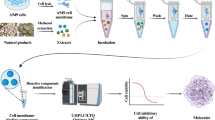
Abstract
A novel stability-enhanced graphene quantum dot (GQD)-decorated epidermal growth factor receptor (EGFR) cell membrane chromatography was constructed to study the potential application of GQDs in bioaffinity chromatography, and to screen active components acting on EGFR from traditional Chinese medicine (TCM). The carboxyl groups on the surface of GQDs reacted with the amino groups of the amino-silica gel (SiO2-NH2) to form a covalent bond, thereby preparing the GQD-decorated silica gel (SiO2-GQDs). The EGFR cell membrane was further immobilized on the SiO2-GQDs through the same covalent binding method to obtain the GQD-decorated cell membrane stationary phase (SiO2-GQDs-CMSP). In this way, the cell membrane was firmly immobilized on the decorated silica carrier. The life span and stability of the GQD-decorated cell membrane chromatographic (SiO2-GQDs-CMC) column were both enhanced, and the optimal immobilization conditions of the EGFR cell membrane were also determined. This model was then verified by establishing a SiO2-GQDs-CMC online liquid chromatography-ion trap-time-of-flight (LC-IT-TOF) system to screen possible active components in Peucedanum praeruptorum Dunn. As a result, praeruptorin B (Pra-B) was screened out, and its inhibitory effect against EGFR cell growth was evaluated by the cell counting kit-8 (CCK-8) assay. Molecular docking assay was also conducted to further estimate the interaction between Pra-B and EGFR. Overall, this research indicated that GQDs may be a promising nanomaterial to be used in prolonging the life span of the CMC column, and Pra-B could be a potential EGFR inhibitor so as to treat cancer.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell membrane chromatography (CMC) is an effective chromatographic method applied for the screening of active components acting on specific receptors from TCM [1,2,3]. A growing body of literature has recognized the application of CMC and established various CMC models to study drug-receptor interactions, and screen active ingredients from a complex system [4,5,6,7,8,9]. Of note, the preparation of cell membrane stationary phase (CMSP) is performed by immobilizing the cell membrane highly expressing one or more [10] specific receptors onto the surface of silica gel or other carriers. TCM injections [11] or the extracts of complex samples such as medicinal herbs are injected into the CMC model; the retained fractions are then further analyzed by reversed-phase chromatography and mass spectrometry (MS). Theoretically, the stronger the components are retained, the more biologically active they are likely to be on the specific receptors [1, 12].
Despite these advantages and applications, the short column life span of the CMC model still restricts its application to a certain extent. In a typical CMC model, the cell membrane is fixed on the silica carrier by physical adsorption-hydrophobic interaction [1, 13]. However, with the elution of the mobile phase, this weak and unstable binding force can easily cause the fall-off of the cell membrane, or the immobilized receptors may gradually lose biological activities—both can lead to poor column repeatability and short life span (around 48–72 h) [14,15,16,17]. In recent years, several attempts using chemically modified silica-based carriers have been made to solve these problems [18,19,20,21,22]. For instance, 3-aminopropyltriethoxysilane (APTES) was applied as a bridge to connect silica gel and glutaraldehyde, thereby improving the adhesion of cell membrane through the covalently binding between aldehyde groups of glutaraldehyde and amino groups on the cell membrane [18, 19, 21]; 3-mercaptopropyltrimethoxysilane (MPTS)-modified silica gel was obtained to realize covalently binding between an active ester group of MPTS and the amino groups on the surface of the cell membrane [20]; polyvinyl alcohol (PVA) possessing high bioaffinity activities was mixed with poly dimethyl diallyl ammonium chloride (PDAC) and glutaraldehyde to form stable polymer structure, thereby acting as a stable carrier for cell membrane immobilization [22]. Although these methods have prolonged the column life and increased cell membrane coverage, the high requirements of the reaction conditions and the toxicity of the reagents in these approaches still have to be considered.
Graphene, a promising carbon nanomaterial [23], is composed of a single-atom-thick and two-dimensional sheet of hexagonally arranged carbon atoms [24]. It plays an increasingly important role in the modification of stationary phase in analytical chemistry [25]. Functional graphene nanomaterials (FGNs) exhibit huge potential in biomolecule adsorption compared with other nanomaterials because of various functional groups, high specific surface area, and good interface properties. Graphene quantum dots (GQDs), as one of the family members of FGNs, are nanometer-sized single-layer fragments of graphene with lateral size less than 10 nm [26, 27]. They are deemed as a kind of promising loading carrier for various molecules such as protein and drug molecules through covalent or noncovalent immobilization [27]. GQDs have also been generally used in fluorescent sensing and bioimaging [28,29,30] on account of its unique fluorescence. Although GQDs are now widely applied in multiple research areas, the roles they play in affinity chromatographic material science remain mostly unexamined. Furthermore, the unique physical and chemical properties of GQDs, such as chemical stability [28], high thermostability, and good dispersibility [31], and the existence of oxygen-rich functional groups [32], enable them to be immobilized on the surface of silica matrix. These indicate that GQDs have huge potential in the preparation of novel CMSP.
The overexpression of EGFR has been recognized as the driver mechanism in many cancer diseases such as breast cancer, lung cancer, and pancreatic cancer [33]. Therefore, EGFR is an attractive therapeutic target in the development of anti-cancer drugs [34]. Peucedanum praeruptorum Dunn is a famous herbal medicine which has significant contributions to the treatment of cough in traditional medicinal practice [35]. It has also been recognized to have various pharmacological activities such as anti-hypertensive, anti-myocardial ischemia [36], and anti-tumor effects [35]. Several studies suggest that the extracts of Peucedanum praeruptorum Dunn may contain compounds showing cell growth inhibitory activities in cancer cell lines [37, 38].
In this study, a covalently GQD-decorated EGFR cell membrane chromatographic (CMC) column combined with LC-IT-TOF system was developed for the first time to screen active anti-cancer components from Peucedanum praeruptorum Dunn. The characterization of the synthesized stationary phase and the optimization of immobilization conditions were performed. Eventually, the CCK-8 assay and molecular docking assay were conducted for the verification of the screened component.
Methods
Regents and materials
GQDs (JCGOD-2-6n) were obtained from Nanjing JCNO Technology Co., Ltd. (Nanjing, China). SiO2-NH2 (IN850010-0) was acquired from Shaanxi Taibo Scientific Instrument Co., Ltd. (Shaanxi, China). Gefitinib (≥ 98%) was procured from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Praeruptorin B (≥ 98%, Lot#: 130225) was purchased from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China). Phentolamine mesylate, benzhexol hydrochloride (≥ 98%), and prazosin hydrochloride (≥ 98%) were purchased from Macklin (Shanghai, China). Peucedanum praeruptorum Dunn was gained from a TCM store (Xi’an, China), and authenticated by the Department of Pharmacognosy, Xi’an Jiaotong University (Xi’an, China). Silica gel (ZEX-II, 5 μm, 200 Å) was purchased from Qingdao Meigao Chemical (Qingdao, China). HPLC grade methanol was supplied by Thermo Fisher Scientific (Pittsburgh, USA). A MK-459 Millipore Milli-Q Plus ultrapure water system was used to prepare ultrapure water. The ultrasonic cell grinder (JY 92-II) obtained from Ningbo Scientz Biotechnology Co., Ltd. (Ningbo, China) was applied for cell rupture.
Instrument configuration and conditions
A two-dimensional system was established by combining the SiO2-GQDs-CMC column and LC-IT-TOF with a 2-position 6-port valve (892–0269, Hitachi High-Tech, Japan) and one Shim-pack VP-ODS pre-column (10 mm × 2.0 mm I.D., 5 μm, Shimadzu Corporation, Kyoto, Japan). The SiO2-GQDs-CMC column (10 mm × 2.0 mm I.D., 5 μm) was prepared by an RPL-10ZD column packing pump (Dalian Replete Science and Technology Co., Ltd., Dalian, China). It was placed as the first-dimensional screening part, while the instrumental parts (all made by Hitachi High-Tech in Japan, the same below) contained a 6110 pump (with manual purge), a plunger washing pump, a low-pressure gradient unit for pump, a 6-channel degassing unit 6, a 6210 autosampler, a 6310 column oven, a 6410 ultraviolet (UV) detector, and an interface control board. The second dimension was mainly consisted of a Shimadzu Shim-pack VP-ODS column (250 mm × 4.6 mm I.D., 5 μm, Kyoto, Japan), a 6110 pump (with manual purge), a 6430 diode array detector (DAD), and an IT-TOF (Shimadzu Corporation, Kyoto, Japan), which served as the identification part.
The schematic figure of the SiO2-GQDs-CMC online LC-IT-TOF system was shown in Fig. S1 (see Supplementary Information (ESM)). In position 2, the CMC column is mainly used for the screening of samples, while the ODS column is equilibrated by the second-dimensional mobile phase. When the system is in position 1, the components retained in the CMC column will be enriched into the pre-column. The valve is then turned to the position 2 again, and the components in the pre-column will be eluted to the ODS column for analysis and to MS for further identification.
All the first-dimensional affinity columns used ultrapure water as the mobile phase (flow rate 0.5 mL/min). The column temperature was set at 37 °C. The mobile phase in reversed-phase chromatographic column was comprised of ultrapure water (A) and acetonitrile (B) (flow rate 0.8 mL/min). The gradient elution was as follows: 0–60 min, 30–90% B; 60–70 min, 90–90% B; 70–70.1 min, 90–30% B; 70.1–90 min, 30–30% B. The MS settings can be found in the ESM.
Standard solution and sample preparation
The pre-made standard solutions (1 mg/mL, prepared with methanol) of gefitinib, praeruptorin B (Pra-B), phentolamine mesylate, benzhexol hydrochloride, and prazosin hydrochloride were stored at − 20 °C. All solutions of standards to be injected were diluted to suitable concentrations before use.
Peucedanum praeruptorum Dunn was extracted according to the following methods: 10 g of Peucedanum praeruptorum Dunn was pulverized, and the powder was thrown into 100 mL 60% ethanol. Refluxing was then performed repeatedly at 65 °C for 2 h [36]. The filtrates were collected in a conical flask. Working sample solutions were prepared as follows: concentrating 1 mL of filtrates to dryness with rotary evaporation and the dried residue was dissolved in 1 mL methanol, and then filtered using a Millipore filter (0.22 μm) before use. The above-prepared solutions were saved at − 20 °C.
Preparation of GQD-decorated silica gel
The schematic illustration of the preparation of the SiO2-GQDs-CMSP was exhibited in Fig. 1. A coupling agent, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS), was employed to covalently bond the amino groups contained in the SiO2-NH2 with the carboxyl groups of GQDs to obtain the SiO2-GQDs. In brief, 0.207 g EDC (1 mmol) and 0.124 g NHS (1 mmol) were dissolved by 72 mL of GQD aqueous dispersion (1 mg/mL), and the mix was stirred for 30 min to activate the carboxyl groups of GQDs. This step was followed by the mixing of the SiO2-NH2 (0.6 g) with the above solution and then the mixed solution was stirred at room temperature for 24 h. Finally, the resulting substance was washed with ultrapure water and methanol via the vacuum filtration for many times and dried at 60 °C under vacuum for 12 h [39].
Preparation of the SiO 2 -GQDs-CMSP
The EGFR-high expressed HEK293 (EGFR-HEK293) cell lines were constructed in our laboratory [40]. The EGFR-HEK293 cells were cultured in Dulbecco’s modified Eagle medium with the add-on of fetal bovine serum (10%), penicillin, and streptomycin (both 100 U/mL) at 37 °C under sterile conditions in 5% CO2 atmosphere. After the cell density reached to 80–100%, the cells were digested and collected. The cell digestion solution was centrifuged, and the cell pellet was suspended in physiological saline (pH 7.4). To fully collect the cells digested, the centrifugal force was placed at 3000g (10 min, 4 °C). The above procedure was repeated for three times. Next, the cleaned cell pellet was resuspended in Tris-HCl (50 mM, pH 7.4), ultrasonically ruptured for 60 min and further ruptured by an ultrasonic cell grinder (time 3 s; gap 1 s; work times 6). The cell suspension was centrifuged for 10 min under 3000g so as to avoid the adhesion of organelles. The pellet was discarded, and the supernate was centrifuged at 15000g (20 min, 4 °C). The sediment was resuspended with cold normal saline (pH 7.4) and centrifuged at the same centrifugal force to obtain the EGFR-HEK293 cell membrane.
Activating the carboxyl groups of the SiO2-GQDs in the same way as described above and the EGFR cell membrane was then slowly added, stirred at 4 °C for 6 h, and retained at 4 °C overnight. The prepared SiO2-GQDs-CMSP was cleaned with cold normal saline (pH 7.4) for three times and packed into a column (10 mm × 2.0 mm I.D., 5 μm) through wet packing machine.
Characterization of the prepared materials
The morphologies of the SiO2-NH2, SiO2-GQDs and SiO2-GQDs-CMSP were investigated with field emission scanning electron microscope (SEM, Zeiss Sigma HD). The functional groups of the carriers above were characterized by Fourier transform infrared spectrophotometer (FT-IR, Nicolet iS50). The chemical composition of the stationary phases prepared was analyzed by organic element analyzer (EA, PerkinElmer 2400 II) respectively. The fluorescence intensity of the carriers was detected by a fluorescence spectrophotometer due to the strong fluorescence of GQDs.
Maximization of the amount of immobilized EGFR cell membrane
In order to achieve the best immobilization of the EGFR cell membrane onto the SiO2-GQDs, the immobilization time, EDC/NHS mass ratio, and cell membrane amount were investigated. The amount of free cell membranes was determined by a Bradford Protein Assay Kit. The amount of immobilized cell membrane can be calculated by subtracting the amount of free cell membranes suspended in the reaction solution from the total added cell membrane amount.
The EGFR cell membrane was suspended into 3 mL cold normal saline (pH = 7.4) to obtain cell membrane suspension with a concentration of 1.24 mg/mL. Different cell membrane amounts (100 μL, 200 μL, 400 μL, 600 μL, 800 μL from cell membrane suspension mentioned above) were investigated while reacted for 6 h and used 0.207/0.124 (g/g) of EDC/NHS mass ratio.
Different immobilization times (1 h, 2 h, 4 h, 6 h, 8 h) of the EGFR cell membrane were investigated under the conditions of 0.207/0.124 (g/g) of EDC/NHS mass ratio and cell membrane amount of 600 μL 1.24 mg/mL/50 mg carriers.
Different EDC/NHS mass ratios including 0.069/0.041, 0.207/0.124, and 0.006/0.003 (g/g) were explored under the conditions of 6-h immobilization time and cell membrane amount of 600 μL 1.24 mg/mL/50 mg carriers.
System suitability of the SiO 2 -GQDs-CMC column
Gefitinib, an epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitor, was applied as a positive drug for the investigation of system suitability. Drugs with specific acting receptors (phentolamine mesylate acting on α adrenergic receptor, benzhexol hydrochloride acting on M cholinoceptor, and prazosin hydrochloride acting on postsynaptic α receptor) were selected as negative compounds to investigate the selectivity of the SiO2-GQDs-CMC column. Gefitinib was injected into the SiO2-NH2 column, SiO2-GQDs column, and SiO2-GQDs-CMC column, respectively, to evaluate the specificity of the SiO2-GQDs-CMC column, and the retention time was used as an indicator. The intra-column repeatability of a SiO2-GQDs-CMC column was evaluated by injecting gefitinib for continuous 5 times in 1 day (n = 5). The inter-column differences were tested by comparing the retention time of gefitinib on three batches (n = 3) of SiO2-GQDs-CMC columns. The life span of the SiO2-GQDs-CMC column was investigated by continuously injecting gefitinib, and the chromatographic behavior was applied as a pointer of the activity of the cell membrane. Same methods were used to test the intermediate precision, intra- and inter-column differences, and life span of the traditional CMC column.
The application of the SiO 2 -GQDs-CMC online LC-IT-TOF system
The two-dimensional system of the SiO2-GQDs-CMC coupled with LC-IT-TOF was verified by the injection of gefitinib. After verification, the system was applied to screen and identify potential components acting on EGFR from the above extracts of Peucedanum praeruptorum Dunn. Condition settings for chromatography and IT-TOF were listed in the section “Instrument configuration and conditions.”
CCK-8 assay
In order to evaluate the effect of active compounds screened out, the CCK-8 assay was conducted according to the manufacturer’s instructions. Gefitinib was employed as a positive control. Briefly, the EGFR-HEK293 cells (5 × 103) were seeded in a 96-well plate and incubated for 24 h at 37 °C. The treatment of different concentrations of gefitinib and the standard solution of the active compound (0.4 μM, 1 μM, 3 μM, 5 μM, 10 μM, 25 μM) to the cells was conducted respectively followed by incubation for 48 h. Then, the CCK-8 agent (10 μL) was mixed with the solution in each well of the 96-well plates and incubated for 40 min (37 °C). Finally, the absorbance values of the cells were obtained by converting the optical density of cells detected via a microplate reader (Bio-Rad Instruments, USA) at 450 nm.
Molecular docking assay
The binding potency of the screened compound to EGFR was evaluated by employing the Surflex-Dock module on SYBYL version × 2.0 (Tripos Inc.). The detailed operation steps were carried out according to the previous literature [41]. The selected PDB ID for EGFR is 2ITY.
Results and discussion
Characterization analysis of stationary phase
Figure 2 shows the SEM images of the SiO2-NH2, SiO2-GQDs and SiO2-GQDs-CMSP respectively. With the binding of GQDs and the coating of cell membrane, the surface of the carriers gradually becomes rough. The result indicates that the SiO2-GQDs and the SiO2-GQDs-CMSP were successfully prepared.
The FT-IR spectra (ESM Fig. S2A) demonstrate the characterization of functional groups of the SiO2-NH2, SiO2-GQDs, and SiO2-GQDs-CMSP. The peaks at 1650 cm−1 and 3500 cm−1 are derived from the stretching vibration of C=O bond in amide bond and the stretching vibration of O–H bond in hydroxyl group respectively. This indicates the successful synthesis of the SiO2-GQDs. The peak at 1550 cm−1 is related to the bending vibration of N–H bond in protein amide II band, which means the successful immobilization of the EGFR cell membrane on the SiO2-GQDs.
EA characterization of the chemical element composition of the SiO2-NH2, SiO2-GQDs, and SiO2-GQDs-CMSP is listed in Table S1 (see ESM). The increased carbon content and hydrogen content indicate the successful preparation of the SiO2-GQDs. Meanwhile, the increased content of the three elements (N, C, H) in the SiO2-GQDs-CMSP can be attributed to the successful coating of cell membrane.
From the result of the fluorescence spectrophotometer (ESM Fig. S2B), substantial fluorescence property of GQDs can be seen while the SiO2-GQDs shows significantly reduced fluorescence. With the coating of the cell membrane, fluorescence intensity decreases further. This may be due to the binding of silica gel and cell membrane, both of which can significantly lower the fluorescence intensity emitted by GQDs. Therefore, we can take the advantage of the unique fluorescence of GQDs to characterize materials easier and simpler.
Selection of the preferable preparation conditions for the SiO 2 -GQDs-CMSP
In order to explore the best reaction conditions for cell membrane to be immobilized onto the SiO2-GQDs, the immobilization time, the amount of cell membrane added, and the mass ratio of the crosslinking agent were investigated separately.
The amount of cell membrane covalently bonded was largely affected by the immobilization time. In this study, different immobilization times (1 h, 2 h, 4 h, 6 h, 8 h) were explored while other reaction conditions remained unchanged. As shown in Fig. 3a, before the reaction time is 6 h, the amount of cell membrane immobilized increases in a positive trend, but after 6 h of reaction, the amount remains basically unchanged. For this reason, 6 h was selected as the reaction time.
EDC/NHS activation of carboxyl groups has been widely used in various kinds of materials, and different concentrations of EDC/NHS can produce different crosslinking effects [42]. Based on the EDC/NHS concentrations applied in the covalent immobilization of silicon [39], different mass ratios of EDC/NHS including 0.069/0.041, 0.207/0.124, and 0.006/0.003 (g/g) were studied. From Fig. 3b, the reaction efficiency using 0.207 g EDC and 0.124 g NHS is obviously higher than the other two ratios. 0.207/0.124 g/g was hence chosen as the mass ratio of the crosslinking agent.
The amount of cell membrane involved in the synthesis also plays a vital role in the reaction efficiency. Theoretically, the more cell membranes are added to the reaction, the more cell membranes will be immobilized. However, the reaction will no longer proceed when it reaches a certain level, because the active reaction sites have all been occupied. This means that the amount of bonded cell membrane will reach “a plateau” and at this time the maximal amount of cell membrane can be immobilized. A positive relationship between 0.124 and 0.744 mg in the amount of immobilized cell membrane can be seen in Fig. 3c, while there is no significant increase after 0.744 mg. Therefore, 0.744 mg cell membrane was applied, and 0.511 mg cell membrane could be immobilized on 50 mg SiO2-GQDs.
System suitability of the SiO 2 -GQDs-CMC model
The retention time of gefitinib on each column was used as an indicator. It can be seen from Fig. 4a that the SiO2-GQDs-CMC column has noticeable retention of gefitinib (the retention time is about 26.765 min). In comparison, the SiO2-NH2 column and the SiO2-GQDs column do not show apparent retention (1.660 min and 2.997 min, respectively). This result implicates that the SiO2-GQDs-CMC column has satisfied specificity. Similarly, the fact (Fig. 4b) that drugs acting on known receptors are not retained on the SiO2-GQDs-CMC column also indicates the good selectivity of the SiO2-GQDs-CMC column.
The suitability of the SiO2-GQDs-CMC online LC-IT-TOF system. a Retention behavior of gefitinib on the SiO2-NH2 column, SiO2-GQDs column, and SiO2-GQDs-CMC column. b Retention behavior of phentolamine mesylate, benzhexol hydrochloride, and phentolamine mesylate on the SiO2-GQDs-CMC column. c Retention time of the traditional CMC column and the SiO2-GQDs-CMC column prepared with maximal cell membrane amount within 8 days (n = 3)
As indicated in Table S2 (see ESM), the relative standard deviation (RSD) values of the retention time for 5 injections of a SiO2-GQDs-CMC column and a traditional CMC column are 1.03% (n = 5) and 2.75% (n = 5) respectively. The RSD values for the retention time of different batches of these two columns are 1.13% (n = 3) for the SiO2-GQDs-CMC column, and 4.59% (n = 3) for the traditional CMC column. These results demonstrate comparatively enhanced intermediate precision and stability of the SiO2-GQDs-CMC column, and this novel prepared column can meet the analytical requirements for the experiments.
The traditional CMC column achieves the purpose of wrapping the cell membrane on the carrier through the hydrophobic interaction between the cell membrane and the silica gel. However, the weak physical adsorption makes the cell membrane easy to drop from the carrier, bringing about low reproducibility and limited lifetime within 3 days [18]. In a bid to verify the covalent binding force of the SiO2-GQDs-CMC column, the life span of the column was investigated for 8 days with 3 injections for each day (n = 3) and compared with the traditional CMC column under same conditions. As shown in Fig. 4c, the immobilization of the cell membrane to the novel carrier is significantly strengthened by covalent binding, increasing the lifetime of the CMC column to at least 8 days.
Validation of the SiO 2 -GQDs-CMC online LC-IT-TOF system
Gefitinib was applied to verify the availability of the two-dimensional SiO2-GQDs-CMC online LC-IT-TOF system. Figure 5a shows the retention behavior of gefitinib on the SiO2-GQDs-CMC column. The retained component (R, retention time about 27.343 min) was enriched into the trapped column. Through the two-dimensional switching valve, the enriched fraction was switched to the reversed-phase column for analysis and identification by MS. Figure 5b depicts the HPLC chromatogram of the enriched component and the result of MS identification. Compared with the direct chromatogram of gefitinib standard solution (Fig. 5c), the enriched compound is proved to be gefitinib. All these results demonstrate that this system could be used to identify, analyze, and authenticate potential effective components acting on EGFR.
Validation of the SiO2-GQDs-CMC online LC-IT-TOF system with gefitinib. a Chromatogram of gefitinib on the SiO2-GQDs-CMC column. b Chromatogram of the retained component R switched to the LC-IT-TOF. R was identified as gefitinib. c Chromatogram of gefitinib standard solution directly analyzed by the LC-IT-TOF
Screening of targeted components using the SiO 2 -GQDs-CMC online LC-IT-TOF system from Peucedanum praeruptorum Dunn
After validation, the SiO2-GQDs-CMC column was then devoted to identifying and analyzing targeted compounds acting on EGFR from the extracts of Peucedanum praeruptorum Dunn. Figure 6a exhibits the retention result. It shows one visible retained fraction (R, retention time about 20.613 min) on the SiO2-GQDs-CMC column. The retained constituent was then enriched to the LC-IT-TOF system and to be identified. Figure 6b and c respectively depict the chromatogram of the retained fraction R enriched to LC-IT-TOF, and the chromatogram of the extracts of Peucedanum praeruptorum Dunn directly injected into the second dimension of the system. The chromatographic peak marked with Pra-B in the figure is identified as praeruptorin B (Pra-B).
The application of the SiO2-GQDs-CMC online LC-IT-TOF system for screening active components from Peucedanum praeruptorum Dunn. a Chromatogram of the total extracts of Peucedanum praeruptorum Dunn on the SiO2-GQDs-CMC column. b Chromatogram of the retained component R switched to the LC-IT-TOF. R was identified as Pra-B. c Chromatogram of the total extracts of Peucedanum praeruptorum Dunn directly analyzed by the LC-IT-TOF
The standard solution of Pra-B was applied to further verify the screening result. As exhibited in Fig. S3A (see ESM), the Pra-B standard solution shows a consistent retention behavior on the SiO2-GQDs-CMC column with that of the above extracts in Fig. 6a. The MS data and the retention time on the HPLC column (ESM Fig. S3B and S3C) are also matched with those in Fig. 6b and c. Pra-B extracted from Peucedanum praeruptorum Dunn can hence be recognized as the screened component binding on EGFR.
Pharmacological effect of the active component
Pra-B is likely to interact with EGFR according to the above results, so the in vitro cell viability assay was conducted to verify the inhibitory effect of Pra-B on EGFR. As demonstrated in Fig. 7a and b, in the range of drug concentration from 0.4 to 25 μmol/L, Pra-B and gefitinib both show dose-dependent inhibition effect against EGFR-HEK293 cells. These results further support the outcomes of the above assays, indicating that Pra-B can act on EGFR, and may be a potential cancer inhibitor.
Pharmacological effect and molecular docking results of gefitinib as well as the active component in Peucedanum praeruptorum Dunn screened by the SiO2-GQDs-CMC model. a Gefitinib against EGFR cell viability. b Pra-B against EGFR cell viability. c Pocket result of molecular docking for gefitinib. d Pocket result of molecular docking for Pra-B. e Striped result of molecular docking for gefitinib. f Striped result of molecular docking for Pra-B
Molecular docking assay
The molecular docking between receptors and ligands is usually used to predict the binding sites as well as the affinity between ligands and receptors. In this research, molecular docking assay served the purpose of analyzing the binding sites of gefitinib and Pra-B with EGFR protein, respectively. SYBYL-X1.1 was used to select EGFR-TK from the protein database. Automatic docking of gefitinib and Pra-B with macromolecular EGFR was performed. The results in Fig. 7 show that the binding method of Pra-B and EGFR is similar to that of gefitinib and EGFR. Gefitinib and Pra-B can form four and one covalent bonds with EGFR respectively. Figure 7c and e show that gefitinib fits into the active pocket of EGFR protein with the total molecular docking score of 8.5558, while that of Pra-B with EGFR is 5.7914 (Fig. 7d and f). In all, the predicted results of the molecular docking affinity of the two compounds are consistent with the results of the pharmacological experiments and chromatographic retention behavior.
Conclusion
In this research, we have successfully constructed a new SiO2-GQDs-CMSP by covalent immobilization. This synthetic method has prolonged the life span of the CMC column to at least 8 days. Besides, we established the SiO2-GQDs-CMC online LC-IT-TOF system to screen potential active components acting on EGFR from the extracts of Peucedanum praeruptorum Dunn, while Pra-B was finally screened out. This compound was further identified to have EGFR inhibitory activity by CCK-8 assay. In conclusion, the enhanced method of the preparation of CMSP has improved the applying efficiency of CMC and provided valuable insights into the application of graphene nanomaterial in the chromatography science.
References
He LC, Wang SC, Geng XD. Coating and fusing cell membranes onto a silica surface and their chromatographic characteristics. Chromatographia. 2001;54(1–2):71–6.
Hou XF, Zhou MZ, Jiang Q, Wang SC, He LC. A vascular smooth muscle/cell membrane chromatography-offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines. J Chromatogr A. 2009;1216(42):7081–7.
Han SL, Zhang P, Wei F, Wang SC. Screening active components acting on alpha(1A) adrenergic receptors from agrimony using a Sprague-Dawley rat prostate cell membrane chromatography online coupled HPLC/MS method. Anal Methods-Uk. 2012;4(10):3351–7.
He JY, Han SL, Yang FF, Zhou N, Wang SC. Prostate cell membrane chromatography-liquid chromatography-mass spectrometry for screening of active constituents from Uncaria rhynchophylla. J Chromatogr Sci. 2013;51(10):905–9.
Du H, Ren J, Wang SC, He LC. Cell membrane chromatography competitive binding analysis for characterization of alpha(1A) adrenoreceptor binding interactions. Anal Bioanal Chem. 2011;400(10):3625–33.
Han SL, Zhang T, Feng LX, Lv N, Wang SC. Screening of target compounds from Fructus Piperis using high alpha(1A) adrenoreceptor expression cell membrane chromatography online coupled with high performance liquid chromatography tandem mass spectrometry. J Pharmaceut Biomed. 2013;81–82:133–7.
Hou XF, Wang SC, Hou JJ, He LC. Establishment of A431 cell membrane chromatography-RPLC method for screening target components from Radix Caulophylli. J Sep Sci. 2011;34(5):508–13.
Han SL, Wei F, Huang J, Wang SC. Characterization of compounds acting on the alpha 1A adrenergic receptor from Caulis spatholobi by cell membrane chromatography with possible application for treatment of benign prostatic hyperplasia. Anal Lett. 2014;47(10):1661–9.
Han SL, Lv YN, Kong LY, Sun YM, Fu J, Li LJ, et al. Simultaneous identification of the anaphylactoid components from traditional Chinese medicine injections using rat basophilic leukemia 2H3 and laboratory of allergic disease 2 dual-mixed/cell membrane chromatography model. Electrophoresis. 2018;39(9–10):1181–9.
Fu J, Lv YN, Jia QQ, Lin YY, Han SL. Dual-mixed/CMC model for screening target components from traditional Chinese medicines simultaneously acting on EGFR & FGFR4 receptors. Talanta. 2019;192:248–54.
Lin YY, Wang C, Hou YJ, Sun W, Che DL, Yang L, et al. Simultaneous identification of three pseudoallergic components in Danshen injection by using high-expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled online to HPLC-ESI-MS/MS. J Sep Sci. 2018;41(11):2488–97.
Han SL, Zhang T, Huang J, Hu ZG, Wang SC. Screening target components from Radix salviae miltiorrhiae using an EGFR/CMC-online-HPLC/MS method. Anal Methods-Uk. 2012;4(4):1078–83.
He LC, Yang GD, Wang SC, Wei YM, Geng XD. Mixed-model of affinity and hydrophobic interaction for drug retention in cell membrane chromatography. J Liq Chromatogr R T. 2005;28(11):1651–68.
He LC, Yang GD, Geng XD. Enzymatic activity and chromatographic characteristics of the cell membrane immobilized on silica surface. Chin Sci Bull. 1999;44(9):826–31.
Chen XF, Cao Y, Zhang H, Zhu ZY, Liu M, Liu HB, et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal Chem. 2014;86(10):4748–57.
Ding X, Chen XF, Cao Y, Jia D, Wang DY, Zhu ZY, et al. Quality improvements of cell membrane chromatographic column. J Chromatogr A. 2014;1359:330–5.
Wang XY, Chen XF, Gu YQ, Cao Y, Yuan YF, Hong ZY, et al. Progress of cell membrane chromatography and its application in screening active ingredients of traditional Chinese medicine. Chinese J Anal Chem. 2018;46(11):1695–702.
Ding X, Cao Y, Yuan YF, Gong ZR, Liu Y, Zhao L, et al. Development of APTES-decorated HepG2 cancer stem cell membrane chromatography for screening active components from Salvia miltiorrhiza. Anal Chem. 2016;88(24):12081–9.
Wang XY, Ding X, Yuan YF, Zheng LY, Cao Y, Zhu ZY, et al. Comprehensive two-dimensional APTES-decorated MCF7-cell membrane chromatographic system for characterizing potential anti-breast-cancer components from Yuanhu-Baizhi herbal medicine pair. J Food Drug Anal. 2018;26(2):823–33.
Gu YQ, Chen X, Wang Y, Liu Y, Zheng LY, Li XQ, et al. Development of 3-mercaptopropyltrimethoxysilane (MPTS)-modified bone marrow mononuclear cell membrane chromatography for screening anti-osteoporosis components from Scutellariae Radix. Acta Pharm Sin B. 2020;10(10):1856–65.
Liu Y, Wang XY, Gu YQ, Zhang MY, Cao Y, Zhu ZY, et al. Covalent design of cell membrane stationary phase with enhanced stability for fast screening P-glycoprotein inhibitors. ACS Appl. Bio Mater. 2020;3(8):5000–6.
Wu C, Wang N, Xu P, Wang X, Shou D, Zhu Y. Preparation and application of polyvinyl alcohol-decorated cell membrane chromatography for screening anti-osteoporosis components from Liuwei Dihuang decoction-containing serum. J Sep Sci. 2020;43(11):2105–14.
Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6(3):183–91.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–9.
Zhang ML, Qiu HD. Progress in stationary phases modified with carbonaceous nanomaterials for high-performance liquid chromatography. Trac-Trend Anal Chem. 2015;65:107–21.
Cheng C, Li S, Thomas A, Kotov NA, Haag R. Functional graphene nanomaterials based architectures: biointeractions, fabrications, and emerging biological applications. Chem Rev. 2017;117(3):1826–914.
Du D, Wang K, Wen Y, Li Y, Li YY. Photodynamic graphene quantum dot: reduction condition regulated photoactivity and size dependent efficacy. Acs Appl Mater Inter. 2016;8(5):3287–94.
Wang XY, Lei R, Huang HD, Wang N, Yuan L, Xiao RY, et al. The permeability and transport mechanism of graphene quantum dots (GQDs) across the biological barrier. Nanoscale. 2015;7(5):2034–41.
Tan XY, Li YC, Li XH, Zhou SX, Fan LZ, Yang SH. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem Commun. 2015;51(13):2544–6.
Huang ZC, Shen YT, Li Y, Zheng WJ, Xue YJ, Qin CQ, et al. Facile synthesis of analogous graphene quantum dots with sp(2) hybridized carbon atom dominant structures and their photovoltaic application. Nanoscale. 2014;6(21):13043–52.
Wu Q, Gao J, Chen LX, Dong SQ, Li H, Qiu HD, et al. Graphene quantum dots functionalized beta-cyclodextrin and cellulose chiral stationary phases with enhanced enantioseparation performance. J Chromatogr A. 1600;2019:209–18.
Zheng P, Wu NQ. Fluorescence and sensing applications of graphene oxide and graphene quantum dots: a review. Chem-Asian J. 2017;12(18):2343–53.
Bhatia P, Sharma V, Alam O, Manaithiya A, Alam P, Kahksha, et al. Novel quinazoline-based EGFR kinase inhibitors: a review focussing on SAR and molecular docking studies (2015–2019). Eur J Med Chem. 2020;204:112640.
Poursheikhani A, Yousefi H, Tavakoli-Bazzaz J, Seyed HG. EGFR blockade reverses cisplatin resistance in human epithelial ovarian cancer cells. Iran Biomed J. 2020;24(6):370–8.
Song Y, Jing W, Yan R, Wang Y. Research progress of the studies on the roots of Peucedanum praeruptorum Dunn (Peucedani radix). Pak J Pharm Sci. 2015;28(1):71–81.
Han SL, Li CL, Huang J, Wei F, Zhang Y, Wang SC. Cell membrane chromatography coupled with UHPLC-ESI-MS/MS method to screen target components from Peucedanum praeruptorum Dunn acting on alpha 1A adrenergic receptor. J Chromatogr B. 2016;1011:158–62.
Li XY, Zu YY, Ning W, Tang MX, Gong C, Niu SL, et al. A new xanthyletin-type coumarin from the roots of Peucedanum praeruptorum. J Asian Nat Prod Res. 2020;22(3):287–94.
Liang TG, Yue WY, Li QS. Chemopreventive effects of Peucedanum praeruptorum DUNN and its major constituents on SGC7901 gastric cancer cells. Molecules. 2010;15(11):8060–71.
Wu Q, Chen LX, Gao J, Dong SQ, Li H, Di DL, et al. Graphene quantum dots-functionalized C-18 hydrophobic/hydrophilic stationary phase for high performance liquid chromatography. Talanta. 2019;194:105–13.
Zhang T, Han S, Huang J, Wang S. Combined fibroblast growth factor receptor 4 cell membrane chromatography online with high performance liquid chromatography/mass spectrometry to screen active compounds in Brassica albla. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;912:85–92.
Shi YD, Sun W, Pan XY, Hou XF, Wang SC, Zhang JB. Establishment of thrombin affinity column (TAC)-HPLC-MS/MS method for screening direct thrombin inhibitors from Radix Salviae Miltiorrhiae. J Chromatogr B. 2020;1139.
Huang HH, Lee TH. Electrochemical impedance spectroscopy study of Ti-6Al-4V alloy in artificial saliva with fluoride and/or bovine albumin. Dent Mater. 2005;21(8):749–55.
Acknowledgments
The authors thank Prof. Langchong He and Prof. Shengli Han for their valuable suggestions and assistance with the experiments. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This work was supported by National Natural Science Foundation of China (81973278 and 81930096), China Postdoctoral Science Foundation (2019T120923 and 2018M641003), and Natural Science Foundation of Shaanxi Province (Grant Number: 2020SF-309).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 731 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Yi, X., Wang, S. et al. Construction of graphene quantum dots-decorated EGFR cell membrane chromatography for screening active components from Peucedanum praeruptorum Dunn. Anal Bioanal Chem 413, 1917–1927 (2021). https://doi.org/10.1007/s00216-021-03161-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03161-6