Abstract
Aminomethylphosphonic acid (AMPA) is the main metabolite of glyphosate (GLYP) and phosphonic acids in detergents. GLYP is a synthetic herbicide frequently used worldwide alone or together with its analog glufosinate (GLUF). The general public can be exposed to these potentially harmful chemicals; thus, sensitive methods to monitor them in humans are urgently required to evaluate health risks. We attempted to simultaneously detect GLYP, AMPA, and GLUF in human urine by high-resolution accurate-mass liquid chromatography mass spectrometry (HRAM LC-MS) before and after derivatization with 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl) or 1-methylimidazole-sulfonyl chloride (ImS-Cl) with several urine pre-treatment and solid phase extraction (SPE) steps. Fmoc-Cl derivatization achieved the best combination of method sensitivity (limit of detection; LOD) and accuracy for all compounds compared to underivatized urine or ImS-Cl-derivatized urine. Before derivatization, the best steps for GLYP involved 0.4 mM ethylenediaminetetraacetic acid (EDTA) pre-treatment followed by SPE pre-cleanup (LOD 37 pg/mL), for AMPA involved no EDTA pre-treatment and no SPE pre-cleanup (LOD 20 pg/mL) or 0.2–0.4 mM EDTA pre-treatment with no SPE pre-cleanup (LOD 19–21 pg/mL), and for GLUF involved 0.4 mM EDTA pre-treatment and no SPE pre-cleanup (LOD 7 pg/mL). However, for these methods, accuracy was sufficient only for AMPA (101–105%), while being modest for GLYP (61%) and GLUF (63%). Different EDTA and SPE treatments prior to Fmoc-Cl derivatization resulted in high sensitivity for all analytes but satisfactory accuracy only for AMPA. Thus, we conclude that our HRAM LC-MS method is suited for urinary AMPA analysis in cross-sectional studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glyphosate (GLYP; N-(phosphonomethyl)glycine) and glufosinate (GLUF; dl-homoalanin-4-yl-(methyl)phosphinate) are non-selective, synthetic post-emergence herbicides widely used in agricultural, forestry, and urban settings [1, 2]. Aminomethylphosphonic acid (AMPA) is the main (70%) GLYP degradation product of soil microbes [1, 3] and the key metabolite of phosphonate-containing household and industrial detergents [4, 5]. GLYP, AMPA, and GLUF have been detected in numerous foodstuffs and environmental water sources [1, 2, 5,6,7,8,9,10], indicating avenues by which these compounds may come into contact with humans who would otherwise not be exposed. Despite this, data on exposure to these compounds via urinary measurement among non-occupationally or toxically exposed individuals is scant [11,12,13,14,15,16].
Various methods to analyze GLYP, AMPA, and GLUF individually or together in human urine have been described. They include immunoassays [13, 17, 18], high-performance liquid chromatography (HPLC) with fluorescence [19] or ultraviolet [20] detection, LC with mass spectrometry (MS) [15, 21,22,23] or tandem MS (MS/MS) [16, 24,25,26,27,28] detection, and gas chromatography (GC) with MS [29], MS/MS [11, 12, 30], or electron capture [31] detection. GLYP, AMPA, and GLUF are amphoteric, polar compounds with low molecular weights (169, 111, and 181, respectively) and poor volatility, which make their detection at low levels very challenging. Therefore, derivatization is frequently applied to improve detection sensitivity as well as enable analysis by conventional reverse phase (RP) LC-MS methodologies. 9-Fluorenylmethoxycarbonyl chloride (Fmoc-Cl) is a derivatizing agent commonly used during amino acid analysis due to its quick reaction with primary and secondary amines, and has been applied mainly to environmental water and soil samples to increase the sensitivity of GLYP, AMPA, and GLUF during LC-MS analysis (reviewed in [32]). 1-Methylimidazole-sulfonyl chloride (ImS-Cl) is a derivatizing agent used by us previously to extensively improve (> 50-fold) the LC-MS sensitivity of phenolic molecules including steroidal estrogens, bisphenol A, triclosan, and parabens [33, 34]. However, we are unaware of analytical methods using Fmoc-Cl or ImS-Cl to derivatize GLYP, AMPA, and GLUF for quantitation of GLYP, AMPA, or GLUF from human urine.
In this study, we attempted to simultaneously detect GLYP, AMPA, and GLUF by high-resolution accurate-mass (HRAM) LC-MS in underivatized urine and in urine after derivatization with Fmoc-Cl or ImS-Cl. Urine was chosen as a matrix because it presents many important advantages compared with blood including higher analyte concentrations, non-invasive collection, and integration over longer periods of exposure [35]. Urine pre-treatment with hydrochloric acid (HCl) and ethylenediaminetetraacetic acid (EDTA) with and without following SPE cleanup was tested as a means to release analytes from mineral complexation and to improve assay sensitivity and accuracy.
Materials and methods
Chemicals and instrumentation
GLYP, AMPA, GLUF, and AMPA-13C-15N-d2 were purchased from Sigma-Aldrich (St. Louis, MO). GLYP-13C-15N was purchased from Cambridge Isotope Laboratory (Tewksbury, MA); GLUF-d3 was purchased from Medical Isotopes (Pelham, NH). Sodium tetraborate and ammonium hydroxide were purchased from Sigma-Aldrich (St. Louis, MO). All other HPLC grade solvents including acetonitrile (ACN), ethyl acetate (EtOAc), hexanes, acetone, dichloromethane (DCM), methanol (MeOH), concentrated HCl, formic acid, potassium hydroxide, and EDTA were obtained from Fisher Scientific (Waltham, MA). Analyte levels were adjusted for urinary creatinine (determined using a Roche Cobas MiraPlus clinical autoanalyzer) to account for differences in urine volume [35]. Isotope dilution electrospray ionization (ESI) HRAM LC-MS analysis was performed with a model Accela LC system including a CTC HTS PAL autosampler coupled to an orbitrap model Q-Exactive mass spectrometer, all controlled by Xcalibur software (all from Thermo Scientific Inc., Waltham, MA). Deidentified urine samples from pre- and post-menopausal women obtained from a previous discontinued study were pooled according to menopausal status.
Sample preparation by SPE and LC-MS analysis without derivatization
In total, 500 μL of urine were spiked with 5 μL of the authentic standard mixture (GLYP, AMPA, and GLUF, 100 ng/mL in water) or not spiked followed by mixing with 25 μL of the three internal standards (ISs; GLYP-13C1, AMPA-13C-15N-d2, and GLUF-d3, 500 ng/mL of each in water) and diluting with 500 μL of water. The mixture was loaded onto a Strata-SAX SPE cartridge (100 mg/1 cc; Phenomenex, Torrance, CA) after preconditioning the cartridge with 1 mL of MeOH and 1 mL of water. The cartridge was then dried by air under vacuum for 5 min, followed by washing with 1 mL of water and drying by air under vacuum for another 5 min. The cartridge was subsequently washed with 1 mL of MeOH followed by air drying under vacuum. The residue was eluted with 1.2 mL of 1 M HCl in MeOH, dried under nitrogen at 45 °C, then re-dissolved in 100 μL of 20% aq. ACN. In total, 25 μL of this re-dissolved extract was injected onto an Asahipak NH2P-40 3E column (250 x 3.0 mm; 4 μm) connected to an Asahipak NH2P-50G 3A (2.0 × 10 mm; 4 μm) pre-column (Shodex, New York, NY). Separation was performed with mobile phases consisting of ACN (A) and 0.1 M aq. ammonium hydroxide at pH 11 (B). The flow rate was 500 μL/min with the following linear gradient (%B): from 0 to 5 min at 20%, from 5.1 to 15 min to 80%, then back to initial conditions and equilibrate for 5 min. ESI-HRAM-MS analysis was performed in negative mode with parallel reaction monitoring (PRM) using the following high collision dissociation (hcd) settings: GLYP (m/z 168.0054 → m/z 124.0153, m/z 149.99465; hcd@15), GLYP-IS (m/z 170.0061 → m/z 126.01567, m/z 151.99505; hcd@15), AMPA (m/z 109.9997 → m/z 62.96246, m/z 78.95734 hcd@19), AMPA-IS (m/z 114.0218 → m/z 62.96246, m/z 78.95734 hcd@19), GLUF (m/z 180.0421 → m/z 119.02516, m/z 136.05169 hcd@22), GLUF-IS (m/z 183.0611 → m/z 122.044, m/z 139.07053 hcd@22).
Sample preparation and LC-MS analysis after derivatization with 1-methylimidazole-sulfonyl chloride (ImS-Cl)
In total, 50 μL of urine were diluted with 50 μL of water and spiked with 10 μL of IS. The mixture was treated with 50 μL of sodium phosphate buffer (0.1 M, pH 11) then derivatized with 20 μL of ImS-Cl (20 mg/mL in ACN) and incubated at 65 °C for 15 min according to our recently published methodology [33, 34]. In total, 10 μL of the crude ImS adduct was injected onto an Ascentis C18 analytical column (150 x 3.0 mm; 2.7 µm, Supelco, St. Louis, MO) and separated with a mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B) at a flow rate of 400 μL/min with the following linear gradient (%B): from 0 to 5 min 5% to 15%, increase to 25% in 2 min, then 50% in 0.1 min, stay at 50% for 1 min, then back to initial conditions of 5% and equilibrate for 5 min. LC-MS analyses were performed under positive ESI mode, with spray voltage at 4 kV, sheath gas flow rate at 30, and Aux flow rate at 5. Capillary temperature was set at 320 °C. ESI-HRAM-MS analysis was performed in negative mode with targeted SIM at the monoisotopic masses of the deprotonated analytes (± 5 ppm to account for MS inaccuracies) [M-H]− (m/z): GLYP-ImS (312.00554), GLYP-IS-ImS (314.00593), AMPA-ImS (254.00006), AMPA-IS-ImS (258.01300), GLUF-ImS (324.04192), and GLUF-ImS (327.06075).
Sample preparation by SPE and LC-MS analysis after derivatization with Fmoc-Cl and SPE purification
In total, 500 μL of urine were spiked with 10 μL of the authentic standard mixture (GLYP, AMPA, and GLUF, 100 ng/mL in water) or not spiked followed by mixing with 25 μL of the three ISs (GLYP-13C1, AMPA-13C-15N-d2, and GLUF-d3, 500 ng/mL of each in water) and diluting with 1 mL of water. This solution was loaded onto a Strata-X polymeric RP SPE column (60 mg/3 mL; Phenomenex) after preconditioning with 2 mL of MeOH and 2 mL of water. The SPE cartridge was washed with 700 μL of water. The combined pass-through and wash fractions were vortex-mixed with 100 μL of sodium borate buffer (2.6% in water) and 150 μL of Fmoc-Cl (6 mg/mL in ACN) and incubated at 50 °C for 20 min. After cooling to room temperature, 5 μL of formic acid was added to the reaction mixture and centrifuged at 800×g for 5 min. The supernatant was then loaded onto a SepPak C18 RP SPE cartridge (50 mg/1 mL; Waters, Milford, MA) after preconditioning with 1 mL of MeOH and 1 mL of water. The SPE cartridge was dried under vacuum for 10 min followed by washing with 700 μL of DCM and drying under vacuum for 10 min. The analytes were eluted with 1 mL of MeOH and 400 μL of 0.1% formic acid in MeOH. Both eluent fractions were combined, dried under nitrogen, then re-dissolved in 100 μL of 25% aq. ACN. In total, 50 μL of this extract was separated on a Kinetex C18 analytical column (150 × 3 mm, 2.6 μm, Phenomenex). The mobile phase consisted of (A) 5 mM ammonium acetate buffer pH 9 mixed with MeOH and ACN (90/5/5), and (B) MeOH and ACN (50/50), and was operated at a flow rate of 300 μL/min with the following gradient (%B): starting with 0% to 15% in 7 min and increasing to 30% in 6 min, then holding for 2 min at 30% and back to initial conditions to equilibrate for 5 min. LC-MS analyses were performed under positive ESI mode, with spray voltage at 4 kV, sheath gas flow rate at 30, and Aux flow rate at 5. Capillary temperature was set at 320 °C. ESI-HRAM-MS analysis was performed in negative mode with targeted SIM by monitoring the monoisotopic masses of the protonated analytes (± 5 ppm to account for MS inaccuracies): GLYP-Fmoc (m/z 392.08937), GLYP-13C-15N-Fmoc (m/z 394.089760), AMPA-Fmoc (m/z 334.083890), AMPA-13C-15N-d2-Fmoc (m/z 338.09683), GLUF-Fmoc (m/z 404.12575), GLUF-d3-Fmoc (m/z 407.14458).
Sample preparation with acid and base treatment followed by SPE and LC-MS analysis after derivatization with Fmoc-Cl and SPE purification
In total, 500 μL of urine spiked with an authentic standard mixture (GLYP, AMPA, and GLUF, 100 ng/mL in water) at 0.5 ng/mL and 2 ng/mL in duplicate were combined with 25 μL of the three ISs (500 ng/mL). The mixture was acidified with 5 μL of 6 M HCl to pH 1, then vortexed and equilibrated at room temperature for 1 min. The mixture was neutralized to pH 6~7 with 6.6 μL of 6 M KOH then diluted with 1 mL of water. This solution was loaded onto a Strata-X SPE column (60 mg/3 mL; Phenomenex) after preconditioning with 2 mL of MeOH and 2 mL of water. The SPE cartridge was washed with 700 μL of water. The combined wash fractions were then subjected to derivatization with Fmoc-Cl, SepPak C18 SPE purification, and LC-MS analysis as described above.
Sample preparation with EDTA treatment followed by SPE and LC-MS analysis after derivatization with Fmoc-Cl and SPE purification
In total, 500 μL of urine spiked with an authentic standard mixture (GLYP, AMPA, and GLUF, 100 ng/mL in water) at 0.5 ng/mL and 2 ng/mL in duplicate were combined with 25 μL of the three ISs (500 ng/mL). The mixture was treated with EDTA to reach a final concentration of 0.2 mM (60 μL of 1 mM EDTA), 0.4 mM (150 μL of 1 mM EDTA), and 28 mM (80 μL of 100 mM EDTA). The mixture was vortexed and equilibrated at room temperature for 30 min. The mixture was then neutralized to pH 6~7 with 0~2 μL of 1 M KOH then diluted with 1 mL of water. This solution was loaded onto a Strata-X SPE (60 mg/3 mL; Phenomenex) after preconditioning with 2 mL of MeOH and 2 mL of water. The SPE cartridge was washed with 700 μL of water. The combined wash fractions were then subjected to derivatization with Fmoc-Cl, SepPak C18 SPE purification, and LC-MS analysis as described above.
Results and discussion
Humans can be exposed to GLYP, AMPA, and GLUF in many ways (Fig. 1) since these compounds have been detected in numerous foodstuffs [6, 7, 36,37,38,39] and also in surface [5, 40,41,42] and ground waters [5], which supply the majority of drinking water to US households [43]. AMPA is the major metabolite (70%) of GLYP formed from soil microbial degradation [1, 3], and is the key metabolite of phosphonates—functional agents of household and industrial laundry and cleaning detergents [4, 5] that can inadvertently enter surface waters [4]. In 2015, GLYP and GLYP-based herbicides (GBH) were deemed “probable human carcinogens” (category 2A) by the International Agency for Research on Cancer (IARC) due to strong evidence demonstrating their ability to induce DNA damage and oxidative stress [44]. IARC also cited evidence from in vitro and animal studies showing that AMPA can induce oxidative stress—a situation that can lead to cancer in humans [45]. Thus, examination of AMPA exposure deserves close attention as this metabolite may have many underlying health implications. The use of GLYP and GBH will likely continue or even increase around the world [45]. Due to the high global use and current controversial carcinogenicity status of GLYP, monitoring of this compound and particularly its metabolite AMPA to the general public is urgently warranted.
GLYP, AMPA, and GLUF are very small and polar compounds that lack chromophores or other heteroatoms that can facilitate their sensitive detection. In addition, the amphoteric nature of these agents makes their concentration and purification by normal phase or RP SPE very difficult. For these reasons, Fmoc-Cl derivatization has been frequently applied, mainly to environmental water and soil samples [32], to increase lipophilicity thereby allowing for better RP SPE retention and increased sensitivity during MS analysis. In this study, we tested Fmoc-Cl and ImS-Cl as derivatization reagents to increase the lipophilicity of GLYP, AMPA, and GLUF and thereby enhance retention during RP SPE and sensitivity during LC-MS analysis of human urine.
Urine analysis without derivatization
We evaluated various SPE cartridges to extract and purify GLYP, AMPA, and GLUF from urine samples. These cartridges included RP (Strata-X, SepPak C18), cation exchange (Strata-X-C), anion exchange (Strata-X-A, Oasis-MAX), and silica-based weak anion exchange (Strata-SAX). Aqueous standards of these analytes were not retained on the RP cartridge (Strata-X) and the majority was found in the loading pass-through. We were also unable to obtain good recovery for the analytes using the cation exchange SPE. While the anion exchange SPEs could retain GLYP with relatively good yield (> 40%), the recoveries for AMPA and GLUF were poor (< 1%). The silica-based weak anion exchange SPE (Strata-SAX) showed the best recovery compared with other SPEs with more than 40% recovery for all analytes.
LC separations were found to be selective and with good peak shapes using the poly-amino-based Asahipak NH2P-40 3E column, which functions with a mixed chromatographic mechanism of both hydrophilic interaction and ion exchange. Using the Asahipak column, we were able to separate the analytes with good retention (9~10 min) and good resolution. Other conventional HILIC columns we tested (Luca-HILIC, or zic-c-HILIC) did not provide good retention or better peak shapes. The mobile phase was also optimized by using 0.1 M ammonium hydroxide and ACN. These chemicals yielded better peak shapes and MS sensitivity than using ammonium bicarbonate.
The molecular weights of GLYP, AMPA, and GLUF are low (< 200) and in the same range as the inherent MS noise. In order to achieve selective MS detection, we applied PRM in negative mode and scanned the product ions formed in the high collision cell of the mass spectrometer. The collision energy for each analyte was optimized accordingly as described in the “Materials and methods” section. The LODs in standards were 500 pg/mL for GLYP and AMPA, and 1000 pg/mL for GLUF while the LODs in urine were much higher (> 5000 pg/mL) due to urinary interferences that were not removed by chromatography. Due to these high LODs, we decided to pursue derivatization methods to increase MS sensitivity.
Urine analysis after derivatization with ImS-Cl
We previously succeeded in using ImS-Cl to derivatize a variety of molecules (including steroids and bisphenol A) to products with improved chromatographical features and obtained more than 50-fold increases in LC-MS sensitivity compared with their educts [33, 34]. In this study, we used the ImS tag again in hopes of improving both SPE recovery and MS sensitivity by increasing the lipophilicity of the highly polar GLYP, AMPA, and GLUF analytes. After derivatization, the ImS adducts retained their zwitterionic characteristic and could, therefore, be detected in both negative and positive ESI modes with a similar intensity, but with less noise in negative mode, which was subsequently applied for all analyses. The LODs of these adducts in neat standards were 100 pg/mL. However, the LODs in urine were much higher (100 ng/mL), which was most likely due to interferences in the urine matrix. Diluting the urine two-fold with water prior to derivatization improved sensitivity two-fold to 50 ng/mL, while increasing the dilution another four-fold improved the sensitivity an additional 50-fold to 1 ng/mL.
We attempted to further improve the sensitivity of this method in urine by (1) pre-cleaning urine by SPE before derivatization and (2) applying liquid-liquid extraction (LLE) or SPE to purify the ImS-adducts after derivatization. Several different cartridge types were used for SPE including RP (Oasis HLB, Waters), anion exchange (Oasis-MAX) and Strata X-A, and weak anion exchange (Strata X-AW). However, neither LLE nor any tested SPE method was able to lower the method LOD and produce satisfactory recoveries. We also tested other sulfonyl chloride reagents including dansyl chloride and biphenyl sulfonyl chloride; however, these products also failed to improve method sensitivity. Due to these unsatisfying results, we decided to apply Fmoc-Cl as a derivatizing agent.
Urine analysis after derivatization with Fmoc-Cl
The Fmoc group was previously reported to be a good tag for GLYP, AMPA, and GLUF analysis in water, soil, and/or foodstuff [46,47,48,49] to increase MS sensitivity. However, to our knowledge, derivatization with Fmoc-Cl for the simultaneous sensitive analysis of these analytes in human urine has not been reported. In this study, the Fmoc group was able to increase the lipophilicity of the very polar GLYP, AMPA, and GLUF compounds (Fig. 2 and Fig. 3), which resulted in better retention during SPE with the RP material and, correspondingly, better sample purification compared with not applying derivatization. In addition, GLYP, AMPA, and GLUF were better retained on the RP Kinetex EVO C18 column during analytical chromatographic separations (Fig. 4a) compared with the ImS-adducts, which improved MS sensitivity due to the presence of sharper and very symmetrical peaks.
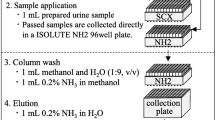
Work flow scheme showing different urine pre-treatment and SPE steps to improve recovery and accuracy of all analytes derivatized with Fmoc-Cl. IS, internal standard; ACN, acetonitrile; DCM, dichloromethane; RT, room temperature. All steps are detailed in the “Materials and methods” section
LC-MS traces of (a) analyte adducts and their corresponding internal standards (b) AMPA monitored at their monoisotopic masses of m/z 334.08389 and m/z 338.09683, respectively. The upper 2, middle 2, and lower 2 traces show authentic standard (20,000 pg/mL), spiked urine (final 2000 pg/mL), and un-spiked urine (230 pg/mL), respectively. RT, retention time; NL, absolute abundance of the peak size
To further improve the assay, we applied SPE to purify the Fmoc adduct. The SepPak C18 cartridge showed the best recovery for all analyte adducts compared with other tested cartridges (polymer-based Strata-X, mixed-mode Oasis-MAX). Using the SepPak C18 SPE cartridge, we obtained satisfactory LODs from urine of 2000 pg/mL, 500 pg/mL, and 1000 pg/mL for GLYP, AMPA, and GLUF, respectively. However, these LODs were still much higher compared to the Fmoc-adducts in standards without SPE (4~18 pg/mL based on S/N = 5). We attributed the higher LOD to ion suppression, which we assumed was due to the analytes getting lost during sample workup or remaining unreacted during the derivatization process. Therefore, we further optimized this procedure by including an initial SPE pre-cleanup step before derivatization using a Strata-X SPE column. The Strata-X column was able to bind the colored pigments and possibly other interferences and did not irreversibly retain the analytes. The resulting colorless wash fraction from the SPE pre-cleanup step which was then derivatized with Fmoc-Cl. Derivatization was followed by another SPE step with SepPak C18 to purify the reaction adduct (Fig. 2). This procedure (i.e., SPE pre-cleanup–derivatization–SPE for adduct purification) greatly improved the reaction yield, however, resulted in poor GLYP recovery in urine (31%). Recovery improved slightly (34–54%, Table 1 method #1) when the SPE pre-cleanup step was removed but, overall, was still poor. This poor GLYP recovery may have been due to the formation of a complex between urinary substances and/or metal ions with GLYP resulting in less free GLYP available for derivatization [50].
To improve GLYP recovery in urine, we attempted different pre-treatment methods of the crude urine sample with or without subsequent SPE pre-cleanup followed by Fmoc-Cl derivatization (Fig. 2, Table 1). These pre-treatment methods included acidifying urine to pH 1 using HCl to prevent the formation of GLYP-metal ion complexes [50] (Table 1; method #2), and pre-treating urine with EDTA to have it act as a proxy ligand for GLYP and, therefore, keep GLYP in its free form and thereby available for derivatization [51] (Table 1, methods #3–5).
Pre-treating urine with HCl prior to derivatization, as previously suggested to improve recovery of GLYP in groundwater [50], resulted in very poor recoveries for all three compounds with or without a subsequent SPE pre-cleanup step (Table 1, method #2). Moreover, the LOD for GLYP with HCl pre-treatment led to LODs more than twice that without pre-treatment (130–155 pg/mL with HCl pre-treatment vs. 60–75 pg/mL without pre-treatment, Table 1). Thus, we focused on pre-treatment methods using EDTA (Table 1, methods #3–5).
The best method for GLYP involved 0.4 mM EDTA pre-treatment and subsequent SPE pre-cleanup (LOD 37 pg/mL). The best method for AMPA involved no EDTA pre-treatment and no SPE pre-cleanup (LOD 20 pg/mL) or 0.2–0.4 mM EDTA pre-treatment with no SPE pre-cleanup (LOD 19–21 pg/mL). The best method for GLUF (LOD 7 pg/mL) involved 0.4 mM EDTA pre-treatment and no SPE pre-cleanup. However, for these stated methods, accuracy was sufficient only for AMPA (101–105%), while being modest for GLYP (61%) and GLUF (63%).
Overall, among the different combinations of EDTA pre-treatment and SPE pre-cleanup steps, we found that the combination that worked best for one analyte typically compromised the LOD and/or recovery of another analyte. No single pre-treatment/SPE pre-cleanup combination could be found that resulted in the best outcome for all three compounds (Table 1, method #1, 3–5). Thus, we found that the simultaneous analysis of GLYP, AMPA, and GLUF in urine in one procedure was not possible if both sensitivity and accuracy are to be maximized. Due to the high sensitivity and excellent recovery of AMPA, we conclude that our optimized HRAM LC-MS method is well suited for urinary AMPA analysis (Fig. 4b). Using this method, we were able to detect AMPA in un-spiked urine (n = 122) up to approximately 400 pg/mL from an ongoing study of post-menopausal women (manuscript under review ). Our AMPA values are in the range reported by Mills et al. (2017) [15], who used LC-MS without disclosing method details and detected between 114 and 482 pg/mL from elderly adults, but on the lower end of the range compared to Conrad [11] and Hoppe [12] who measured < 100–1880 pg/mL and < 150–2630 pg/mL, respectively, by GC-MS/MS (Table 2).
Conclusion
In our study, urine derivatized with Fmoc-Cl achieved the best combination of method sensitivity (LOD) and accuracy for all three analytes (GLYP, AMPA, and GLUF) compared with underivatized urine or urine derivatized with ImS-Cl or other tested sulfonyl-based reagents. Different combinations of EDTA pre-treatment and/or subsequent SPE pre-cleanup steps were needed for each analyte to selectively improve assay performance. Using the optimized method for each analyte, accuracy was modest for GLYP (61%) and GLUF (63%) but excellent for AMPA (101–105%). For this reason, we conclude that our HRAM LC-MS method in urine is suited only for AMPA analysis in cross-sectional studies.
Data availability
The data that support the reported findings of this study are available from AAF and XL.
References
Bai SH, Ogbourne SM. Glyphosate: environmental contamination, toxicity and potential risks to human health via food contamination. Environ Sci Pollut Res Int. 2016;23(19):18988–9001.
Masiol M, Gianni B, Prete M. Herbicides in river water across the northeastern Italy: occurrence and spatial patterns of glyphosate, aminomethylphosphonic acid, and glufosinate ammonium. Environ Sci Pollut Res Int. 2018;25(24):24368–78.
Duke SO. Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J Agric Food Chem. 2011;59(11):5835–41.
Jaworska J, Van Genderen-Takken H, Hanstveit A, van de Plassche E, Feijtel T. Environmental risk assessment of phosphonates, used in domestic laundry and cleaning agents in The Netherlands. Chemosphere. 2002;47(6):655–65.
Battaglin WA, Meyer M, Kuivila K, Dietze J. Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. JAWRA J Am Water Resour Assoc. 2014;50(2):275–90.
Chen MX, Cao ZY, Jiang Y, Zhu ZW. Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J Chromatogr A. 2013;1272:90–9.
Han Y, Song L, Zhao P, Li Y, Zou N, Qin Y, et al. Residue determination of glufosinate in plant origin foods using modified Quick Polar Pesticides (QuPPe) method and liquid chromatography coupled with tandem mass spectrometry. Food Chem. 2016;197(Pt A):730–6.
Hogendoorn EA, Ossendrijver FM, Dijkman E, Baumann RA. Rapid determination of glyphosate in cereal samples by means of pre-column derivatisation with 9-fluorenylmethyl chloroformate and coupled-column liquid chromatography with fluorescence detection. J Chromatogr A. 1999;833(1):67–73.
Grandcoin A, Piel S, Baures E. Aminomethylphosphonic acid (AMPA) in natural waters: its sources, behavior and environmental fate. Water Res. 2017;117:187–97.
Grunewald K, Schmidt W, Unger C, Hanschmann G. Behavior of glyphosate and aminomethylphosphonic acid (AMPA) in soils and water of reservoir Radeburg II catchment (Saxony/Germany). J Plant Nutr Soil Sci. 2001;164(1):65–70.
Conrad A, Schroter-Kermani C, Hoppe HW, Ruther M, Pieper S, Kolossa-Gehring M. Glyphosate in German adults - time trend (2001 to 2015) of human exposure to a widely used herbicide. Int J Hyg Environ Health. 2017;220(1):8–16.
Hoppe HW. Determination of glyphosate residues in human urine samples from 18 European countries. Medical Laboratory Bremen, D-28357 Bremen/Germany; 2013.
Jayasumana C, Gunatilake S, Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16:103.
McGuire MK, McGuire MA, Price WJ, Shafii B, Carrothers JM, Lackey KA, et al. Glyphosate and aminomethylphosphonic acid are not detectable in human milk. Am J Clin Nutr. 2016;103(5):1285–90.
Mills PJ, Kania-Korwel I, Fagan J, McEvoy LK, Laughlin GA, Barrett-Connor E. Excretion of the herbicide glyphosate in older adults between 1993 and 2016. Jama. 2017;318(16):1610–1.
Parvez S, Gerona RR, Proctor C, Friesen M, Ashby JL, Reiter JL, et al. Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ Health. 2018;17(1):23.
Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, et al. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann Occup Hyg. 2007;51(1):53–65.
Rendon-von Osten J, Dzul-Caamal R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: a survey in Hopelchen, Campeche, Mexico. Int J Environ Res Public Health. 2017;14(6).
Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, et al. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 2004;112(3):321–6.
Hori Y, Fujisawa M, Shimada K, Sato M, Kikuchi M, Honda M, et al. Quantitative determination of glufosinate in biological samples by liquid chromatography with ultraviolet detection after p-nitrobenzoyl derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;767(2):255–62.
Chen D, Miao H, Zhao Y, Wu Y. A simple liquid chromatography-high resolution mass spectrometry method for the determination of glyphosate and aminomethylphosphonic acid in human urine using cold-induced phase separation and hydrophilic pipette tip solid-phase extraction. J Chromatogr A. 2019;1587:73–8.
Kazui Y, Seto Y, Inoue H. Phosphorus-specific determination of glyphosate, glufosinate, and their hydrolysis products in biological samples by liquid chromatography–inductively coupled plasma–mass spectrometry. Forensic Toxicol. 2014;32(2):317–22.
Mesnage R, Moesch C, Grand R, Lauthier G, Vendômois J, Gress S, et al. Glyphosate exposure in a farmer’s family. J Environ Prot. 2012;9:1001–3.
Connolly A, Jones K, Galea KS, Basinas I, Kenny L, McGowan P, et al. Exposure assessment using human biomonitoring for glyphosate and fluroxypyr users in amenity horticulture. Int J Hyg Environ Health. 2017;220(6):1064–73.
Connolly A, Basinas I, Jones K, Galea KS, Kenny L, McGowan P, et al. Characterising glyphosate exposures among amenity horticulturists using multiple spot urine samples. Int J Hyg Environ Health. 2018;221(7):1012–22.
Jensen PK, Wujcik CE, McGuire MK, McGuire MA. Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. J Environ Sci Health B. 2016;51(4):254–9.
Watanabe D, Ohta H, Yamamuro T. Solid-phase extraction of phosphorous-containing amino acid herbicides from biological specimens with a zirconia-coated silica cartridge. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:69–76.
Tsao YC, Lai YC, Liu HC, Liu RH, Lin DL. Simultaneous determination and quantitation of paraquat, diquat, glufosinate and glyphosate in postmortem blood and urine by LC-MS-MS. J Anal Toxicol. 2016;40(6):427–36.
Saito T, Miura N, Namera A, Oikawa H, Miyazaki S, Nakamoto A, et al. Mixed-mode C–C 18 monolithic spin-column extraction and GC–MS for simultaneous assay of organophosphorus compounds, glyphosate, and glufosinate in human serum and urine. Forensic Toxicol. 2012;30(1):1–10.
Kruger M, Schledorn P, Schrödl W, Hoppe H-W, Lutz W, Shehata AA. Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol. 2014;4(2):1–5.
Jauhiainen A, Rasanen K, Sarantila R, Nuutinen J, Kangas J. Occupational exposure of forest workers to glyphosate during brush saw spraying work. Am Ind Hyg Assoc J. 1991;52(2):61–4.
Raina-Fulton R. A review of methods for the analysis of orphan and difficult pesticides: glyphosate, glufosinate, quaternary ammonium and phenoxy acid herbicides, and dithiocarbamate and phthalimide fungicides. J AOAC Int. 2014;97(4):965–77.
Li X, Franke AA. Improvement of bisphenol A quantitation from urine by LCMS. Anal Bioanal Chem. 2015;407(13):3869–74.
Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids. 2015;99:84–90.
Franke A, Halm B, Ashburn L. Isoflavones in children and adults consuming soy. Arch Biochem Biophys. 2008;476:161–70.
Liao MF, Chaou WT, Tsao LY, Nishida H, Sakanoue M. Ultrasound measurement of the ventricular size in newborn infants. Brain and Development. 1986;8(3):262–8.
Xu J, Smith S, Smith G, Wang W, Li Y. Glyphosate contamination in grains and foods: an overview. Food Control. 2019;106710.
Cessna A, Darwent A, Townley-Smith L, Harker K, Kirkland K. Residues of glyphosate and its metabolite AMPA in field pea, barley and flax seed following preharvest applications. Can J Plant Sci. 2002;82(2):485–9.
Cessna A, Darwent A, Townley-Smith L, Harker K, Kirkland K. Residues of glyphosate and its metabolite AMPA in canola seed following preharvest applications. Can J Plant Sci. 2000;80(2):425–31.
Medalie L, Baker NT, Shoda ME, Stone WW, Meyer MT, Stets EG, et al. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci Total Environ. 2020;707:136008.
Kolpin DW, Thurman EM, Lee EA, Meyer MT, Furlong ET, Glassmeyer ST. Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci Total Environ. 2006;354(2–3):191–7.
Aparicio VC, De Geronimo E, Marino D, Primost J, Carriquiriborde P, Costa JL. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere. 2013;93(9):1866–73.
U.S. Geological Survey. Surface water use in the United States 29015. Available from: https://www.usgs.gov/special-topic/water-science-school/science/surface-water-use-united-states?qt-science_center_objects=0#qt-science_center_objects
IARC. IARC monographs on the evaluation of the carcinogenic risks to humans-volume 112: some organophosphate insecticides and herbicides. IARC, World Health Organization: Lyon, France; 2017.
Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. 2016;28(1):3.
Hanke I, Singer H, Hollender J. Ultratrace-level determination of glyphosate, aminomethylphosphonic acid and glufosinate in natural waters by solid-phase extraction followed by liquid chromatography-tandem mass spectrometry: performance tuning of derivatization, enrichment and detection. Anal Bioanal Chem. 2008;391(6):2265–76.
Ibanez M, Pozo OJ, Sancho JV, Lopez FJ, Hernandez F. Residue determination of glyphosate, glufosinate and aminomethylphosphonic acid in water and soil samples by liquid chromatography coupled to electrospray tandem mass spectrometry. J Chromatogr A. 2005;1081(2):145–55.
Ehling S, Reddy TM. Analysis of glyphosate and aminomethylphosphonic acid in nutritional ingredients and milk by derivatization with fluorenylmethyloxycarbonyl chloride and liquid chromatography-mass spectrometry. J Agric Food Chem. 2015;63(48):10562–8.
Skeff W, Recknagel C, Schulz-Bull DE. The influence of salt matrices on the reversed-phase liquid chromatography behavior and electrospray ionization tandem mass spectrometry detection of glyphosate, glufosinate, aminomethylphosphonic acid and 2-aminoethylphosphonic acid in water. J Chromatogr A. 2016;1475:64–73.
Ibanez M, Pozo OJ, Sancho JV, Lopez FJ, Hernandez F. Re-evaluation of glyphosate determination in water by liquid chromatography coupled to electrospray tandem mass spectrometry. J Chromatogr A. 2006;1134(1–2):51–5.
Gros P, Ahmed AA, Kuhn O, Leinweber P. Influence of metal ions on glyphosate detection by FMOC-Cl. Environ Monit Assess. 2019;191(4):244.
Connolly A, Leahy M, Jones K, Kenny L, Coggins MA. Glyphosate in Irish adults - a pilot study in 2017. Environ Res. 2018;165:235.
Funding
This study received funding from the National Cancer Institute (P30 CA71789).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors and all authors approved the final version.
Corresponding author
Ethics declarations
Deidentified urine samples in this study were obtained from pre- and post-menopausal women, who provided written, informed consent, from a study that was approved by the University of Hawaii Office of Research Compliance and has since been discontinued. Quality control samples were pooled according to menopausal status.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franke, A.A., Li, X. & Lai, J.F. Analysis of glyphosate, aminomethylphosphonic acid, and glufosinate from human urine by HRAM LC-MS. Anal Bioanal Chem 412, 8313–8324 (2020). https://doi.org/10.1007/s00216-020-02966-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02966-1