Abstract
Histamine (HA) is a biogenic amine associated with allergies and food poisoning. It is an important indicator of food freshness and quality. In recent years, a series of medical negligence cases have been reported to be related to the intravenous injection of antibiotics produced via fermentation with fish peptone due to HA contamination. To detect HA efficiently, mouse monoclonal antibody was developed. An enzyme-linked immunosorbent assay (ELISA) and a chemiluminescence enzyme immunoassay (CLEIA) were developed and compared with conventional HPLC analysis. Both immunoassays showed low cross-reactivity, low 50% inhibitive concentration (IC50; 1.2 μg/mL and 1.1 μg/mL), low limits of detection (LODs, IC10; 89.0 ng/mL and 73.4 ng/mL), and appreciable recoveries in spiked foods and drugs (from 73.4 to 131.0% and from 77.0 to 119.0%, espectively), demonstrating that the developed methods are sensitive, specific, fast, and reliable for HA detection in complicated real samples.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biogenic amines (BAs) are nonvolatile, heat-stable bases of low molecular weight commonly present in living organisms possessing essential physiological functions [1]. Moderate levels of BAs are essential for bio-function, but high concentrations of BAs can cause toxicological effects [2]. Histamine (HA, 4-(2-aminoethyl)-1H-imidazole) is the most important BA in relation to allergies and food poisonings. HA levels in processed food products are strongly affected by the quality of raw material, conditions, and handling techniques during processing [3], which was formed by decarboxylation of the essential amino acid l-histidine after bacterial action and has been considered as an important indicator of food freshness and quality [4]. HA poisoning is an acute allergy-like food poisoning with typical symptoms including sweating, nausea, facial flushing, a burning or peppery taste in the mouth, rash, diarrhea, and abdominal cramps, which usually disappear within several hours without medical intervention. More severe symptoms (e.g., blurred vision, swelling of the tongue and throat, and respiratory distress) can occur and require medical treatment with anti-histamines [5].
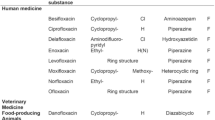
In recent years, HA is also involved in many adverse drug reactions (ADRs) associated with the administration of drugs, which was produced after a fermentation process. After a life-threatening ADR occurred at the beginning of 2017 (a woman died after intravenous administration of an 80 mg/2 mL solution of gentamicin from a batch produced by an Italian manufacturer), the Italian Official Medicines Control Laboratory was asked to ascertain the quantity of HA present in two batches of injectable gentamicin medicinal product (40 mg/mL as free base) and two batches of gentamicin-active pharmaceutical ingredient (API) [6]. When gentamicin is administered intravenously with a maximum allowable dose of 520 mg per day, only 13 ppm of histamine in gentamicin is sufficient to reach a 7-μg hazard threshold [7]. Different countries have regulations on the maximum limit of HA permitted in food (Table 1).
Several analytical methods have been reported to identify and determine HA in foods and biological systems (Table 2). Most of them were usually limited by low specificity and sensitivity or by requiring costly instrumental equipment. Antibody-based immunoassays are well established as rapid methods which have been developed for analysis of antibiotics, pesticides, and other harmful chemicals with high sensitivity, rapidity, low cost, and applicability [12]. However, the production of sensitive and specific antibody against HA is a big challenge due to the simple structure of HA without sufficient epitome to elicit sensitive and specific antibody. Hammar et al. [13] produced anti-HA mAb that shows a high cross-reaction with 1-methylhistamine. Most of antibodies against the derivatives of HA, such as p-benzoquinone-histamine [14] and p-nitrobenzoylated histamine [15], have been generated and used for developing immunoassays for HA via a pre-derivatization. However, all the reported derivatization of HA suffered from some drawbacks: long time, low yield, along with side reactions, and unstable of products.
This study aimed to generate a high-specific anti-HA mAb and develop immunoassays for the quantification of HA without pre-derivatization in drug products and food samples.
Material and methods
Antigen preparation
HA was conjugated to the carrier protein fetal bovine serum albumin (BSA) or ovalbumin (OVA) via N-(3-maleimidobenzoyloxy)succinimide (MBS). HA (3.3 mg) and MBS (5.1 mg) were dissolved in 1 mL carbonate–bicarbonate buffer (CBS, pH = 9.0) and shaken at room temperature for 3 h. BSA/OVA (10 mg) reduced by dithiothreitol (DTT) was added to the mixture and reacted for 3 h at room temperature. Finally, the obtained conjugate was dialyzed against four changes of phosphate-buffered saline (PBS) for 3 days and stored frozen at − 20 °C. The conjugates were confirmed by high-performance gel filtration chromatography (HPGFC).
BALB/c mice immunization
The 6–7-week-old female BALB/c mice were injected subcutaneously with 80 μg of conjugates and equivalent Freund’s complete adjuvant (FCA; Sigma, St. Louis, MO, USA). After the initial immunization, booster injections were given four times at biweekly intervals, using 55 μg of conjugates in 500 μL of Freund’s incomplete adjuvant (FIA; Sigma, St. Louis, MO, USA) per mouse per booster dose. Post-immunization blood was collected from the tail vein. Blood was clot for 30 min and centrifuged at 5000g for 5 min, and the serum was collected. The titers of antiserum were determined using indirect competitive enzyme-linked immunosorbent assay (ic-ELISA).
Cell fusion and preparation of ascites
Spleen cells were collected from the mouse showing the highest antibody titer and fused with myeloma cell SP2/0 in the logarithmic growth phase at a ratio of 8:1. Pre-heated 50% PEG 4000 (1 mL) was slowly added to the mixture of spleen cells and myeloma cell SP2/0 for fusion. DMEM (Solarbio, Beijing, China) containing 20% fetal bovine serum (Sigma, St. Louis, MO, USA) was added into the fused cells, cultured in a 5% CO2 incubator at 37 °C for 16–20 h, then transferred to HAT medium. Under the microscope, the monoclonal cells that have grown in the HAT medium were picked up to a 96-well plate (Thermo, Shanghai, China) containing HT medium [22]. When the hybridoma cells grew to 40% of the pore area, the cell culture supernatant was taken and screened for anti-HA antibody by indirect ELISA. The positive hybridoma cells were subcloned three times by limiting dilution process. Finally, the specific hybridoma cells were expanded for the preparation of ascetic fluid.
Seven days after the injection of pristane (0.6 mL), 8–10-week-old female BALB/c mice were intraperitoneally injected with hybridoma cells (1 mL). When the mice had bulged abdomen, tight skin, and sluggish response, ascites was collected from the abdominal cavity of mice. The obtained ascites was centrifuged at 1500g for 10 min to remove cellular components, oils, fats, and other precipitates. The supernatants were further purified by ion exchange chromatography and gel filtration according to standard protocols [23]. The anti-HA antibody concentration was quantified by Bio-Rad protein assay kit. The isotype of the mAb was identified using IsoStrip mouse monoclonal antibody isotyping kit.
ic-ELISA
The wells of ELISA plates (Corning Inc., Cambridge, MA, USA) were coated with HA-OVA (100 μL/well) in CBS at 4 °C overnight. The plate was washed three times with PBS containing 0.05% Tween 20 (PBST) and dried. Free protein binding sites were blocked with 2% (w/v) BSA in PBS (200 μL per well) at 37 °C for 2 h. After washing three times, HA standards (50 μL) and the diluted antibody (50 μL) in PBS were added to each well and incubated at 37 °C for 40 min. The same washing step was implemented. The plates were incubated with goat anti-mouse HRP (100 μL per well, diluted 1:5000) dissolved in PBS containing 1% BSA at 37 °C for 40 min. The plates were washed again. Subsequently, 100 μL per well of 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma, St. Louis, MO, USA) substrate solution was added to plates, which were then incubated at 37 °C for 10 min. The reaction was terminated by the addition of 2 M H2SO4 (50 μL per well). The absorbance values were measured at 450 nm using a microplate reader (ELx800; BioTek, Vermont, USA).
Cross-reactivity
The cross-reactivity of the anti-HA antibody with analogs was tested by ic-ELISA. Different concentrations (50 ng/mL, 100 ng/mL, 200 ng/mL, 500 ng/mL, 1 μg/mL, 2 μg/mL, 5 μg/mL, and 10 μg/mL in PBS) of tyramine, putrescine, cadaverine, l-tryptophan, phenylethylamine, l-histidine, and tryptamine were analyzed.
Cross-reactivity (CR) % = [IC50 (histamine) / IC50 (competitor)] × 100%
Indirect competitive chemiluminescence enzyme immunoassay
The steps of the indirect competitive chemiluminescence enzyme immunoassay (ic-CLEIA) process were as follows [24]. Microtiter plates were coated with optimized concentrations of HA-OVA in CBS (pH 9.6) and incubated at 4 °C overnight. Then, the plates were blocked with 2% BSA in PBS (200 μL per well) at 37 °C for 2 h. Histamine standards (50 ng/mL, 100 ng/mL, 200 ng/mL, 500 ng/mL, 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, and 20 μg/mL) in PBS (50 μL) and the diluted antibody (50 μL) in PBS were added to each well and incubated at 37 °C for 40 min. After washing three times, the plates were incubated with goat anti-mouse HRP (100 μL per well, 1:5000 dilution) dissolved in PBS containing 1% BSA at 37 °C for 40 min. The mixture was incubated for 40 min. The plates were washed again. Subsequently, 100 μL of chemiluminescent substrate solution (luminal solution; Heliosense Biotechnology, Xiamen, China) was pipetted into each well. The emitted photons were read after 3 min of incubation at room temperature in the dark.
Histamine analysis by HPLC
A calibration curve for HA analysis was constructed as follows: serial concentrations (1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL, 40 μg/mL, 50 μg/mL, 80 μg/mL, and 100 μg/mL) of HA standard solutions in distilled water were prepared and subjected to the derivatization. Sodium hydroxide solution (2 mol/L) and 600 μL of 10 mg/mL dansyl chloride solution were subsequently added to 300 μL standard solution (pH = 9.5) and placed in a 40 °C water bath for 20 min. Ammonia (100 μL) was added to the mixed solution and diluted with acetonitrile (ACN) to 1.5 mL. Finally, the solutions were filtered with a 0.22-μm filter for HPLC analysis. The liquid chromatographic system (Agilent 1260, Santa Clara, CA, USA) for HA was equipped with an LC pump delivering the mobile phase (\( {V}_{\mathrm{ACN}}:{V}_{{\mathrm{H}}_2\mathrm{O}}=75:25 \)) at a flow rate of 1 mL/min and with an automatic injector with a loop of 25 μL. The stationary phase was packed in a stainless steel column (100 × 4.6 mm, id 3.5 μm). The column temperature was maintained at 25 °C. An integrator was connected to the diode array detector (DAD) to record the signals of 254 nm. The results were plotted against the concentration of HA to obtain a calibration curve.
Sample preparation
Food samples (carp, shrimp, soy sauce, and fish ball) and drug samples (gentamicin, amikacin, penicillin injection, fish peptone) were purchased from the local supermarket and pharmacy. Antibiotic fermentation was prepared by our laboratory. For sample preparation, each tissue sample (1 g in 9 mL distilled water) spiked with HA standards at different concentrations (10 mg/kg, 50 mg/kg, and 100 mg/kg) was homogenized for 1 min. The mixture was sonicated for 1 h at 60 °C, and the suspension was centrifuged (Eppendorf, Hamburg, Germany) at 3000g for 10 min at 4 °C. The supernatant was diluted with PBS for the immunoassay analysis immediately. For soy sauce preparation, distilled water (9 mL) and soy sauce (1 mL) were mixed and shaken for 1 min. Subsequently, the mixture was centrifuged at 3000g for 10 min and the supernatant was filtered with a 0.22-μm filter for analysis. Samples for HPLC were derivatized as described before.
Results
Characterization of the artificial antigen
Two peaks (BSA and HA-BSA) were observed after the HPGFC analysis. HA-BSA had characteristic peaks at 7 min. BSA exhibited absorption peaks at 7.5 min. The shift of the retention time proved that the antigen synthesis was successful (Fig. S1, see Electronic Supplementary Material, ESM).
Antibody production
The cell fusion was performed for hybridoma from mice immunized with HA-BSA. The hybridoma supernatants were screened using indirect ELISA. Six positive hybridomas were further screened by ic-ELISA. Two stable hybridoma cell lines showing competitive response against HA were cloned three times by limited dilution. The hybridoma cell line showing the lowest half inhibitory concentration (designated as 2H1) was amplified and cultured for the preparation of anti-HA ascites (Fig. S2, see ESM). The anti-HA mAb was purified from mice ascites using ammonium sulfate precipitation method and protein G column affinity chromatography. The concentration of mAb tested by the Bradford protein assay kit was 2.0 mg/mL. The titer of the mAb was 1:128,000. The isotype of mAb tested by mouse monoclonal antibody isotype kit was IgG1 with kappa light chain.
ic-ELISA
The sensitivity of ELISA was determined under optimal concentrations of HA-OVA and antibody (HA-OVA, 1 μg/mL; antibody dilution, 1: 32,000), which were determined by chessboard. In the representative competitive inhibition curve for HA (Fig. 1), the regression curve equation of the anti-HA mAb was Y = − 0.354X + 1.59 (R2 = 0.987, n = 3), with an IC50 value of 1.2 μg/mL and the limit of detection (IC10 value) of 89.0 ng/mL. The IC50 value demonstrated that the antibody could be used for HA detection with a linear range from 100.0 ng/mL to 10.0 μg/mL.
ic-CLEIA
The sensitivity of CLEIA was determined under optimal concentrations of HA-OVA and antibody (HA-OVA, 1 μg/mL; antibody dilution, 1: 32,000). Representative competitive inhibition curve (Fig. 2) revealed the regression curve equation of Y = − 0.335X + 1.525 (R2 = 0.991, n = 3) with the IC50 value of 1.1 μg/mL and the limit of detection (IC10) value of 73.4 ng/mL. The linear range was 100.0 ng/mL to 10.0 μg/mL.
Cross-reactivity
The anti-HA mAb showed no cross-reactivity with HA analogs (Table S1, see ESM).
HPLC analysis of HA
A reversed-phase HPLC was developed for HA determination. The retention time of HA was 4.605 min. The regression curve equation was Y = 13.767X + 11.549 (R2 = 0.997, n = 3, Fig. 3). The linearity ranged from 1.0 to 100.0 μg/mL. The limit of detection was 1 μg/mL.
Spike recovery
To evaluate the accuracy and precision of the developed test system in matrices, the spiked food samples (carp, shrimp, soy sauce, and fish Ball) and drug samples (gentamicin, amikacin, penicillin injection, fish peptone, and fermentation liquid) containing different concentrations of HA (10 mg/kg, 50 mg/kg, and 100 mg/kg, respectively) were detected using the proposed ic-ELISA and ic-CLEIA, respectively, and both methods showed high recoveries (Table 3) and low coefficients of variation (Table 3).
Comparison of ELISA, CLEIA, and HPLC results for HA in samples
The samples (carp, shrimp, scallop, cooking wine) were taken and stored at 25 °C for 36 h; then, HA content was measured every 6 h by three methods (ELISA, CLEIA, and HPLC). The histamine content in the carp changed less in the first 12 h and increased significantly after 12 h. The difference of histamine in three methods was not significant, and the agreement of the three methods was excellent at the concentrations < 1000 mg/kg (Fig. 4).
Discussion
HA can be produced by heat or bacterial enzymatic decarboxylation of histidine during production, storage, or transportation of protein-rich foods. Foodborne poisoning caused by HA intake has been repeatedly reported around the world (Table 4). The only way to prevent histamine accumulation is to store fishery and food products below 4 °C [25]; an effective way to reduce the level of histamine in fish is to quickly remove the viscera and wash the fish [26].
Special attention is paid to the levels of free histidine in the pharmaceutical industry as fish peptones have been used during many drug productions (fermentation). In the process of gentamicin fermentation, the presence of free histidine may lead to HA formation in certain conditions [27]. The specific case of gentamicin described in this paper shows that all antibiotics produced via fermentation with fish peptones could be contaminated by histamine at toxic levels and thus need to be properly screened (Table 4) [28]. A series of drug regulatory authorities, including the Medicines and Healthcare Products Regulatory Agency (MHRA) [29], Health Canada [30], and the Australian Department of Health [31], have issued alerts that specific batches of the products containing gentamicin have an increased level of HA. Healthcare professionals were reminded to report suspected adverse reactions associated with this product in accordance with the national spontaneous reporting system. As a precautionary measure, Irvine Scientific tested raw material inventory lots of gentamicin sulfate received from the supplier [32]. Since the end of 2015, many ADRs associated with intravenous injection of gentamicin into horses have been reported; the EU reported that ADR was an allergic reaction [33]. It has been reported that ADR was caused by HA, which was higher in certain batches of gentamicin API. Therefore, some antibiotics produced by fermentation and fish protein peptone required in the production process were detected in the samples.
Compared with commercial enzyme immunoassays (MaxSignal enzymatic assay, Neogen Veratox, ELISA, and RIDASCREEN ELISA) [41, 42] and previously reported indirect derivatization methods, the two developed immunoassay methods that did not need to convert HA to its derivative could omit the derivatization steps of sample pre-treatment and save the consumption of organic reagents and analysis time, with lower LOD compared to most of other immunoassays (Table 2). Our work addressed the HA detection issue not only in food but also, more importantly, in drug product, as this is a new issue not yet very openly discussed, but the concerns have been given by European Medicines Agency, Germany Federal Institute for Drugs and Medical Device, Italian National Institute of Health and drug manufactures, and Chinese pharmaceutical company and drug regulator, due to the histamine contamination during the fermentation process of antibiotic (intermediate material) production with reported lethal consequence. Presently, only a Q-TOF LC/MS method has been developed for the identification and quantitation of HA at ppm level in gentamicin API and medicinal products [6]. Our work could be the first attempt in determining HA using immunoassays in drug samples.
Conclusions
The obtained antibody can directly recognize HA without obvious CR with other HA structural analogs. The recoveries from spiked food and drug samples ranged from 73.4 to 131.0%. The LODs were 89.0 ng/mL (ic-ELISA) and 73.4 ng/mL (ic-CLEIA), which showed higher sensitivity than HPLC-DAD. The developed immunoassays were able to detect a trace amount of HA in foods and drugs that were well below the maximum residue limits of different regulations.
References
Gagic M, Jamroz E, Krizkova S, Milosavljevic V, Kopel P, Adam V. Current trends in detection of histamine in food and beverages. J Agr Food Chemi. 2019;67(3):773–83.
Alizadeh N, Kamalabadi M, Mohammadi A. Determination of histamine and tyramine in canned fish samples by headspace solid-phase microextraction based on a nanostructured polypyrrole fiber followed by ion mobility spectrometry. Food Anal Methods. 2017:1–8.
Lin L, Xu ZL, Yang JY, Xiao ZL, Li YJ, Beier RC, et al. Synthesis of novel haptens and development of an enzyme-linked immunosorbent assay for quantification of histamine in foods. J Agr Food Chemi. 2014;62(51):12299–308.
Martuscelli M, Arfelli G, Manetta AC, Suzzi G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013;140(3):590–7.
Lehane L, Olley J. Histamine fish poisoning revisited. Int J Food Microbiol. 2000;58(1):1–37.
Gaudiano MC, Valvo L, Rodomonte AL. A Q-TOF LC/MS method for identification and quantitation of histamine in the antibiotic gentamicin at ppm level: validation and uncertainty evaluation. J Pharm Biomed Anal. 2019;162:158–63.
Guidelines for the dosing and monitoring of gentamicin, vancomycin and teicoplanin 2010. https://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/Dosing_of_Gentamicin_Vancomycin_and_Teicoplanin.pdf. Accessed March 2010.
Hörman A. Regulation (EC) no 2073/2005 on microbiological criteria for foodstuffs 2008. Available from: http://www.sice.oas.org/TPD/CACM_EU/Negotiations/IIround_microbiological_e.pdf. Accessed 26 February 2008.
GB 5009.208-2016: determination of biogenic amines in food 2016. http://www.doc88.com/p-3387486346572.html. Accessed 23 June 2017.
Fish and fisheries products hazards and controls guidance 4th Ed 2019. https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM251970.pdf. Accessed August 2019.
FAO. Public health risks of histamine and other biogenic amines from fish and fishery products 2013. http://www.fao.org/fileadmin/user_upload/agns/pdf/Histamine/Histamine_AdHocfinal.pdf. Accessed 23 July 2012.
Ricardo Jorge DO. Heterogeneous and homogeneous immunoassays for drug analysis. Bioanalysis. 2014;6(21):2877–96.
Hammar E, Berglund A, Hedin A, Norrman A, Rustas K, Ytterström U, et al. An immunoassay for histamine based on monoclonal antibodies. J Immunol Methods. 1990;128(1):51–8.
Guesdon JL, Chevrier D, Mazié JC, David B, Avrameas S. Monoclonal anti-histamine antibody. Preparation, characterization and application to enzyme immunoassay of histamine. J Immunol Methods. 1986;87(1):69–78.
Luo L, Xu ZL, Yang JY, Xiao ZL, Wang H. A sensitivity-enhanced heterologous immunochromatographic assay based on monoclonal antibody for the rapid detection of histamine in saury samples. RSC Adv. 2015;5(96):78833–40.
Sandra P, Jordi B, Esteve F. Amperometric biosensor for the determination of histamine in fish samples. Food Chem. 2013;141(4):4066–72.
Patange SB, Mukundan MK, Kumar KA. A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control. 2005;16(5):465–72.
CHIN KDH, Koehler PE. Identification and estimation of histamine, tryptamine, phenethylamine and tyramine in soy sauce by thin-layer chromatography of dansyl derivatives. J Food Sci. 2010;48(6):1826–8.
Altieri I, Semeraro A, Scalise F, Calderari I, Stacchini P. European official control of food: determination of histamine in fish products by a HPLC–UV-DAD method. Food Chem. 2016;211:694–9.
Poli C, Laurichesse M, Rostan O, Rossille D, Jeannin P, Drouet M, et al. Comparison of two enzymatic immunoassays, high resolution mass spectrometry method and radioimmunoassay for the quantification of human plasma histamine. J Pharm Biomed Anal. 2016;118:307–14.
Manz G, Booltink E. Validation study of a HistaSure™ ELISA (Fast Track) for the determination of histamine in fish samples. J AOAC Int. 2014;97(6):1601–14.
Yuan B, C-X A, Yuan L, Gao W, J-P H, Chen J, et al. Preparation of monoclonal antibody of anti-feline calicivirus and establishment of double-antibody sandwich enzyme-linked immunosorbent assay detecting method. Genet Mol Res. 2014;13(3):7388–97.
Purification of immunoglobulins and preparation of Fab fragments. Laboratory Techniques in Biochemistry & Molecular Biology. 1985:95–121.
Kim JK, Adam A, Loo JCK, Ong H. A chemiluminescence enzyme immunoassay (CLEIA) for the determination of medroxyprogesterone acetate in human serum. J Pharm Biomed Anal. 1995;13(7):885–91.
Vagelis E, Brett MM, Chrissanthy P, Stathis F, Tom N. Changes in histamine and microbiological analyses in fresh and frozen tuna muscle during temperature abuse. Food Addit Contam. 2007;24(8):820–32.
Kim SH, Price RJ, Morrissey MT, Field KG, Wei CI, An H. Histamine production by Morganella morganii in mackerel, albacore, Mahi-mahi, and salmon at various storage temperatures. J Food Sci. 2010;67(4):1522–8.
Rapid implementation of the monograph products of fermentation (1468) 2018. https://www.edqm.eu/sites/default/files/supplementcomments96_e.pdf. Accessed June 2018.
Henao CMG, Morales CIH, Villa RC, Henao AMC. Gentamicin induced anaphylaxis, a case report. World Allergy Organ J. 2015;8(S1):A269.
Gentamicin: potential for histamine-related adverse drug reactions with some batches Medicines and Healthcare products Regulatory Agency. https://www.gov.uk/drug-safety-update/gentamicin-potential-for-histamine-related-adverse-drug-reactions-with-some-batches. Accessed 24 November 2017.
Canada Health Gentamicin injection USP (10 mg/mL and 40 mg/mL) – potential for adverse reactions with 12 lots contaminated with histamine 2017. http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2017/63768a-eng.php. Accessed 3 July 2017.
Health ADo. Recall - potential for allergic reactions due to higher than expected amounts of histamine. https://www.tga.gov.au/alert/gentamicin-injection-bp-80-mg-2-ml-steriluer. Accessed 6 October 2017.
Technology AR. Information on gentamicin-Histamin products 2018. http://www.irvinesci.com/news/information-on-gentamicin-histamin-products. Accessed 29 January 2018.
Committee for Medicinal Products for Veterinary Use (CVMP) meeting of 17–19 April 2018 2018. Available from: https://www.ema.europa.eu/en/news/committee-medicinal-products-veterinary-use-cvmp-meeting-17-19-april-2018. Accessed 19 April 2018.
Becker K, Southwick K, Reardon J, Berg R, Maccormack JN. Histamine poisoning associated with eating tuna burgers. JAMA. 2001;285(10):1327–30.
Kan K, Ushiyama H, Shindo T, Uehara S, Yasuda K. Outbreak of histamine poisoning due to ingestion of fish, “Abura-sokomutsu”. Lepidocybium flavobrunneum. J Food Hyg Soc Jpn. 2000;41(2):116–21.
Macan J, Vucemilović A, Turk R, Medugorac B, Milković-Kraus S, Gomzi M, et al. Occupational histamine poisoning by fish flour: a case report. Occup Med. 2000;50(1):22.
Tursi A, Modeo ME, Cascella AM, Cuccorese G, Spinazzola AM, Miglietta A. Scombroid syndrome with severe and prolonged cardiovascular involvement. Recenti Prog Med. 2001;92(9):537.
Hall M. Something fishy: six patients with an unusual cause of food poisoning! Emerg Med (Fremantle). 2010;15(3):293–5.
Tsai YH, Hsieh HS, Chen HC, Cheng SH, Chai TJ, Hwang DF. Histamine level and species identification of billfish meats implicated in two food-borne poisonings. Food Chem. 2007;104(4):1366–71.
Histamine poisoning caused by tuna in Luxembourg. 2018. https://www.foodnavigator.com/Article/2018/05/28/Tuna-linked-to-foodborne-outbreak-inLuxembourg. Accessed 28 May 2018.
Hungerford J, Wu WH. Comparison study of three rapid test kits for histamine in fish: BiooScientific MaxSignal enzymatic assay, Neogen Veratox ELISA, and the Neogen reveal histamine screening test. Food Control. 2012;25(2):448–57.
Rahimi E, Nayebpour F, Alian F. Determination of histamine in canned tuna fish using ELISA method. AEJTS. 2012;4(2):64–6.
Funding
This work is financially supported by the National Natural Science Foundation of China (Nos. 31572556 and 31873006); the Key Program for International S&T Cooperation Project of Shaanxi Province (No. 2017KW-ZD-10); the Ministry of Education and Science of the Russian Federation (No. RFMEFI60417X0198); the FCT I.P, the Ministério da Ciência, Tecnologia e Ensino Superior (MCTES); and the European Regional Development Fund (ERDF) (No. POCI-01-0145-FEDER-007569).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All procedures performed in this study involving mice were in accordance with the experimental animal regulation of China and according to the measures for the administration of experimental animal in Shaanxi Province concerning protection of animals used for scientific purposes. All experimental animal protocols were reviewed and approved by the Ethics Committee of Shaanxi University of Technology for the use of laboratory animals (No. 2019-010).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 92.4 kb)
Rights and permissions
About this article
Cite this article
Xu, L., Zhou, J., Eremin, S. et al. Development of ELISA and chemiluminescence enzyme immunoassay for quantification of histamine in drug products and food samples. Anal Bioanal Chem 412, 4739–4747 (2020). https://doi.org/10.1007/s00216-020-02730-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02730-5