Abstract
An amperometric l-ascorbic acid biosensor utilizing ascorbate oxidase (AOx) immobilized onto poly(l-aspartic acid) (P(l-Asp)) film was fabricated on carbon nanofiber (CNF) and nanodiamond particle (ND)-modified glassy carbon electrode (GCE). Effects of AOx, ND, and CNF amounts were investigated by monitoring the response currents of the biosensor at different amounts of AOx, ND, and CNF. The electropolymerization step of l-aspartic acid on CNF-ND/GCE surface was also optimized. Scanning electron microscopy (SEM), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) techniques were used to enlighten the modification steps of the biosensor. The effects of pH and applied potential were studied in detail to achieve the best analytical performance. Under optimized experimental conditions, the AOx/P(L-Asp)/ND-CNF/GCE biosensor showed a linear response to l-ascorbic acid in the range of 2.0 × 10−7–1.8 × 10−3 M with a detection limit of 1.0 × 10−7 M and sensitivity of 105.0 μAmM−1 cm−2. The novel biosensing platform showed good reproducibility and selectivity. The strong interaction between AOx and the P(l-Asp)/ND-CNF matrix was revealed by the high repeatability (3.4%) and good operational stability. The AOx/P(l-Asp)/ND-CNF/GCE biosensor was successfully applied to the determination of l-ascorbic acid in vitamin C effervescent tablet and pharmaceutical powder containing ascorbic acid with good results, which makes it a promising approach for quantification of l-ascorbic acid.

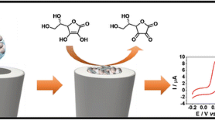
Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-Ascorbic acid or vitamin C is a water-soluble vitamin found in citrus and other fruits, and in vegetables [1]. It is widely utilized as an antioxidant in some foods, pharmaceutical formulations, and cosmetics [2]. l-Ascorbic acid plays a crucial role in some biological processes such as hormone biosynthesis, collagen formation, and amino acid metabolism [3]. The excess of ascorbic acid can lead to several disorders such as gastric irritation, urinary stones, and diarrhea, whereas its deficiency can cause muscle degeneration, anemia, Parkinson’s, Alzheimer’s, infections, or poor wound healing [3, 4]. Ascorbic acid cannot be synthesized in human body and must be provided exogenously in the diet [1]. Thus, the development of rapid and reliable techniques for the quantitative determination of ascorbic acid is of great importance in clinical chemistry, pharmaceutical, cosmetic, and food industries.
Different analytical techniques such as spectrophotometry [5], chromatography [6], colorimetry [7], and chemiluminescence [8] have been reported for the quantification of ascorbic acid. Nevertheless, most of these techniques suffer from cumbersome and time-consuming sample pretreatment steps or expensive equipment. As compared to them, the electrochemical determination of ascorbic acid is more practical, sensitive, and rapid and does not need complex sample preparation procedure [3]. The determination of ascorbic acid by amperometric technique is based on its direct oxidation at conventional solid electrodes such as glassy carbon or platinum. However, this approach has some drawbacks including high overpotential required for direct oxidation of ascorbic acid, low selectivity, and poor sensitivity [4]. In this sense, chemically modified electrodes have been proposed to lower the overpotential [9, 10]. On the other hand, the use of amperometric biosensors which integrate biological recognition through enzyme specificity with the simplicity of fabrication is another attractive approach for l-ascorbic acid determination [11,12,13,14]. These devices have gained increasing attention in the last few decades for their favorable properties such as high sensitivity, rapid response, low cost, and good selectivity.
Nowadays, carbon nanomaterials such as graphene and its derivatives, carbon nanotubes, or carbon nanofibers (CNFs) are widely used as sensing materials in the development of amperometric biosensors of good sensitivity and high selectivity [15, 16]. Among various carbon nanomaterials, CNFs exhibit distinguished properties such as large surface area, good mechanical stability, high electric conductivity, broad chemical modification capacity, and biocompatibility, which make them good candidates for electrode modification [16, 17]. Compared with carbon nanotubes, CNFs have inherent advantages including higher mechanical stability, easier surface functionalization, lower-cost mass production, and more edge sites on the outer wall [18].
Nanodiamonds (NDs), a member of the carbon nanomaterial family, have attracted researcher’s attention in different fields such as biomedicine, sensor development, drug delivery, and energy storage [19]. NDs exhibit excellent characteristics including small particle size (about 5 nm), high biocompatibility, thermal stability, large specific surface area, chemical inertness, facile surface functionalization, wide potential window, and low background current [20,21,22]. NDs also contain a small fraction of carbon atoms with sp2 hybridization which causes the nanomaterial to be an electric conductor, unlike conventional diamond [20]. The surface of NDs can be functionalized with a variety of functional groups such as carboxyl and hydroxyl groups which can be used for conjugation with biological molecules [23]. For instance, the bioconjugation of surface carboxylic groups present in NDs with enzyme through coupling agents (1-ethyl-3–(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) has been reported [24]. These outstanding properties of NDs make them favorable materials to fabricate modified electrodes for biosensors. Moreover, the use of NDs in electrochemical sensing has been reported to improve various electrode characteristics such as effective surface area and detection limit [25]. Various efforts have been made to show the appropriateness of both CNFs [16, 17] and NDs [20, 26] individually in electrochemical sensing but no significant research has been conducted on modified electrodes based on the combination of CNFs and NDs.
Polymer-modified electrodes have shown to be an effective tool in biosensor fabrication due to their advantages including good selectivity and sensitivity, chemical stability, improved electrocatalysis, easy preparation, and absence of surface fouling. Moreover, polymers are reported as suitable platforms for the immobilization of biomolecules [27, 28]. l-Aspartic acid (l-Asp) is an amino acid that contains two carboxyl groups (–COOH) and one amino group (–NH2). l-Asp can be easily electropolymerized on the GCE surface by CV to form a P(l-Asp) layer [29]. P(l-Asp) exhibits various favorable properties such as biocompatibility, low toxicity, easiness of preparation, low cost, and biodegradability [30, 31]. Moreover, the functional groups on the membrane enable the immobilization of a high number of enzyme molecules on the surface of modified electrodes. Both the carboxyl and amino groups are involved in electropolymerization of l-aspartic acid which enables the immobilization of a high number of enzyme molecules on the surface of modified electrodes [30].
A novel trend in biosensor development is the combination of polymers and carbon nanomaterials in electrode modification, with the aim of combining the advantageous properties of different materials. These composites are expected to possess complementary properties of each component with a synergistic effect and to exhibit improved performance in biosensor fabrication. In this context, our attention was directed toward the application of a composite containing CNFs, NDs, and P(l-Asp) in electrode modification to benefit from the properties of both carbon nanomaterials and polymer. To the best of our knowledge, there is no study on the application of CNFs, NDs, and P(l-Asp) composite for the determination of l-ascorbic acid. Herein we report, for the first time, the construction and application of an ascorbic acid biosensor using a composite matrix containing CNF, ND, and P(l-Asp) as supporting material for immobilization of ascorbate oxidase (AOx) on glassy carbon electrode (AOx/P(l-Asp)/ND-CNF/GCE). Optimization of conditions that might affect the biosensing of ascorbic acid was performed. The analytical performance of the AOx/P(l-Asp)/ND-CNF/GCE biosensor was evaluated in terms of linear dynamic range, the limit of detection, specificity, stability, and reproducibility. In addition, the reliability of the resulting biosensor was investigated in the real sample by measuring ascorbic acid concentration in vitamin C effervescent tablets and pharmaceutical powder containing ascorbic acid.
Materials and methods
Reagents and instrumentation
Ascorbate oxidase (E.C. 1.10.3.3. from Cucurbita sp. 1000–3000 U mg−1), l-ascorbic acid, carbon nanofibers (D × L 100 nm × 20–200 μm), (1-ethyl-3–(3-dimethylaminopropyl)-carbodiimide hydrochloride, monodispersed nanodiamond particles (10 mg mL−1 in H2O, carboxylated), N-hydroxysuccinimide, potassium hexacyanoferrate(III), potassium chloride, potassium hexacyanoferrate(II) trihydrate, sodium hydroxide, glucose, caffeine, paracetamol, citric acid, oxalic acid, urea, glycine, sodium chloride, disodium hydrogen phosphate, hydrochloric acid, sodium dihydrogen phosphate, chitosan, and l-aspartic acid were obtained from Sigma-Aldrich. Vitamin C effervescent tablets and pharmaceutical powder containing ascorbic acid were bought from a pharmacy. Double distilled water obtained from ELGA Purelab Option-S System was used throughout the experiments.
All electrochemical studies were performed using a Metrohm Autolab electrochemical analyzer (Eco-Chemie, Utrecht, The Netherlands) controlled by the NOVA software. A traditional three-electrode arrangement consists of a modified glassy carbon working electrode (3.0 mm in diameter, BASi MF2012, Bioanalytical Systems, Inc., USA), platinum wire auxiliary electrode (BASi MW1032), and Ag/AgCl reference electrode (BASi MF2052) was used for the electrochemical experiments. The SEM images were captured by a Carl Zeiss AG, EVO® 40 Series scanning electron microscope.
Electrochemical measurements
Amperometric measurements were carried out in phosphate buffer solution (PBS) (pH 6.5, 0.05 M) at a constant operating potential of + 0.25 V under stirring. Before aliquots of ascorbic acid were added to the stirred PBS, the background current was allowed to reach a constant level. The steady-state current responses were recorded after the addition of ascorbic acid and the response current was marked as the difference (Δi) between the steady-state and background currents. Ascorbic acid solutions were prepared freshly prior to each measurement. Cyclic voltammograms and electrochemical impedance spectra were obtained in 100 mM KCl solution containing 5.0 mM Fe (CN)63−/4−. A potential window from − 0.20 to + 0.60 V and a scan rate of 50 mV s−1 were used for CV measurements. EIS measurements were performed within a frequency range of 0.05–105 Hz. All measurements were accomplished at room temperature.
Preparation of the biosensor
AOx/P(l-Asp)/ND-CNF/GCE biosensor was fabricated as described below.
-
(i)
GCE was polished with alumina slurry on a wet microcloth to obtain a mirror-like surface. Then, the electrode was rinsed with double distilled water, sonicated in double distilled water and ethanol, and dried at room temperature. 100.0 mg chitosan (CS) was dissolved in 20.0 mL pH 5.0 acetate buffer solution under magnetic stirring. Chitosan was used as a dispersive agent to obtain stable CNF-ND dispersions due to its advantageous properties such as good film-forming ability and biocompatibility [32]. Eight milligrams of the CNF was dispersed into 1.0 mL CS solution by ultrasonic agitation for 2 h. 6 mg mL−1 ND solution was prepared by diluting the 10 mg mL−1 ND solution with double distilled water. 0.5 mL of diluted ND solution (6 mg mL−1) was added to 0.5 mL of CNF-CS suspension and the final mixture was sonicated for 1 h to get a uniform dispersion. Five microliters of the ND-CNF-CS suspension was cast onto the GCE surface and allowed to dry.
-
(ii)
Electropolymerization of L-Asp on the ND-CNF/GCE was carried out by CV between −1.5 and + 2.0 V at a scan rate of 100 mV s−1 for 10 cycles in PBS (pH 6.0) containing 2.0 mM L-Asp [27]. P(L-Asp)/ND-CNF/GCE was rinsed repeatedly with double distilled water and dried in air.
-
(iii)
EDC/NHS coupling chemistry [33] was used for the immobilization of AOx onto the modified electrode. For this purpose, 3 μL of EDC (100 mM)-NHS (100 mM) mixture was pipetted onto the P(l-Asp)/CNF-ND/GCE to activate the unbound and free –COOH groups of P(l-Asp)/ND-CNF composite film. AOx solution was prepared in pH 6.0 PBS and stored at − 18 °C until usage to prevent activity loss. Five microliters of AOx solution (100 U mL−1) was casted onto the P(l-Asp)/ND-CNF/GCE and left to be dried at + 4 °C to construct the biosensor. The enzyme electrode was washed with PBS (pH 6.5) to eliminate the unbound components. Three microliters of Nafion solution (0.25%) was drop coated on the biosensor to minimize the effect of interfering species and prevent enzyme leakage. The fabrication process of AOx/P(l-Asp)/ND-CNF/GCE is presented in Scheme 1.
Results and discussion
Optimization of the electrode surface composition
The analytical performance of the AOx/P(l-Asp)/ND-CNF/GCE biosensor is directly affected by the amounts of (a) CNF and (b) ND in the composite, (c) the cycle number used for the electropolymerization of l-Asp, and (d) AOx loading. Therefore, in the first step, the amounts of CNF, ND, and AOx and the cycle number of the P(l-Asp) were optimized to achieve good analytical characteristics. The optimization study was performed by changing the amount of each component at one time and keeping others constant. In order to determine the optimum condition, amperometric responses of the biosensors to successive ascorbic acid additions were recorded and the sensitivities were evaluated.
i. CNF amount
To find the optimum CNF amount, 2.0, 4.0, 6.0, 8.0, and 10.0 mg of CNFs were dispersed in 1.0 mL of CS solution and these mixtures were used in biosensor construction. Figure 1A depicts the ascorbic acid responses of the biosensors constructed with different amounts of CNF. The sensitivity of the biosensor increased with the increase in CNF amount until 8.0 mg and decreased thereafter. The decrease observed at higher amounts of CNF can be attributed to the diffusion problem for the substrate. Hence, 8.0 mg mL−1 of CNF was selected for the fabrication of the biosensor.
The effects of (a) CNF and (b) ND amounts on the response of AOx/P(l-Asp)/ND-CNF/GCE. (c) Cyclic voltammograms for the electropolymerization of l-Asp at ND-CNF/GCE (2.0 mM l-Asp, 10 cycles between − 1.5 and + 2.0 V at 100 mV s−1 scan rate). Effects of (d) cycle number for polymerization and (e) AOx loading on the response of AOx/P(l-Asp)/ND-CNF/GCE (at + 0.25 V, in 0.05 M PBS pH 6.5). Error bars represent the standard deviation across the biosensors for each assay (N = 3)
ii. ND amount
The effect of ND amount on the response of AOx/P(l-Asp)/ND-CNF/GCE biosensor was also determined. For this purpose, different ND amounts as 4.0, 6.0, 8.0, and 10.0 mg mL−1 were used for the construction of the biosensor and amperometric responses of the biosensors to ascorbic acid were recorded (Fig. 1B). The highest sensitivity was obtained with the biosensor constructed with 6.0 mg mL−1 ND and this optimum amount was used in the construction of AOx/P(l-Asp)/ND-CNF/GCE biosensor. Camargo et al. [26] reported that an increase in ND amount on GCE surface leads to a decrease in peak currents of cyclic voltammograms which is consistent with our findings.
iii. Effect of cycle number
Electropolymerization of l-Asp on ND-CNF/GCE was performed by continuous cyclic voltammograms which were recorded between − 1.5 and + 2.0 V at 100 mV s−1 scan rate for 10 cycles in a 0.05 M pH 6.0 PBS containing 2.0 mM l-Asp (Fig. 1C). The redox behaviors of the polymer are shown in Fig. 1C. As can be seen, a cathodic peak at − 0.8 V and an anodic peak at + 1.3 V were observed due to the formation of P(l-Asp). Furthermore, the gray film observed on the electrode surface after modification confirmed the formation P(l-Asp) on the surface of ND-CNF/GCE. It was reported that monomer containing amino group can be electropolymerized at the surface of GCE to form a film via carbon-nitrogen (C-N) linkages between the electrode and –NH2 [34]. In our study, the possible electropolymerization mechanism can be detailed as follows: the oxidation of the –NH2 group could convert to its corresponding cation radical and these radicals can form C-N linkages at the ND-CNF/GCE surface to form P(l-Asp) films [30]. The voltammograms obtained during the modification of the ND-CNF/GCE surface with P(l-Asp) are similar to those obtained by polymerizing l-Asp on bare GCE and multiwalled carbon nanotube-modified GCE [27, 30].
Polymer thickness is an important parameter in enzyme immobilization since very thick or thin polymeric layers may not preserve the enzyme molecules properly [35]. The thickness of the polymer film could be controlled by changing the cycle number of voltammetric scans. Thus, the optimum cycle number for the electropolymerization step was determined from amperometric measurements at varying cycle numbers from 5 to 20 cycles. The highest biosensor sensitivity was obtained at a cycle number of 10 and this value was used as the optimum cycle number (Fig. 1D). It was also observed that, by increasing the cycle number, the thickness of the polymer film was increased, which makes it difficult to perform enzyme immobilization procedures in the next step. Moreover, the stability of the electrode surface was not satisfactory at thick polymer layers.
iv. AOx loading
Enzyme loading should also be optimized to achieve the highest biosensor performance. To optimize the AOx loading, five different biosensors having 0.00, 0.05, 0.10, 0.50, and 1.00 unit AOx were constructed by keeping other parameters constant. Figure 1E shows the dependence of sensitivity on AOx loading. The sensitivity of the biosensor improved as the loading of AOx increased up to 0.50 unit where the highest response was obtained and then decreased at 1.00 unit. The decrease in sensitivity at high enzyme loadings can be ascribed to the increased film thickness, which increased the resistance to the diffusion of the substrate molecule [35]. Consequently, an AOx loading of 0.50 U immobilized on the P(l-Asp)/ND-CNF/GCE was chosen for future experiments.
Surface morphology
Morphological characterization of electrode surfaces by SEM is an effective tool to confirm the construction steps of modified electrodes. SEM images of (a) CNF/GCE, (b) ND-CNF/GCE, (c) P(l-Asp)/ND-CNF/GCE, and (d) AOx/P(l-Asp)/ND-CNF/GCE are shown in Fig. 2. In the image, a typical morphology of CNFs [36] can be observed. When NDs were added to CNF-CS mixture, there were spherical structures between CNFs as shown in image b. These structures can be attributed to the presence of NDs and it is obvious that the incorporation of NDs changes the microstructure of films. The ND-CNF/GCE surface presents a very rough appearance, suggesting that the ND-CNF matrix provided an increased surface area for the modified GCE. After the electropolymerization of l-aspartic acid on ND-CNF/GCE, the morphology of the electrode changed significantly and the polymer layer covered the electrode surface (image c). However, CNFs and NDs are still visible and the modified electrode surface reveals a porous structure which is important for the effective immobilization of the enzymes onto the electrode surface. In image d, a different morphology with bright clustered structures was observed on the electrode surface revealing the successful immobilization of AOx onto P(l-Asp)/ND-CNF/GCE.
Electrochemical characterization
The biosensor fabrication process and charge transfer properties of the modified electrodes were also probed by CV and EIS techniques. Figure 3A presents the cyclic voltammograms of different electrodes recorded in redox probe Fe(CN)63−/4−. At the bare GCE (curve a), well-defined redox peaks of the ferri/ferrocyanide couple were observed. The modification of the GCE surface by ND (curve b) and CNF (curve c) led to an increase in the peak currents and decrease in peak to peak separation (ΔEp) as a result of larger surface area and better conductivity. The peak currents of ND-CNF/GCE (curve d) increased much more than those of bare GCE, CNF/GCE, and ND/GCE due to the synergistic effect of CNFs and NDs. In contrast, the introduction of P(l-Asp) onto ND-CNF matrix resulted in a decrease in the peak current signal (curve e) which is probably due to the negative effect of thick polymer film layer on the conductivity of the electrode surface. Moreover, the electrostatic repulsion between the negatively charged carboxyl groups of the polymer layer and the negatively charged redox probe contributed to the decrease of the peak current [30].
EIS is a valuable tool to enlighten the interface properties of surface-modified electrodes. A typical Nyquist plot includes a semicircular part obtained at high frequencies representing the electron transfer-limited process and a linear part obtained at low frequencies representing the diffusion process. The diameter of the semicircle corresponds to the charge transfer resistance, Rct [37]. Figure 3B depicts the impedance spectra of (a) GCE, (b) ND/GCE, (c) CNF/GCE, (d) ND-CNF/GCE, and (e) P(l-Asp)/ND-CNF/GCE recorded in 5 mM [Fe(CN)6]3−/4−. A high Rct value of 910 Ω was observed at bare GCE due to the low electron transfer rate of glassy carbon. According to the fitting results, the Rct of ND/GCE was 210 Ω indicating the increased conductivity of the electrode interface. The increase in conductivity observed after the incorporation of NDs into the modification matrix is consistent with the EIS results reported for nanodiamond-potato starch-modified GCE [26]. On the other hand, CNF/GCE exhibited a Rct value of 35 Ω which can be attributed to the improved electron transfer rate of [Fe(CN)6]3−/4− at the electrode surface due to the presence of CNFs. The Rct value of ND-CNF/GCE was 16 Ω which suggested that NDs and CNFs had a synergistic effect and ND-CNF matrix shows the highest electron transfer efficiency. After l-Asp was electrodeposited on the surface of ND-CNF/GCE, an increase in Rct (22 Ω) was observed (curve e) suggesting that the polymer film increases the resistance of the electrode. A similar observation was also reported for P(l-Asp)/MWCNT bionanocomposite-modified GCE [30].
Optimization of experimental conditions
The operating potential and pH of the working buffer were also optimized to improve the performance of the ascorbic acid biosensor. Figure 4A shows the bioelectrocatalytic oxidation of ascorbic acid at AOx/P(l-Asp)/ND-CNF/GCE biosensor. According to the figure, the bioelectrocatalytic oxidation of ascorbic acid began to appear from 0.0 V and reached the maximum value at about + 0.20 V. In addition, peak current increased with increasing the concentration of ascorbic acid, suggesting that the AOx/P(l-Asp)/ND-CNF/GCE biosensor exhibited a good bioelectrocatalytic performance toward ascorbic acid oxidation. In order to determine an appropriate operating potential, the amperometric response of biosensor was also investigated at different potentials at 0.10, 0.15, 0.20, and 0.25 V (Fig. 4B). This potential range was selected according to the bioelectrocatalytic oxidation of ascorbic acid depicted in Fig. 4A. As can be seen, the sensitivity increased as the operating potential shifts from 0.10 to 0.20 V and reached a plateau around 0.20–0.25 V which can be ascribed to the rate-limiting process of enzyme kinetics and substrate diffusion [38]. Therefore, an operating potential of + 0.25 V was selected for further studies. This relatively low operating potential would help to decrease the effect of interferences from electrochemically active species normally coexist in real samples.
(a) The cyclic voltammograms of the AOx/P(l-Asp)/ND-CNF/GCE biosensor in 0.05 M PBS containing different concentrations (0.0, 1.0, 1.5, and 2.0 mM) of ascorbic acid. The effects of (b) operating potential and (c) buffer pH on the AOx/P(l-Asp)/ND-CNF/GCE response (0.05 M PBS, error bars represent the standard deviation across the biosensors for each assay, N = 3)
In this study, the response mechanism of the AOx/P(l-Asp)/ND-CNF/GCE biosensor is based on the biocatalytic oxidation of ascorbic acid to l-dehydroascorbic acid and H2O by AOx in the presence of O2. Several authors have reported that the biocatalytic oxidation of ascorbic acid by AOx follows a double displacement reaction mechanism which occurred in the presence of two substrates (ascorbic acid and O2) and two reactants (first: ascorbic acid; second: O2) resulting in products of dehydroascorbic acid and H2O [1, 13]. The reaction mechanism can be presented by the following reaction:
This mechanism is also named as the “ping-pong” reaction mechanism where the substrates appear to bounce on and off from enzyme, just like a ping-pong ball [1]. According to the reaction mechanism, ascorbic acid first binds to AOx(ox) to form AOx(ox)-ascorbic acid, then transforms into AOx(red)-dehydroascorbic acid, next releases product dehydroascorbic and forms AOx(red). The other substrate O2 binds to AOx(red) to form AOx(red)-O2 which transforms into AOx(ox)-H2O then releases another product H2O and forms AOx(ox). In this reaction, the concentration of O2 was held constant, whereas ascorbic acid varied. Therefore, the concentration of O2 was kept constant in air-saturated PBS in all amperometric measurements.
pH of the working buffer is a crucial parameter for biosensing studies since it affects the bioactivity of the enzyme [39]. The effect of buffer pH on the amperometric response of the AOx/P(l-Asp)/ND-CNF/GCE biosensor was explored over the range from pH 6.0–9.0 in 0.05 M PBS (Fig. 4C). The sensitivity increased with increasing pH from 5.0 to 6.5, where the highest sensitivity was achieved, then decreased afterwards. The optimum sensitivity for AOx/P(l-Asp)/ND-CNF/GCE biosensor was observed at pH 6.5 and this pH was used as the optimum value for l-ascorbic acid biosensing. The optimum pH for the free AOx is pH 5.5–7.0 [40]. After the immobilization of AOx, no significant change was observed at the optimum pH of the enzyme indicating that the immobilization matrix or procedure did not affect the microenvironment or quaternary structure of the enzyme. Several biosensor studies reported pH 6.5 as the optimum pH value for ascorbic acid determination [14, 41].
Analytical performance of the biosensor
After the optimization of experimental conditions, the response of AOx/P(l-Asp)/ND-CNF/GCE to ascorbic acid was investigated. For this purpose, amperometric current-time (i-t) responses of AOx/P(l-Asp)/ND-CNF/GCE for successive additions of ascorbic acid were recorded in a stirred 0.05 M PBS (pH 6.5) at an operational potential of + 0.25 V. The i-t response of the biosensor shows that a stable, rapid (< 10 s), and well-defined amperometric response was observed after each addition of ascorbic acid, indicating the effectiveness of the biosensing platform for the biorecognition event (Fig. 5A). Figure 5B depicts the corresponding calibration curve plotted from i-t data. According to the calibration curve, AOx/P(l-Asp)/ND-CNF/GCE biosensor displayed a linear relationship between the current and ascorbic acid concentration in the range of 2.0 × 10−7–1.8 × 10−3 M with a sensitivity of 7.42 μA mM−1 (105.0 μA mM−1 cm−2). The detection limit of the biosensor was calculated according to the 3sb/m formula where m represents the slope of the calibration graph and sb represents the standard deviation of the chronoamperometric responses from different solutions of ascorbic acid at the concentration level corresponding to the lowest concentration of the calibration plot [42]. The detection limit of the AOx/P(l-Asp)/ND-CNF/GCE biosensor was found to be 1.0 × 10−7 M.
(a) Current-time plots of the AOx/P(l-Asp)/ND-CNF/GCE biosensor to successive additions of ascorbic acid and (b) calibration curve of the biosensor (inset: relationship between the response current and the concentration; 0.05 M pH 6.5, PBS at + 0.25 V, error bars represent the standard deviation across the biosensors for each assay, N = 3)
Figure 5B (inset) shows that the calibration curve of AOx/P(l-Asp)/ND-CNF/GCE biosensor follows a hyperbolic trend with a plateau observed at high ascorbic acid concentrations which is typical for Michaelis-Menten kinetics. The apparent Michaelis-Menten constant (KMapp) can be calculated from the Lineweaver-Burk equation:
In this equation, Is represents the steady-state current after ascorbic acid is added, C represents the bulk concentration of ascorbic acid, and Imax is the maximum biosensor current recorded under saturated ascorbic acid conditions [43]. In our study, KMapp value was calculated as 1.21 mM. This KMapp value was found to be smaller than those of poly(3,4-ethylenedioxythiophene)/single-walled carbon nanotube/Nafion/AOx-modified platinum electrode (18.35 mM) [44] and polypyrrole/multiwalled carbon nanotubes/AOx-modified platinum electrode (21.18 mM) [14]. According to the characteristics of KMapp, the lesser the value of KMapp, the stronger will be the affinity between AOx and ascorbic acid. Thus, the low value of KMapp indicated that the AOx immobilized on the P(l-Asp)/ND-CNF composite showed a high bioaffinity to ascorbic acid.
The repeatability and reproducibility of the AOx/P(l-Asp)/ND-CNF/GCE biosensor were also studied. Five consecutive calibration plots were performed under the optimal experimental conditions using the same biosensor to investigate the repeatability. The change observed in sensitivity (slope of the linear calibration) in terms of relative standard deviation (RSD) was 3.4% between the 1st and the 5th calibration. This result indicated the high repeatability of the presented biosensor which can be attributed to the good interaction between the AOx enzyme and P(l-Asp)/ND-CNF/GCE matrix. In case of reproducibility, five calibration curves were plotted, using five different similarly fabricated electrodes. The RSD values obtained with AOx/P(l-Asp)/ND-CNF/GCE biosensor was 3.3% indicating that the ascorbic acid biosensor also exhibited good fabrication reproducibility. In order to prove the operational stability, the biosensor response to 0.1 mM ascorbic acid solution was recorded for a hundred times. Only 12% loss in activity was observed after 100 measurements which confirms the good operational stability of the ascorbic acid biosensor.
The selectivity of the AOx/P(l-Asp)/ND-CNF/GCE biosensor to various potential interferants such as oxalic acid, glucose, citric acid, paracetamol, glycine, caffeine, and sodium chloride was investigated in 0.05 M pH 6.5 PBS (Fig. 6). The interference effect was calculated by comparison of the response obtained for 0.1 mM ascorbic acid solution vs. the response obtained for solutions of the same concentration of those interferants. The results showed that the interference of oxalic acid, citric acid, paracetamol, glycine, and sodium chloride was negligible (< 5%). On the other hand, glucose and caffeine showed only 2% and 5% interference on the ascorbic acid response of the AOx/P(l-Asp)/ND-CNF/GCE biosensor. The results of the selectivity study showed that the presented biosensor exhibited good anti-interference ability.
A comparison between the analytical performance of previously reported ascorbic acid biosensors and AOx/P(l-Asp)/ND-CNF/GCE biosensor is presented in Table 1. It can be seen from the table that the AOx/P(l-Asp)/ND-CNF/GCE biosensor offered a wider linear dynamic range for ascorbic acid than some of the previous ascorbic acid biosensors [4, 11,12,13]. Moreover, AOx/P(l-Asp)/ND-CNF/GCE biosensor exhibited lower detection limit than all other ascorbic acid biosensors presented in Table 1 except Nafion-MWCNT/AOx/PEDOT/GCE [13]. This good analytical performance may be attributed to the following reasons, (i) the high conductivity of CNFs, (ii) synergistic effect of CNFs and NDs to improve the electron transfer rate, (iii) the good interaction between the enzyme and –COOH groups of P(l-Asp)/ND-CNF matrix which improved the covalent bond formation between electrode and enzyme, and (iv) the stability of the P(l-Asp)/ND-CNF matrix on GCE surface.
The analytical performance of the presented AOx/P(l-Asp)/ND-CNF/GCE biosensor was also compared with the earlier ascorbic acid sensors based on the non-enzymatic determination (Table 2). Particularly worth mentioning was that our proposed biosensor’s detection limit was lower than most of the other published ascorbic acid sensors. This low detection limit is important for the detection of small amounts of ascorbic acid in some real samples. Furthermore, the AOx/P(l-Asp)/ND-CNF/GCE biosensor shows a wider linear range [47, 52,53,54,55] and higher sensitivity [47, 48, 50,51,52] compared with some of the listed works.
Real sample analysis
The analytical applicability of the AOx/P(l-Asp)/ND-CNF/GCE biosensor for real sample determinations was studied by analyzing vitamin C effervescent tablet and pharmaceutical powder containing ascorbic acid. The ascorbic acid contents of these samples were determined using the standard addition method. Sample 1 was a vitamin C effervescent tablet which contains 1000 mg of ascorbic acid per tablet with vitamin D and zinc. One tablet was taken, dissolved in 0.05 M pH 6.5 PBS, and diluted to 250.0 mL. The resulting solution was diluted 1:50 with PBS, and aliquots from stock sample 1 solution were injected into the test cell containing 5.0 mL of PBS. Then, aliquots of standard ascorbic acid solution were added to the cell and a multiple addition calibration curve was plotted. The ascorbic acid concentration in tablet solution calculated from the calibration curve was 1005.8 ± 18.8 mg tablet−1 (N = 3) with an average recovery of 100.6 ± 1.9%. Sample 2 was a pharmaceutical powder with 300 mg ascorbic acid per sachet. One sachet of powder was transferred to a calibrated flask, dissolved with 0.05 M pH 6.5 PBS and diluted to 100.0 mL. The resulting solution was diluted 1:10 for the analysis. The concentration of ascorbic acid in sample 2 was found to be 299.0 ± 4.2 mg sachet−1 (N = 3). The mean recovery was calculated as 99.7 ± 1.4. The performance of the biosensor was also checked out by calculation of texp value using the results obtained from samples 1 and 2 applications and the nominal value. The texp value was calculated as 1.01 for sample 1 and 1.33 for sample 2 at 95% confidence level while the tcritic was 4.30. The results indicate that no significant difference observed between the nominal value and the results obtained from the proposed biosensor at the 95% confidence level. It can be concluded that AOx/P(l-Asp)/ND-CNF/GCE biosensor is a promising approach for the rapid, accurate, and practical analysis of ascorbic acid in real samples.
Conclusion
In this study, a novel l-ascorbic acid biosensor based on AOx, P(l-Asp), CNF, and ND-modified GCE was fabricated. The presented biosensor combined the advantageous properties of both carbon nanomaterials and polymer and exhibited good analytical characteristics in terms of wide linear dynamic range, low detection limit, high sensitivity, excellent repeatability, and good operational stability. Moreover, the strong attraction between the enzyme and composite matrix contributed to the analytical performance of the biosensor. It can be concluded that the P(l-Asp)/CNF-ND matrix is a promising immobilization platform for enzyme-based sensors. The biosensor also showed anti-interference ability which is crucial in real sample applications. In addition, the biosensor was tested on vitamin C effervescent tablets and pharmaceutical powder to determine their ascorbic acid content. The results of the real sample analysis indicated that the proposed biosensor can be used for the rapid and reliable analysis of ascorbic acid.
References
Liu M, Wen Y, Xu J, He H, Li D, Yue R, et al. An amperometric biosensor based on ascorbate oxidase immobilized in poly (3,4-ethylenedioxythiophene)/multi-walled carbon nanotubes composite films for the determination of L-ascorbic acid. Anal Sci. 2011;27(5):477.
Prasad BB, Kumar D, Madhuri R, Tiwari MP. Ascorbic acid imprinted polymer-modified graphite electrode: a diagnostic sensor for hypovitaminosis C at ultra trace ascorbic acid level. Sens Actuators B Chem. 2011;160(1):418–27.
Dhara K, Mahapatra DR. Review on nanomaterials-enabled electrochemical sensors for ascorbic acid detection. Anal Biochem. 2019;586:113415.
Chauhan N, Narang J, Pundir CS. Fabrication of multiwalled carbon nanotubes/polyaniline modified Au electrode for ascorbic acid determination. Analyst. 2011;136(9):1938–45.
Jain A, Chaurasia A, Verma KK. Determination of ascorbic acid in soft drinks, preserved fruit juices and pharmaceuticals by flow injection spectrophotometry: matrix absorbance correction by treatment with sodium hydroxide. Talanta. 1995;42(6):779–87.
Spínola V, Mendes B, Câmara JS, Castilho PC. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal Bioanal Chem. 2012;403(4):1049–58.
Kyaw AA. Simple colorimetric method for ascorbic acid determination in blood plasma. Clin Chim Acta. 1978;86(2):153–7.
Chen H, Li R, Lin L, Guo G, Lin JM. Determination of l-ascorbic acid in human serum by chemiluminescence based on hydrogen peroxide–sodium hydrogen carbonate–CdSe/CdS quantum dots system. Talanta. 2010;81(4–5):1688–96.
Fernández L, Carrero H. Electrochemical evaluation of ferrocene carboxylic acids confined on surfactant–clay modified glassy carbon electrodes: oxidation of ascorbic acid and uric acid. Electrochim Acta. 2005;50(5):1233–40.
Kulys J, D’Costa EJ. Printed electrochemical sensor for ascorbic acid determination. Anal Chim Acta. 1991;243:173–8.
Csiffáry G, Fűtő P, Adányi N, Kiss A. Ascorbate oxidase-based amperometric biosensor for L-ascorbic acid determination in beverages. Food Technol Biotechnol. 2016;54(1):31–5.
Chauhan N, Dahiya T, Pundir CS. Fabrication of an amperometric ascorbate biosensor using egg shell membrane bound Lagenaria siceraria fruit ascorbate oxidase. J Mol Catal B Enzym. 2010;67(1–2):66–71.
Wen Y, Xu J, Liu M, Li D, He H. Amperometric vitamin C biosensor based on the immobilization of ascorbate oxidase into the biocompatible sandwich-type composite film. Appl Biochem Biotechnol. 2012;167(7):2023–38.
Li D, Wen Y, He H, Xu J, Liu M, Yue R. Polypyrrole–multiwalled carbon nanotubes composites as immobilizing matrices of ascorbate oxidase for the facile fabrication of an amperometric vitamin C biosensor. J Appl Polym Sci. 2012;126(3):882–93.
Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI. A critical review of glucose biosensors based on carbon nanomaterials: carbon nanotubes and graphene. Sensors. 2012;12(5):5996–6022.
Huang J, Liu Y, You T. Carbon nanofiber based electrochemical biosensors: a review. Anal Methods. 2010;2(3):202–11.
Xie H, Luo G, Niu Y, Weng W, Zhao Y, Ling Z, et al. Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor. Mater Sci Eng C. 2020;107:110209.
Zhang J, Lei J, Liu Y, Zhao J, Ju H. Highly sensitive amperometric biosensors for phenols based on polyaniline–ionic liquid–carbon nanofiber composite. Biosens Bioelectron. 2009;24(7):1858–63.
Kumar S, Nehra M, Kedia D, Dilbaghi N, Tankeshwar K, Kim KH. Nanodiamonds: emerging face of future nanotechnology. Carbon. 2019;143:678–99.
Zambianco NA, Silva TA, Zanin H, Fatibello-Filho O, Janegitz BC. Novel electrochemical sensor based on nanodiamonds and manioc starch for detection of diquat in environmental samples. Diam Relat Mater. 2019;98:107512.
Schrand AM, Hens SAC, Shenderova OA. Nanodiamond particles: properties and perspectives for bioapplications. Crit Rev Solid State Mater Sci. 2009;34(1–2):18–74.
Ramos MMV, Carvalho JH, de Oliveira PR, Janegitz BC. Determination of serotonin by using a thin film containing graphite, nanodiamonds and gold nanoparticles anchored in casein. Measurement. 2020;149:106979.
Alshawafi WM, Aldhahri M, Almulaiky YQ, Salah N, Moselhy SS, Ibrahim IH, et al. Immobilization of horseradish peroxidase on PMMA nanofibers incorporated with nanodiamond. Artif Cells Nanomed Biotechnol. 2018;46(3):973–81.
Wei L, Zhang W, Lu H, Yang P. Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta. 2010;80(3):1298–304.
Kumar V, Kaur I, Arora S, Mehla R, Vellingiri K, Kim KH. Graphene nanoplatelet/graphitized nanodiamond-based nanocomposite for mediator-free electrochemical sensing of urea. Food Chem. 2020;303:125375.
Camargo JR, Baccarin M, Raymundo-Pereira PA, Campos AM, Oliveira GG, Fatibello-Filho O, et al. Electrochemical biosensor made with tyrosinase immobilized in a matrix of nanodiamonds and potato starch for detecting phenolic compounds. Anal Chim Acta. 2018;1034:137–43.
Mekassa B, Tessema M, Chandravanshi BS, Baker PG, Muya FN. Sensitive electrochemical determination of epinephrine at poly (L-aspartic acid)/electro-chemically reduced graphene oxide modified electrode by square wave voltammetry in pharmaceutics. J Electroanal Chem. 2017;807:145–53.
Ates M. A review study of (bio) sensor systems based on conducting polymers. Mater Sci Eng C. 2013;33(4):1853–9.
Mekassa B, Tessema M, Chandravanshi BS, Tefera M. Square wave voltammetric determination of ibuprofen at poly (L-aspartic acid) modified glassy carbon electrode. IEEE Sensors J. 2018;18(1):37–44.
Yazdanparast S, Benvidi A, Abbasi S, Rezaeinasab M. Enzyme-based ultrasensitive electrochemical biosensor using poly (L-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: a meat freshness marker. Microchem J. 2019;149:104000.
Zhang C, Li H, Yu Q, Jia L, Wan LY. Poly (aspartic acid) electrospun nanofiber hydrogel membrane-based reusable colorimetric sensor for Cu (II) and Fe (III) detection. ACS Omega. 2019;4(11):14633–9.
Dalkıran B, Erden PE, Kaçar C, Kılıç E. Disposable amperometric biosensor based on poly-L-lysine and Fe3O4 NPs-chitosan composite for the detection of tyramine in cheese. Electroanalysis. 2019;31(7):1324–33.
Chauhan N, Pundir CS. An amperometric uric acid biosensor based on multiwalled carbon nanotube–gold nanoparticle composite. Anal Biochem. 2011;413(2):97–103.
Shadjou N, Alizadeh S, Hasanzadeh M. Sensitive monitoring of taurine biomarker in unprocessed human plasma samples using a novel nanocomposite based on poly (aspartic acid) functionalized by graphene quantum dots. J Mol Recognit. 2018;31(12):e2737.
Bekmezci SA, Soylemez S, Yilmaz G, Udum YA, Yagci Y, Toppare L. A new ethanol biosensor based on polyfluorene-g-poly (ethylene glycol) and multiwalled carbon nanotubes. Eur Polym J. 2020;122:109300.
Ding W, Wu M, Liang M, Ni H, Li Y. Sensitive hydrazine electrochemical biosensor based on a porous chitosan–carbon nanofiber nanocomposite modified electrode. Anal Lett. 2015;48(10):1551–69.
Lisdat F, Schäfer D. The use of electrochemical impedance spectroscopy for biosensing. Anal Bioanal Chem. 2008;391(5):1555.
Li D, Wen YP, Xu JK, He HH, Liu M. An amperometric biosensor based on covalent immobilization of ascorbate oxidase on biocompatiable and low-toxic poly (thiophene-3-acetic acid) matrix. Chin J Polym Sci. 2012;30(5):705–18.
Kaçar C, Erden PE, Dalkiran B, İnal EK, Kiliç E. Amperometric biogenic amine biosensors based on Prussian blue, indium tin oxide nanoparticles and diamine oxidase–or monoamine oxidase–modified electrodes. Anal Bioanal Chem. 2020;412(8):1933–46.
Nakamura T, Makino N, Ogura Y. Purification and properties of ascorbate oxidase from cucumber. J Biochem. 1968;64(2):189–95.
Wen Y, Xu J, Liu M, Li D, Lu L, Yue R, et al. A vitamin C electrochemical biosensor based on one-step immobilization of ascorbate oxidase in the biocompatible conducting poly (3,4-ethylenedioxythiophene)-lauroylsarcosinate film for agricultural application in crops. J Electroanal Chem. 2012;674:71–82.
Carralero V, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. Amperometric IgG immunosensor using a tyrosinase-colloidal gold-graphite-Teflon biosensor as a transducer. Anal Lett. 2008;41(2):244–59.
Kacar C, Erden PE, Kılıc E. Amperometric L-lysine enzyme electrodes based on carbon nanotube/redox polymer and graphene/carbon nanotube/redox polymer composites. Anal Bioanal Chem. 2017;409:2873–83.
Liu M, Wen Y, Li D, Yu R, Xu J, He H. A stable sandwich-type amperometric biosensor based on poly (3,4-ethylenedioxythiophene)–single walled carbon nanotubes/ascorbate oxidase/nafion films for detection of L-ascorbic acid. Sens Actuators B Chem. 2011;159(1):277–85.
Dodevska T, Horozova E, Dimcheva N. Electrochemical behavior of ascorbate oxidase immobilized on graphite electrode modified with Au-nanoparticles. Mater Sci Eng B. 2013;178(20):1497–502.
Wen YP, Lu LM, Li D, Liu M, He HH, Xu JK. Ascorbate oxidase electrochemical biosensor based on the biocompatible poly (3, 4-ethylenedioxythiophene) matrices for agricultural application in crops. Chin Chem Lett. 2012;23(2):221–4.
Wu GH, Wu YF, Liu XW, Rong MC, Chen XM, Chen X. An electrochemical ascorbic acid sensor based on palladium nanoparticles supported on graphene oxide. Anal Chim Acta. 2012;745:33–7.
Li F, Tang C, Liu S, Ma G. Development of an electrochemical ascorbic acid sensor based on the incorporation of a ferricyanide mediator with a polyelectrolyte–calcium carbonate microsphere. Electrochim Acta. 2010;55(3):838–43.
Yang P, Gao X, Wang L, Wu Q, Chen Z, Lin X. Amperometric sensor for ascorbic acid based on a glassy carbon electrode modified with gold-silver bimetallic nanotubes in a chitosan matrix. Microchim Acta. 2014;181(1–2):231–8.
Babu TS, Varadarajan D, Murugan G, Ramachandran T, Nair BG. Gold nanoparticle–polypyrrole composite modified TiO2 nanotube array electrode for the amperometric sensing of ascorbic acid. J Appl Electrochem. 2012;42(6):427–34.
Zhang H, Huang F, Xu S, Xia Y, Huang W, Li Z. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem Commun. 2013;30:46–50.
Lavanya J, Gomathi N. High-sensitivity ascorbic acid sensor using graphene sheet/graphene nanoribbon hybrid material as an enhanced electrochemical sensing platform. Talanta. 2015;144:655–61.
Harraz FA, Faisal M, Ismail AA, Al-Sayari SA, Al-Salami AE, Al-Hajry A, et al. TiO2/reduced graphene oxide nanocomposite as efficient ascorbic acid amperometric sensor. J Electroanal Chem. 2019;832:225–32.
Pakapongpan S, Mensing JP, Phokharatkul D, Lomas T, Tuantranont A. Highly selective electrochemical sensor for ascorbic acid based on a novel hybrid graphene-copper phthalocyanine-polyaniline nanocomposites. Electrochim Acta. 2014;133:294–301.
Kul D, Ghica ME, Pauliukaite R, Brett CM. A novel amperometric sensor for ascorbic acid based on poly (Nile blue A) and functionalised multi-walled carbon nanotube modified electrodes. Talanta. 2013;111:76–84.
Acknowledgments
The authors thank Prof. Dr. Esma Kılıç from Ankara University for valuable comments and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaçar, C., Erden, P.E. An amperometric biosensor based on poly(l-aspartic acid), nanodiamond particles, carbon nanofiber, and ascorbate oxidase–modified glassy carbon electrode for the determination of l-ascorbic acid. Anal Bioanal Chem 412, 5315–5327 (2020). https://doi.org/10.1007/s00216-020-02747-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02747-w