenous interference [38]. Therefore, strong matrix interference might occur through PPT methods in the case of compounds possessing high polarity or polar group in structure, such as DHE, LIQ, and LIM.
Matrix effect and recovery of the extraction methods for QC standards spiked in blank plasma (method A, PPT with methanol; method B, PPT with acetonitrile; method C, LLE with MTBE-dichloromethane; method D, LLE with ethyl acetate-n-butanol; method E, SALLE with ammonium acetate; method F, SULLE with glucose)
LLE with non-polar mixtures preferably extracted non-polar compounds, and it could be understood as a biased procedure for hydrophilic compounds. Although higher matrix effect values were obtained by LLE methods, the low recovery of LIQ and ILIQ, which was not improved by adjusting pH value with acetic acid nor ammonia, could not be neglected (see Fig. 3, as well as ESM Table S2).
Considering the principles of above approaches and the physicochemical properties of analytes interested, homogeneous LLE with mild condition and excellent extraction performance were evaluated based on several previous studies with minor modifications [17, 19, 20]. In general, the extraction recovery efficiency and matrix effect of SALLE with ammonium acetate were better than those of SULLE with glucose (see Fig. 3, as well as ESM Table S3), which was similar to the reported study [20]. By evaluating the extraction recovery of LIQ, compared with PPT and LLE methods, SALLE with ammonium acetate exhibited the intermediate recovery performance. Both LLE and SALLE are methods to separate compounds based on their relative solubility in two different solvents, which involves a transfer of compounds from one liquid into another liquid phase, generally from aqueous (polar) phase to organic (non-polar or weak-polar) phase. Thus, the relatively lower extraction recovery of LIQ appears to be caused by its hydrophilicity. Unlike LLE, the aqueous and organic solvents employed in SALLE are usually miscible with each other, suggesting that SALLE possessed comparable extraction performance with that of LLE but could be beneficial to hydrophilic compounds. The salting-out effect can be explained by two major hypotheses. One assumes the salts added would be preferentially dissolved in one of the solvents which make this solvent to be insoluble in the other solvent, namely, mutual solubility decrease hypothesis. The other hypothesis assumes that electrostatic attraction could be caused by salt addition because of the polarity difference, leading to the removal of one solvent [39].
Taken together, PPT methods obtained fairly high extraction recovery for all of the analytes, and LLE methods achieved the highest acceptable matrix effect value on average, while SALLE method produced both satisfactory matrix effect and relatively high extraction recovery for analytes with a broad range of polarity. Thus, SALLE was chosen as the optimum approach among the extraction methods assessed above, because of its high recovery, satisfactory matrix interference, and broad coverage of analytes.
Optimization of SALLE
To look deep into the method optimization of SALLE, the influence of type and concentration of salt on extraction performance was investigated. Ammonium salts were selected because of their solubility and compatibility with LC-MS/MS system. A few studies have reported that the application of SALLE with ammonium acetate and ammonium formate indicates satisfactory recovery and matrix interference [18, 25, 26]. In this study, we briefly attempted to quantify very small numbers of samples extracted with the two mentioned ammonium salts, and we found that SALLE with the two kinds of salt both generated acceptable recovery and matrix interference. Notably, a comparison of extraction efficiency between SALLE with ammonium acetate and ammonium formate showed that the latter displayed much poorer method reproducibility for EVO (three levels of QCs with an RSD of 15.53%) and RVN (three levels of QCs with an RSD of 14.02%). Besides, ammonium acetate produced better accuracy and precision (see ESM Fig. S2, and the results of ammonium formate see ESM Table S4). The results showed that higher analytical reproducibility could be achieved by SALLE with ammonium acetate.
Besides, the influence of the composition of organic solvents, including acetonitrile, and acetonitrile-methanol (9:1, v/v), on extraction performance was investigated using the same lot of blank plasma. With the addition of methanol, the precipitate changed from lump structure to floccule (see Fig. 4a). And the interface between acetonitrile and methanol-aqueous layer two-phase was not obvious more than that between acetonitrile-aqueous layer (see Fig. 4b and c). As for matrix effect and extraction recovery, comparable results were obtained by the two organic systems with 2 M ammonium acetate. Therefore, acetonitrile was chosen because of the more apparent interface. Moreover, acetonitrile was pre-cooled in a −20°C freezer to achieve a better separation, which was consistent with the reported studies previously [15]. With respect to the organic layer collection, half of acetonitrile volume of upper layer was withdrawn to avoid phase perturbation and matrix interference.
Comparisons between extraction solvents. (a) The shapes of precipitates formed in SALLE with acetonitrile or acetonitrile-methanol (9:1, v/v) (2M ammonium acetate, before centrifugation). (b) The appearance of extracts obtained immediately by SALLE with acetonitrile or acetonitrile-methanol (9:1, v/v) (2M ammonium acetate, after centrifugation). (c) The appearance of extracts stored 1h at room temperature after extraction
Dhamole et al. reported that high concentration of salt addition favored phase separation [15]. Our results showed that homogeneous solution did not tend to separate into two layers at a low concentration of ammonium acetate. When the concentration of ammonium acetate was increased to a level of approximately 0.5 M in aqueous phase (by calculation), the supernatant started to be separated into two layers and to make salting-out happen (see Fig. 5a and b). Higher concentration of ammonium acetate was preferred for salting-out, but had an adverse effect on the detection sensitivity of LIQN, LIQ, and ILIQ which were detected in negative ion mode. Thus, 2.0 M of ammonium acetate was prepared, and the final aqueous concentration of ammonium acetate was approximately 1.0 M (by calculation) after sample preparation.
Method validation for SALLE with ammonium acetate
In order to evaluate the developed method performance, a validation was performed in accordance with Bioanalytical Method Validation Guidance for Industry [35]. Parameters including selectivity, linearity, accuracy, precision, recovery, matrix effect, and stability were evaluated to ensure the method was suited to the analysis of the study samples.
Selectivity and specificity
Selectivity and specificity of the method were demonstrated by analyzing blank plasms from six individual sources. The typical chromatograms for the blank plasma, and blank plasma spiked with the analytes and the IS are shown in ESM Fig. S3. There was no obvious interference at the retention times of the analytes and the IS in chromatograms.
Linearity and range
Ten levels of standards containing the nine analytes were used for the evaluation of the calibration curves. Weighted least squares linear regression model was used to fit the calibration curves. All calibration standards were within ±15% of nominal concentrations, except the calibrator at LLOQ was ±20% of the nominal concentration. The linear ranges and the runs results are presented in Table 2. The linearity of the nine analytes exhibited good linear relationship with correlation coefficients of determination (R2) within the range of 0.9933~0.9996.
Accuracy and precision
Intra- and inter-batch precision and accuracy were established by assaying three levels of QCs and LLOQ samples in the same batch and three consecutive runs. Six replicates of LLOQ and QCs of each concentration level were evaluated against a fitted calibration curve. Accuracy was expressed as the relative difference between the nominal concentration and found concentration. Precision was expressed by intra-batch and inter-batch RSDs. The results are shown in Table 3. All evaluation QCs were within acceptance.
Matrix effect
In order to confirm that the assay was free of interfering, matrix effect QCs were prepared with the same spiking procedure using six different lots of blank plasma, while matrix effect references were prepared with water. At evaluation, the mean response of matrix effect QCs was compared with that of references at low, medium, and high levels, respectively. The matrix effects for the nine analytes were within 84.09 to 106.37% at three levels (see Table 4). There was no significant ion suppression or enhancement for the analytes.
Extraction recovery
Extraction recovery rates of the nine analytes were evaluated by comparing the response of extracted samples with the response of corresponding extracts of blanks spiked with the analytes post-extraction. The calculated extraction recoveries were within 84.86 to 109.60% at three levels for the analytes except LIQ. The extraction recovery of LIQ was in the range of 59.96~72.60%. Although not all analytes had a 100% recovery, the obtained results were consistent and reproducible with relative standard deviations (RSDs, n = 6) below 7.44% shown in Table 4.
Stability
Given the effect of salt addition, the stability of EVO, RUT, DHE, LIM, RVN, OBA, LIQN, LIQ, and ILIQ during sample preparation (in plasma at room temperature) and chromatographic analysis (extracts at 4 °C) was investigated by analyzing six replicates at LLOQ, and low-, medium-, and high-level concentration. Besides, the stability of samples after three freeze-thaw cycles and long-term storage was also assessed. The results are summarized in ESM Table S5. Under the developed condition, the concentrations for the nine analytes at four levels in plasma sample deviated within −13.10 to 14.18% of nominal values, which indicated the stability during sample preparation and chromatographic analysis.
Incurred sample reproducibility (ISR)
In order to demonstrate the method reliability, 32 incurred SD rat plasma samples were analyzed to produce original values, and then reanalyzed to generate repeat values in separate runs. The percentage difference of results was calculated through the absolute difference divided by the mean of original value and repeat value of each sample. In the ISR evaluation of 32 samples, 32/32 of DHE, RVN, LIM, OBA, LIQ, and ILIQ repeat values and 31/32 of EVO, RUT, and LIQN repeat values were within ±20% of the mean. The mean difference was 0.26% for DHE, −5.33% for EVO, −4.54% for RUT, 1.06% for LIM, −2.25% for RVN, −2.74% for OBA, −4.57% for LIQ, 0.86% for ILIQ, and −7.08% for ILIQ, respectively.
Application in the pharmacokinetic study
The developed method was successfully applied to reveal the pharmacokinetic profiles of the three alkaloids, three terpenoids, and three flavonoids in rat plasma after oral administration of FTR and licorice extracts together. The mean plasma concentration-time curves of the nine compounds are shown in Fig. 6, and the pharmacokinetic parameters are summarized in Table 5. Pharmacokinetic profiles showed that LIM and RVN had good oral absorption effect with maximum plasma drug concentrations of 149.26 and 78.92 ng mL−1, respectively. Moreover, a similar tendency was found in the mean concentration-time curves of DHE and LIQN, which indicated the similar pharmacokinetic behaviors of the two compounds after oral administration of the extracts of FTR and licorice together. Besides, although EVO and RUT had rapid absorption and low concentration levels in plasma, their pharmacokinetics parameters could also be determined through the developed method. Hence, the results exhibited the sensitivity and validity for the developed SALLE method, suggesting the practicability of this sample preparation workflow which could contribute to the pharmacokinetic studies of TCM to get wider analyte coverage.
Conclusions
In this work, we assessed extraction performance on various sample preparation approaches including PPT, LLE, SULLE, and SALLE. On average, PPT produced significantly high extraction recovery and LLE minimized the effects of matrix on ion suppression. However, both of the two methods failed to meet the criteria of matrix effect and recovery for analytes with diverse polarity. The comparison between above-given protocols demonstrated that SALLE appeared to be the optimal method, because of its wide analyte coverage, satisfactory reproducibility, acceptable recovery, and low matrix interference. The addition of ammonium salts during extraction significantly improved the extraction performance of acetonitrile, and there was subtle difference between the extraction performance of ammonium acetate and ammonium formate. Besides, relatively higher salt concentration help improve the extraction performance. Taken together, to our knowledge, the optimized sample preparation method in this study would therefore be probably a better suitable sample pretreatment strategy for plasma high-throughput quantitation with analytes of diverse polarity among conventional techniques.
Using the developed method in this study, the pharmacokinetic profiles of nine compounds in rat plasma after oral administration of FTR and licorice decoction were successfully determined. Ideally, more pharmacokinetic markers of herbal medicines and higher detection sensitivity will be essential for further optimization and validation, which would benefit to investigate the ADMET properties and illustrate the efficacy mechanism responsible for TCM as well as its combined medication and processing technique.
Abbreviations
- ADMET:
-
Absorption, distribution, metabolism, excretion, and toxicity
- HSS:
-
High-strength silica
- ISR:
-
Incurred sample reproducibility
- LLE:
-
Liquid-liquid extraction
- LLOQ:
-
Lower limit of quantitation
- MTBE:
-
Methyl tertiary-butyl ether
- PPT:
-
Protein precipitation
- SALLE:
-
Salting-out-assisted liquid-liquid extraction
- SULLE:
-
Sugaring-out-assisted liquid-liquid extraction
- TCM:
-
Traditional Chinese medicine
References
Shen Y, Luo X, Li H, Chen Z, Guan Q, Cheng L. Simple and reliable serotonin assay in human serum by LC-MS/MS method coupled with one step protein precipitation for clinical testing in patients with carcinoid tumors. J Chromatogr B Anal Technol Biomed Life Sci. 2020;1158:122395. https://doi.org/10.1016/j.jchromb.2020.122395.
Putnam WC, Kallem RR, Subramaniyan I, Beg MS, Edpuganti V. Bioanalytical method development and validation of a liquid chromatography-tandem mass spectrometry method for determination of β-lapachone in human plasma. J Pharm Biomed Anal. 2020;188:113466. https://doi.org/10.1016/j.jpba.2020.113466.
Bin Jardan YA, Mohamed K, Abbas N, El-Gendy M, Alsaif N, Alanazi M, et al. Development and validation of GC-MS method for determination of methcathinone and its main metabolite in mice plasma and brain tissue after SPE: pharmacokinetic and distribution study. J Pharm Biomed Anal. 2021;194:113798. https://doi.org/10.1016/j.jpba.2020.113798.
Lin LC, Li SH, Wu YT, Kuo KL, Tsai TH. Pharmacokinetics and urine metabolite identification of dehydroevodiamine in the rat. J Agric Food Chem. 2012;60(7):1595–604. https://doi.org/10.1021/jf204365m.
Lin Q, Pu H, Guan H, Ma C, Zhang Y, Ding W, et al. Rapid identification and pharmacokinetic studies of multiple active alkaloids in rat plasma through UPLC-Q-TOF-MS and UPLC-MS/MS after the oral administration of Zanthoxylum nitidum extract. J Pharm Biomed Anal. 2020;186:113232. https://doi.org/10.1016/j.jpba.2020.113232.
Andreassen TN, Havnen H, Spigset O, Falch BMH, Skrastad RB. High throughput UPLC®-MSMS method for the analysis of phosphatidylethanol (PEth) 16:0/18:1, a specific biomarker for alcohol consumption, in whole blood. J Anal Toxicol. 2018;42(1):33–41. https://doi.org/10.1093/jat/bkx075.
Wawrzyniak R, Kosnowska A, Macioszek S, Bartoszewski R, Jan MM. New plasma preparation approach to enrich metabolome coverage in untargeted metabolomics: plasma protein bound hydrophobic metabolite release with proteinase K. Sci Rep. 2018;8(1):9541. https://doi.org/10.1038/s41598-018-27983-0.
Zhang Q, Nong Y, Liu Z, Gong L. Proteinase K combining two-step liquid-liquid extraction for plasma untargeted liquid chromatography-mass spectrometry-based metabolomics to discover the potential mechanism of colorectal adenoma. Anal Chem. 2019;91(22):14458–66. https://doi.org/10.1021/acs.analchem.9b03121.
Xu H, Li Q, Yin Y, Lv C, Sun W, He B, et al. Simultaneous determination of three alkaloids, four ginsenosides and limonin in the plasma of normal and headache rats after oral administration of Wu-Zhu-Yu decoction by a novel ultra fast liquid chromatography-tandem mass spectrometry method: application to a comparative pharmacokinetics and ethological study. J Mass Spectrom. 2013;48(4):519–32. https://doi.org/10.1002/jms.3183.
Marcos J, Renau N, Casals G, Segura J, Ventura R, Pozo OJ. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2014;812:92–104. https://doi.org/10.1016/j.aca.2013.12.030.
Bhende SD, Varanasi MB, Abbulu K. A sensitive and efficient LC-MS/MS method for the bioanalysis of fosinopril diacid from human plasma and its application for a bioequivalence study in humans. Biomed Chromatogr. 2021;35(5):e5047. https://doi.org/10.1002/bmc.5047.
Asimakopoulos AG, Thomaidis NS, Bisphenol A. 4-t-octylphenol, and 4-nonylphenol determination in serum by hybrid solid phase extraction-precipitation technology technique tailored to liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2015;986-987:85–93. https://doi.org/10.1016/j.jchromb.2015.02.009.
Xiang C, Qiao X, Wang Q, Li R, Miao W, Guo D, et al. From single compounds to herbal extract: a strategy to systematically characterize the metabolites of licorice in rats. Drug Metab Dispos. 2011;39(9):1597–608. https://doi.org/10.1124/dmd.111.038695.
Gu TY, Gu Y, Zheng YZ. Phase-separation of acetonitrile-water mixture in protein-purification. Sep Technol. 1994;4(4):258–60. https://doi.org/10.1016/0956-9618(94)80031-6.
Dhamole PB, Mahajan P, Feng H. Phase separation conditions for sugaring-out in acetonitrile-water systems. J Chem Eng Data. 2010;55(9):3803–6. https://doi.org/10.1021/je1003115.
Oence DN, Gu TY. Liquid-liquid equilibrium of the acetonitrile-water system for protein purification. Sep Technol. 1996;6(4):261–4. https://doi.org/10.1016/S0956-9618(96)00161-0.
Myasein F, Kim E, Zhang J, Wu H, El-Shourbagy TA. Rapid, simultaneous determination of lopinavir and ritonavir in human plasma by stacking protein precipitations and salting-out assisted liquid/liquid extraction, and ultrafast LC-MS/MS. Anal Chim Acta. 2009;651(1):112–6. https://doi.org/10.1016/j.aca.2009.08.010.
Zhang J, Rodila R, Gage E, Hautman M, Fan L, King LL, et al. High-throughput salting-out assisted liquid/liquid extraction with acetonitrile for the simultaneous determination of simvastatin and simvastatin acid in human plasma with liquid chromatography. Anal Chim Acta. 2010;661(2):167–72. https://doi.org/10.1016/j.aca.2009.12.023.
Zhou W, Wu H, Wang Q, Zhou X, Zhang Y, Wu W, et al. Simultaneous determination of formononetin, biochanin A and their active metabolites in human breast milk, saliva and urine using salting-out assisted liquid-liquid extraction and ultra high performance liquid chromatography-electrospray ionization tandem mass spectrum. J Chromatogr B Anal Technol Biomed Life Sci. 2020;1145. https://doi.org/10.1016/j.jchromb.2020.122108.
Zhang J, Myasein F, Wu HA, El-Shourbagy TA. Sugaring-out assisted liquid/liquid extraction with acetonitrile for bioanalysis using liquid chromatography-mass spectrometry. Microchem J. 2013;108:198–202. https://doi.org/10.1016/j.microc.2012.10.024.
Yoshida M, Akane A, Nishikawa M, Watabiki T, Tsuchihashi H. Extraction of thiamylal in serum using hydrophilic acetonitrile with subzero-temperature and salting-out methods. Anal Chem. 2004;76(16):4672–5. https://doi.org/10.1021/ac040065a.
Wang B, Feng H, Ezeji T, Blaschek H. Sugaring-out separation of acetonitrile from its aqueous solution. Chem Eng Technol. 2008;31(12):1869–74. https://doi.org/10.1002/ceat.200800003.
Tsai WH, Huang TC, Chen HH, Wu YW, Huang JJ, Chuang HY. Determination of sulfonamides in swine muscle after salting-out assisted liquid extraction with acetonitrile coupled with back-extraction by a water/acetonitrile/dichloromethane ternary component system prior to high-performance liquid chromatography. J Chromatogr A. 2010;1217(3):250–5. https://doi.org/10.1016/j.chroma.2009.11.035.
Gu YS, Shih PH. Salt-induced phase separation can effectively remove the acetonitrile from the protein sample after the preparative RP-HPLC. Enzym Microb Technol. 2004;35(6-7):592–7. https://doi.org/10.1016/j.enzmictec.2004.08.011.
Wu H, Zhang J, Norem K, El-Shourbagy TA. Simultaneous determination of a hydrophobic drug candidate and its metabolite in human plasma with salting-out assisted liquid/liquid extraction using a mass spectrometry friendly salt. J Pharm Biomed Anal. 2008;48(4):1243–8. https://doi.org/10.1016/j.jpba.2008.09.002.
Xiong X, Yang L. Salting-out-assisted liquid-liquid extraction with acetonitrile for the determination of trimetazidine in rat plasma using liquid chromatography-mass spectrometry. Biomed Chromatogr. 2015;29(2):268–74. https://doi.org/10.1002/bmc.3271.
Li Y, Wang Y, Tai W, Yang L, Chen Y, Chen C, et al. Challenges and solutions of pharmacokinetics for efficacy and safety of traditional Chinese medicine. Curr Drug Metab. 2015;16(9):765–76. https://doi.org/10.2174/138920021609151201114223.
Li M, Wang C. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: a review. J Ethnopharmacol. 2020;263:113231. https://doi.org/10.1016/j.jep.2020.113231.
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press; 2020.
Shan QY, Sang XN, Hui H, Shou QY, Fu HY, Hao M, et al. Processing and polyherbal formulation of Tetradium ruticarpum (A. Juss.) Hartley: phytochemistry, pharmacokinetics, and toxicity. Front Pharmacol. 2020;11:133. https://doi.org/10.3389/fphar.2020.00133.
Huang W, Sun R. Study on analgesic and accompanying toxic and side effects of Euodiae Fructus based on clinical efficacy dose. China J Chin Mater Med. 2013;38(13):2176–81. (in Chinese). https://doi.org/10.4268/cjcmm20131328.
Yang L, Huang K, Chen X, Yang H, Li K, Zhang Z. Study on analgesic and anti-inflammatory effects of different kinds of Evodia Rutaecarpa (Juss.) Benth. China Pharm. 2013;22(5):4–5. (in Chinese). https://doi.org/10.3969/j.issn.1006-4931.2013.05.002.
Jiang M, Zhao S, Yang S, Lin X, He X, Wei X, et al. An “essential herbal medicine” - licorice: a review of phytochemicals and its effects in combination preparations. J Ethnopharmacol. 2020;249:112439. https://doi.org/10.1016/j.jep.2019.112439.
Li X, Ge J, Zheng Q, Zhang J, Sun R, Liu R. Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine. 2020;68:153180. https://doi.org/10.1016/j.phymed.2020.153180.
U.S. Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry, 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry. Accessed 29 Jul 2021.
Yaroshenko DV, Kartsova LA. Matrix effect and methods for its elimination in bioanalytical methods using chromatography-mass spectrometry. J Anal Chem. 2014;69(4):311–7. https://doi.org/10.1134/S1061934814040133.
Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y. Validation of bioanalytical LC–MS/MS assays: evaluation of matrix effects. J Chromatogr B. 2009;877(23):2198–207. https://doi.org/10.1016/j.jchromb.2009.01.003.
Jemal M, Ouyang Z, Xia YQ. Systematic LC-MS/MS bioanalytical method development that incorporates plasma phospholipids risk avoidance, usage of incurred sample and well thought-out chromatography. Biomed Chromatogr. 2010;24(1):2–19. https://doi.org/10.1002/bmc.1373.
Anthemidis AN, Ioannou K-IG. Recent developments in homogeneous and dispersive liquid-liquid extraction for inorganic elements determination. A review. Talanta. 2009;80(2):413–21. https://doi.org/10.1016/j.talanta.2009.09.005.
Funding
This work was supported by the Three-year Action Plan for the Development of Traditional Chinese Medicine of Shanghai [ZY(2018-2020)-CCCX-5002] awarded to Professor Changhong Wang for financial support of this study.
Author information
Authors and Affiliations
Contributions
Manlin Li: conceptualization, methodology, validation, writing—original draft preparation. Hanxue Wang: investigation. Xiaohan Huan: investigation. Ning Cao: validation. Huida Guan: formal analysis. Hongmei Zhang: resources. Xuemei Cheng: data curation. Changhong Wang: writing—review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Ethics approval
The animal experimental research was approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (Approval Number: PZSHUTCM200731003) and guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 580 kb)
Rights and permissions
About this article
Cite this article
Li, M., Wang, H., Huan, X. et al. Simultaneous LC-MS/MS bioanalysis of alkaloids, terpenoids, and flavonoids in rat plasma through salting-out-assisted liquid-liquid extraction after oral administration of extract from Tetradium ruticarpum and Glycyrrhiza uralensis: a sample preparation strategy to broaden analyte coverage of herbal medicines. Anal Bioanal Chem 413, 5871–5884 (2021). https://doi.org/10.1007/s00216-021-03568-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03568-1