Abstract
The quality of the Dniester River Basin has been seriously impacted by the chemicals released by agriculture, industry, and wastewater discharges. To assess its current chemical pollution status, a transboundary monitoring campaign was conducted in May 2019. Thirteen surface water, 13 sediment, and three biota samples were collected and analyzed using generic sample preparation methods for the determination of organic substances by liquid chromatography high-resolution mass spectrometry (LC-HRMS) and metals by inductively coupled plasma mass spectrometry (ICP-MS). Wide-scope target and suspect screening resulted in detection of Water Framework Directive (WFD) priority substances and emerging contaminants, whereas the raw data were stored in NORMAN Digital Sample Freezing Platform (DSFP) for future retrospective screening. Furthermore, risk assessment was performed to prioritize detected substances and propose a draft list of river basin–specific pollutants. All studied metals (As, Hg, Zn, Cu, Cr, Cd, Pb, Ni) were detected in the surface water and sediments. In total, 139 organic contaminants belonging to various chemical classes (pesticides, pharmaceuticals, drugs of abuse, stimulants, sweeteners, industrial chemicals, and their transformation products) were detected. The highest cumulative concentration of contaminants was observed in surface water from the Byk River, a tributary of the Dniester (Moldova). Concentrations of WFD priority substances diuron and mercury and EU Watch List neonicotinoid compounds imidacloprid and thiamethoxam exceeded their environmental quality standards (EQS), whereas concentrations of 23 emerging substances exceeded their predicted no-effect concentration (PNEC) at minimum one site. Emerging contaminants telmisartan, metolachlor, terbuthylazine, and 4-acetamidoantipyrine were prioritized as potential river basin–specific pollutants.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Dniester River is the second longest river in Ukraine and the main water artery of the Republic of Moldova with total length of 1380 km. It rises on the north side of the Carpathian Mountains in Ukraine close to the border with Poland, runs through Ukraine and then through Moldova (from which it separates the unrecognized breakaway territory of Transnistria), and finally discharges into the Black Sea south-west of Odessa (Ukraine). The total population of the Dniester River Basin is nearly eight million people. Many urban centers lie in the main valley and its tributaries. Surface water of the Dniester is the main source of drinking water for Moldova and an important source of drinking water for a significant part of Ukraine, including 3.5 million people living outside of the basin area [1].
Careful management of the Dniester River Basin resources is needed, including control over chemical pollution. Despite the importance of the Dniester River for the inhabitants, it is facing serious environmental challenges as a result of various anthropogenic activities, deteriorating the water quality and posing threats for the ecosystem and human health. The major pollution sources of the Dniester are intensive agriculture and livestock farming, mining, oil-refining, chemical, woodworking, and food industry, and discharges from municipal wastewater treatment plants [1, 2]. The environmental issues of the Dniester are transboundary, since the river flows through two countries before it discharges into the Black Sea. Another aggravating factor for the chemical pollution status of the Dniester River Basin is the frozen conflict in Transnistria, which makes it difficult for Moldovan and Transnistrian authorities to cooperate at the improvement of existing inefficient infrastructure for wastewater treatment [1].
Comprehensive monitoring of chemical contaminants in combination with risk assessment and identification of pollution sources is a prerequisite for the protection and restoration of the adversely impacted aquatic environment. Ukraine and Moldova have agreed on the joint management and protection of cross-border waters [3], in addition to their national requirements regarding water quality and management [4, 5]. This excludes the Transnistrean Region, which falls outside the laws and policies of Moldova in the sphere of water management [6]. Nevertheless, the two countries aim to harmonize their national water laws with the Water Framework Directive (WFD) [7], as they also share other transboundary river basins and seas with the EU (e.g., Danube River and its tributaries; Black Sea). Since 2017, Ukraine is an EU-associated country [6, 8]. The EU EQS Directive establishes a list of 45 priority substances that must be monitored by Member States [9]. Additional compounds are included in the EU Watch List and their Europe-wide monitoring is required so that sufficient information is collected about their occurrence and risk [10].
In addition to the regulated substances, the so-called contaminants of emerging concern (CECs) might pose a threat to water quality. These substances are currently not included in the routine monitoring programs at EU level, but may be candidates for future regulation depending on research on their (eco)toxicity, potential health effects, and public perception and on monitoring data regarding their occurrence in the various environmental compartments [11]. These contaminants can be classified in different chemical classes (e.g., pharmaceuticals, pesticides, industrial chemicals) including their biotic [12] and abiotic transformation products (TPs) [13], which may be more persistent, more bioaccumulative, and more toxic than the parent compounds.
The Dniester River is one of the less studied rivers in Europe. Basic physico-chemical and microbiological parameters have been reported in the literature [1, 2], supplemented by a limited number of studies focusing on a narrow number of compounds in a single chemical class (e.g., metals [14,15,16,17], aliphatic and polycyclic hydrocarbons [18], polychlorinated biphenyls [15], chlorine, and phosphorous-containing pesticides [14, 15]).
The objectives of this study were to (1) apply state-of-the-art analytical methods and data-processing tools to get a broad view on both inorganic and organic chemical pollution in surface water, sediment, and biota samples from the Dniester River and its tributaries; (2) screen the samples for wide range of suspect contaminants and archive the LC-HRMS chromatograms for future retrospective analysis; (3) evaluate spatial distribution of the detected chemicals, explain the variations in concentration levels, and detect potential pollution sources in the Dniester River Basin; and (4) carry out ecotoxicological risk assessment and prioritize detected substances to establish a list of potential river basin–specific pollutants.
Materials and methods
Sampling
Thirteen surface water, 13 river sediment, and three biota samples were collected from different sampling sites along the Dniester River (Fig. 1). The sampling was carried out in the period from 25 to 28 May 2019. The sampling sites were selected with the aim to cover the entire basin, including inflowing tributaries and the estuary, and include all kinds of anthropogenic pressures (e.g., agricultural sites, industrial areas, wastewater outlets, reservoirs). The sampling period covered the spring season, when there was an increased use of herbicides in agriculture; however, only a slight rainfall and surface runoff were recorded prior to and during the sampling. Detailed description of the sampling sites is provided in section S1 of the Electronic Supplementary Material (ESM).
Sample preparation
Surface water and sediment samples were screened for presence of five (As, Hg, Zn, Cu, Cr) and eight (As, Hg, Zn, Cu, Cr, Cd, Pb, Ni) pre-selected metals, respectively. ICP-MS was used for their determination after acidification (for surface water), microwave digestion and dilution (for sediments). Internal standards were added on-line during analysis. In order to minimize the risk of metal contamination, manipulations were performed inside of a clean room.
All surface water samples and a representative number of sediments and biota samples collected from upper, middle, and lower parts of the basin were extracted following generic sample preparation protocols and then analyzed by LC-HRMS. The sample preparation of 2 L surface water samples included extraction, clean-up, and pre-concentration of the analytes by large-volume solid-phase extraction (SPEDEX HORIZON). Oasis HLB disks were used for the sorption of a wide polarity spectrum of analytes, which were then eluted by methanol. The procedure resulted in 4000-fold concentration of analytes in the final extracts. The sample preparation of sediment samples consisted of an ultrasonic extraction with methanol and Milli-Q water acidified with EDTA. The biota sample preparation comprised of an ultrasonic extraction of the analytes with aqueous solution (0.1% EDTA, 0.1% formic acid)/methanol/acetonitrile, followed by lipid and protein precipitation at low temperature and a defatting process with hexane. All sample preparation methods are described in detail in section S2 of the ESM.
Instrumentation
LC-ESI-QTOF-MS analyses were conducted using an ultra-high-performance liquid chromatographic (UHPLC) system with a HPG-3400 pump (Dionex Ultimate 3000 RSLC, Thermo Fischer Scientific, Dreieich, Germany) coupled to a quadrupole–time-of-flight (QTOF) mass spectrometer (Maxis Impact, Bruker Daltonics, Bremen, Germany).
Chromatographic separation was performed using an Acclaim RSLC C18 column (2.1 × 100 mm, 2.2 μm) from Thermo Fischer Scientific (Dreieich, Germany), preceded by a guard column of the same packaging material, thermostated at 30 °C. For positive ionization mode (PI), the mobile phases consisted of water/methanol 90/10 (solvent A) and methanol (solvent B), both containing 5 mM ammonium formate and 0.01% formic acid. For negative ionization mode (NI), the mobile phases consisted of water/methanol 90/10 (solvent A) and methanol (solvent B), both acidified with 5 mM ammonium acetate. The adopted gradient elution program was the same for both ionization modes, starting with 1% B with a flow rate of 0.2 mL/min for 1 min, and it increased to 39% in 2 min (flow rate 0.2 mL/min), and then to 99.9% (flow rate 0.4 mL/min) in the following 11 min. Then, it was kept constant for 2 min (flow rate 0.48 mL/min); then, the initial conditions were restored within 0.1 min, kept for 3 min; and then, the flow rate decreased to 0.2 mL/min for the last minute. The injection volume was 5 μL.
The QTOF-MS system was equipped with an electrospray ionization interface (ESI), operating in positive and negative mode, with the following operation parameters: capillary voltage 2500 V (PI) and 3500 V (NI); end plate offset 500 V; nebulizer pressure 2 bar; drying gas 8 L/min; and gas temperature 200 °C. The QTOF-MS system was operated in data-independent acquisition mode (broadband collision-induced dissociation (bbCID)), as well as in data-dependent acquisition mode (Auto MS/MS), and recorded spectra over the range of m/z 50–1000, with a scan rate of 2 Hz. A QTOF-MS external calibration was performed daily with the manufacturer’s solution. The instrument provided a typical resolving power (FWHM) between 36,000 and 40,000 at m/z 226.1593, 430.9137, and 702.8636.
Metal determinations were performed by inductively coupled plasma mass spectrometry (ICP-MS) by a Thermo Scientific ICAP Qc (Waltham, MA, USA). Measurements were carried out in a single collision cell mode, with kinetic energy discrimination (KED) using pure He.
LC-HRMS screening and quantification
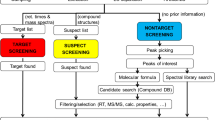
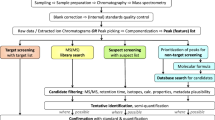
Wide-scope target screening was performed with the use of an in-house developed database of 2273 contaminants (list S21 UATHTARGETS in Suspect List Exchange https://www.norman-network.com/nds/SLE/) and software TASQ Client 2.1 and DataAnalysis 5.1 (Bruker Daltonics, Bremen, Germany). Detection was based on specific screening parameters (mass accuracy < 2 mDa, retention time shift ± 0.2 min, isotopic fitting < 100 mSigma (only for confirmation of positive findings)), whereas the presence of fragment ions confirmed the analytes.
The screening detection limit (SDL) was reported for the contaminants that were screened by the wide-scope LC-QTOF-MS target screening method. The SDL was established as the lowest concentration level tested for which a compound is detected in all spiked samples, at the expected retention time and with a specific mass error of the precursor ion, not exceeding the pre-set threshold values [19]. Standard solutions, spiked samples, and matrix-matched samples (samples spiked with analytes at the end of the sample preparation) at different concentration levels for a representative set of analytes were used for method validation. The SDL was not compound-specific, but a generic reporting value was derived after method validation.
A thorough compound-specific validation was performed for quantification purposes for the compounds detected with the screening method. Selected samples of each matrix were spiked with the detected compounds and structure-related isotope labeled compounds (IS), processed using the above methods and analyzed together with the unspiked samples. The preferred samples used for spiking experiments were those in which only a few compounds were detected. The samples were spiked at three different concentration levels, considering the sensitivity of the method for each analyte in each matrix. Compound-specific LOD and LOQ values were determined as the concentration of a compound in the spiked sample that corresponds to signal-to-noise ratio that equals to 3.3 and 10, respectively. Meanwhile, procedural blank samples were used for the subtraction of eventual laboratory-introduced contamination.
Suspect screening was performed for 6152 environmentally relevant pollutants by archiving all raw chromatograms into NORMAN Digital Sample Freezing Platform (DSFP) (www.norman-data.eu), a novel tool developed for revealing the presence of suspects and identification of unknown compounds [20]. The calibrant substance, producing ion clusters over all m/z, was used to recalibrate the whole chromatogram using HPC fitting algorithm, which is embedded in DataAnalysis 5.1 (Bruker Daltonics, Bremen, Germany). This calibration method ensured mass accuracy below 2 mDa during the whole chromatographic run for m/z = 50–1000. For exporting files in mzML format, CompassXport 3.0.9.2 (Bruker Daltonics, Bremen, Germany) was used. Chromatograms acquired under bbCID were separated in low and high collision energy layer chromatograms. All mzML files and their meta-data (instrumental, sample meta-data, matrix-specific meta-data, and retention time of Retention Time Index (RTI) calibrant substances) were uploaded to DSFP. DSFP utilizes an embedded integrated standard operating procedure (SOP) to process the mzML files and all meta-data for generation of standardized Excel-based Data Collection Templates (DCTs). This data reduction technique resulted in an automatic generation of DCTs, which include condensed relevant information from bulky LC-HRMS files. The data reduction produces acceptable number of false negative results (less than 5%) for the presented suspect screening method. For more details, one is addressed to the previous study [20].
Ecotoxicological risk assessment
The identified substances were evaluated for their probable adverse effects on the environment. Concentration levels of the determined WFD priority substances were checked for their compliance with the legacy EQS values. Assessment of a potential risk of an identified emerging contaminant at a polluted site was based on comparing the measured concentration with its PNEC value. PNEC is regarded as the concentration below which unacceptable effects on living organisms will most likely not occur. PNEC values are usually determined on the basis of results from single species laboratory tests or, in a few cases, established effect and/or no-effect concentrations from model ecosystem tests, taking into account adequate assessment factors. The PNEC can be derived using an assessment factor approach or, when sufficient data is available, using the statistical extrapolation methods [21]. Therefore, in this study, experimental data on PNEC were preferred, and mostly chronic toxicity data were preferred over acute toxicity data. When neither PNEC chronic nor PNEC acute from experimental tests were available, P-PNEC (Provisional-PNEC) was used, which was derived by QSAR (Quantitative Structure-Activity Relationship) toxicity prediction models [22].
Dniester River Basin–specific pollutants
CECs whose concentration levels exceeded ecotoxicity threshold values (lowest PNECs) were prioritized for future monitoring campaigns using adapted NORMAN Prioritisation Framework [23]. The full-scale prioritization could not be applied due to only a limited dataset available from a single monitoring campaign. The adapted prioritization methodology presented recently by Alygizakis et al. [24] was applied using the following three parameters: frequency of appearance (FoA), which is the number of sites at which a contaminant was detected divided by the total number of investigated sites; spatial frequency of exceedance of the lowest PNEC (FoE) calculated as n/N where “n” is the number of sites with maximum environmental concentration per site (MECsite)/lowest PNEC ratios above 1 and “N” is the total number of sites with analytical measurements for the respective compound; extent of exceedance of the lowest PNEC (EoE) where 95th percentile of MEC (MEC95) is divided by the lowest PNEC of the compound. Please, note that at least 20 sites with analysis above the LOQ are required to calculate the MEC95 and, in our simplified approach, only one value per site was used. EoE is calculated for sites with substances with MEC95 exceeding the lowest PNEC as the sum of the fractions MEC95 to the PNEC normalized to 1. Τhe normalization comes from division by the compound with the maximum ratio. Each of the three parameters (FoA, FoE, EoE) is a number between 0 and 1, and their sum total can vary between 0 and 3. In this study, we have set arbitrarily a threshold of “1” to qualify a substance as river basin–specific pollutant [24].
Results and discussion
Occurrence and spatial distribution of metals
All five investigated metals were detected in the surface water samples and the results are provided in Table S3A (see ESM). Concentration of the WFD priority substance mercury exceeded its maximum allowable concentration MAC-EQS (0.07 μg/L) in the sample collected from the headwaters (site 1). The surface water sample collected at the mouth of the Byk River discharging into the Dniester River in Moldova (site 10) was highly dominated by the studied metals, with their total cumulative concentration of 126 μg/L. The industry (electronic goods, building materials, machinery, plastics, rubber, textiles) and agricultural activities around the capital of Moldova Chisinau, which is located on the banks of the Byk River [25] can be considered potential sources of anthropogenic inputs of metals into the aquatic environment.
Metals were also investigated in the sediment samples in order to understand their distribution, transportation, and availability with consideration of metals’ partition between aquatic and sediment phase [26]. All investigated metals were determined in sediments (ESM Table S3B). The highest cumulative concentration (313 mg/kg dry weight (d.w.)) was observed in sediments collected from the Seret River (site 5), a tributary of the Dniester in Ukraine. The second highest cumulative concentration (302 mg/kg d.w.) was observed upstream of the Dniester reservoir (Zalishchyky, Ukraine; site 4). Both sites are located in the Ternopil Oblast, which is a region with significant industrial and agricultural activity such as machine-building, metal-working, construction materials, light, food production, and woodworks. All of these may be among the sources introducing metals into the Dniester River.
Occurrence and spatial distribution of organic contaminants
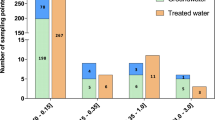
Altogether, 109 targeted organic compounds were detected in at least one of the samples. The overlaid extracted ion chromatograms in a single surface water sample (site 10) are presented in Fig. S1 (ESM). The presence of the compounds was confirmed by the use of reference standards and quantified by standard addition. The SDL for the non-detected compounds was 1.25 ng/L for surface water, 5 μg/kg dry weight (d.w.) for sediments and 5 μg/kg wet weight (w.w.) for biota. Table 1 and ESM Tables S4A and S4B present concentration levels of the detected organic contaminants in surface water, river sediment, and biota samples, respectively, whereas the column chart in Fig. 2 depicts the distribution of the studied classes of contaminants in each surface water sample, as well as the total number of contaminants detected in each sample. When the detected compound was below LOQ, LOQ/2 was used for the statistical treatment of the results [27]. All classes of the detected contaminants are presented in detail in the following sub-chapters.
Pesticides and pesticides’ TPs
In total, 44 pesticides and pesticides’ TPs were detected in the surface water samples, three of which were also detected in one sediment sample. However, only one TP of a pesticide was detected in biota samples. The detected pesticides were mainly herbicides (24 compounds, some of them also used as algicides), fungicides (10 compounds), insecticides (9 compounds, some of them also used as acaricides, molluscicides, nematicides), and insect repellents (2 compounds). The most frequently detected pesticides were metolachlor and DEET (diethyltoluamide), present also in the headwater sample (site 1). The spatial distribution of pesticides proved to be significantly higher in the central part of the basin (concentration range 0.2–14.3 μg/L) comparing with the upper and lower basins (concentration range 0.01–0.1 μg/L). As expected, concentrations of pesticides and their TPs reached their maxima in the vicinity of agricultural sampling stations.
The highest total cumulative concentration was observed at site 6 (14.3 μg/L) at the city Mohyliv-Podilskyi of the Vinnytsia Oblast in Ukraine. The Vinnytsia Oblast has the highest proportion of cultivated agricultural land within the whole Dniester River Basin. The conversion of forest land to agricultural land has led to increased levels of soil contamination and consequent deterioration of surface water quality [1]. Here, it should be considered that in total, ca. 100,000 tons of pesticides is used in Ukraine annually, and 25% of its total pesticides’ market is estimated to be illicit pesticides, both imported and locally produced counterfeit products [28]. The other locations with high total cumulative concentration of pesticides were sites 9 and 10 (4.2 μg/L and 3.0 μg/L, respectively), which are at the mouths of the Reut and Byk rivers discharging into the Dniester River in Moldova. Within the Moldovan part of the Dniester River Basin, 76% of the land is used for agricultural production [1]. The Reut River is located in an area dominated by agriculture [29], and the Byk River is influenced, i.a., by agricultural runoff [30].
Herbicides exhibited the highest total cumulative concentration levels in surface water samples. They were also frequently detected, and proved to be the most commonly used plant protection products in the studied region. Prior to the sampling campaign, the use of herbicides was notably elevated. Beyond the above-mentioned chloroacetanilide herbicide metolachlor, high FoA was observed also for the chlorotriazines terbuthylazine and the TP 2-hydroxypropazine, as well as the herbicides bentazone and dinoterb. The other detected chloroacetanilide and chlorotriazine herbicides, such as acetochlor, metazachlor, atrazine, and simazine, were detected with low FoA. From among other herbicides and their TPs, lenacil, metamitron, metribuzin, nicosulfuron, prometryn, and methyldesphenylchloridazone were detected in one or two water samples at concentrations below 50 ng/L. Dimethenamid and diuron were also detected in only a few samples; however, their maximum concentration exceeded 1000 ng/L. This is especially worrying for the WFD priority substance diuron with annual average EQS. 200 ng/L.
Insecticides were detected with low FoA in surface water samples, but their total cumulative concentration accounted for 33% of the total concentration of pesticides in the river, mainly because of the high concentration levels of the carbamate carbaryl reaching its maximum concentration 5.6 μg/L at site 6 (Mohyliv-Podilskyi). The neonicotinoid insecticides imidacloprid, thiamethoxam, acetamiprid, and thiacloprid were also detected and their occurrence is of importance due to their inclusion in the EU Watch List, relatively low PNECs, and thus high potential of adverse impact on the environment.
Fungicides were detected at lower concentrations compared with herbicides and insecticides. Carbendazim was the substance that stood out of this class because of its high FoA in surface water samples (69%) and relatively high concentration (maximum concentration 755 ng/L at site 10).
The herbicide prometryn and the fungicides azoxystrobin and carbendazim were detected in both surface water and sediment samples of the Dniester River in Zalishchyky, upstream of the Dniester reservoir, in the agricultural Ternopil Oblast in Ukraine (site 4). Their total cumulative concentration in the river sediments was 157 μg/kg (d.w). Moreover, 3-hydroxycarbofuran, which is a TP of carbofuran—carbamate insecticide/acaricide/nematicide, was identified at similar concentration levels in all biota samples.
The findings of our study highlight the importance of screening TPs in river basin and national monitoring campaigns. Many TPs were detected in surface water and biota samples. In selected cases (2-hydroxypropazine, methyldesphenylchloridazone, 3-hydroxycarbofuran), TPs were successfully identified while their parent compounds remained undetected, whereas in case of the photolysis TP of metamitron (desaminometamitron) [31], it was detected at higher concentration and more frequently than the parent compound.
However, in most of the cases, the parent compounds and their TPs were both detected in the samples, especially at sites where the parent pesticide reached its highest concentration. Such was the case of metolachlor, known to be degraded to two major TPs (metolachlor-ESA, metolachlor morpholinone) [32], which were detected along with the parent pesticide in surface water samples. Similar cases were the major TPs of atrazine (2-hydroxyatrazine) [33], simazine (2-hydroxysimazine) [34], terbuthylazine (desethylterbuthylazine and desethyl-2-hydroxyterbuthylazine) [35], azoxystrobin (azoxystrobin acid) [36], and carbendazim (2-aminobenzimidazole) [37]. In all these cases, TPs were detected at lower concentration and in locations where parent pesticides were detected at relatively high concentration.
Pharmaceuticals and pharmaceuticals’ TPs
In total, 40 pharmaceuticals and their TPs were determined in surface water samples. Three pharmaceuticals were found in river sediments (two of which were also detected in surface water), and one was present in biota.
The highest total cumulative concentration of pharmaceuticals and their TPs (26.1 μg/L, 35 pharmaceuticals) was observed in surface water collected from the mouth of the Byk River (site 10). The second highest concentration of pharmaceuticals in surface water (3.6 μg/L, 19 pharmaceuticals) was observed at the mouth of the Tysmenytsia River in Lviv Oblast in Ukraine (site 2). In the rest of the surface water samples, concentration levels of pharmaceuticals were one or two orders of magnitude lower.
Both sites (10 and 2) are heavily impacted by municipal and industrial wastewater discharges [1]. Site 10 receives wastewater from Chisinau and wastewater from pharmaceutical industry. The concentration levels are even comparable with the findings in a European wastewater campaign conducted in the Danube River Basin [24]. Site 10 is obviously impacted by wastewater discharges, which is also supported by the presence of 17 pharmaceuticals, which were detected exclusively at this location at concentrations ranging from 0.02 to 1.5 μg/L. Highest FoA was observed for the TPs 4-acetamidoantipyrine (85%) and 4-formylaminoantipyrine (77%), and the pharmaceutical salicylic acid (100%), all of them reaching their maximum concentration at site 10. It is worth highlighting that the metabolites of metamizole (4-acetamidoantipyrine and 4-formylaminoantipyrine) are known to be frequently detected in the aquatic environment [38], and in our study were detected with high FoA, while the parent compound remained undetected as expected, due to its rapid metabolism in human body [39]. Moreover, the TP of carbamazepine (10,11-dihydro-10,11-dihydroxycarbamazepine) was detected at higher concentration levels than the parent pharmaceutical—a fact that has also been previously reported to occur in wastewater samples [40].
A smaller number of substances were detected in the river sediments. Carbamazepine and salicylamide were determined in both surface water and sediment samples, whereas mabuterol was detected only in the river sediments from sites 4 and 11 (Dniester River sites in Ukraine and Moldova, respectively), and at low concentration levels. Finally, tolycaine was detected in the biota sample collected from site 9 (Reut River, Moldova) at 70.0 μg/kg (w.w.).
Drugs of abuse
Four drugs of abuse were detected in surface water samples. Norephedrine (phenylpropanolamine) was observed with high FoA (62%) and its concentration varied from < 0.66 to 2.6 μg/L (maximum concentration at the wastewater-impacted site 10). The barbiturates barbital (site 10), phenobarbital (sites 2, 10), and secobarbital (site 10) were detected at concentration above LOD but below LOQ. Alike pharmaceuticals, drugs of abuse were dominant in the Byk River in Moldova (site 10).
Stimulants and sweeteners
Four stimulants and stimulants’ TPs (cotinine, hydroxycotinine, caffeine, and theobromine) were detected in surface water. No stimulant was observed in the headwaters (site 1), indicating that headwaters were relatively free of anthropogenic pressures, since cotinine and caffeine are used as marker substances. Cotinine, the predominant metabolite of the stimulant nicotine, was detected with FoA 77% and at maximum concentration in the Tysmenytsia River (site 2), where another minor metabolite, hydroxycotinine, was also detected. Caffeine was detected with the same FoA as cotinine (77%) with concentration ranging from < 0.07 to 1.2 μg/L (maximum at site 2). Theobromine was detected in surface water from the two sites, in which also caffeine was observed at the highest concentration.
Two sweeteners, acesulfame and sucralose, were detected in surface water from the Byk River (site 10), and one sweetener, saccharine, in water from the Tysmenytsia River (site 2).
Industrial chemicals
Eleven industrial chemicals were detected in surface water samples. They were determined at very high cumulative concentration (2.3 μg/L) in water collected from the Byk River (site 10). In this site, a presence of seven industrial chemicals was revealed: benzoic acid, 2-aminobenzothiazole, 2-OH-benzothiazole, 1-H-benzotriazole (1-H-BTR), tolyltriazole (mix of isomers 4- and 5-methylbenzotriazole), 2,4-dinitrophenol (DNP), and triethyl phosphate. This result can be interpreted by the fact that 75% of all Moldovan industries are located along the Dniester River, and only 60% of these have water treatment systems [14]. Part of the pollution (e.g., benzotriazole compounds) can be contributed from municipal sources.
Connection of the Dniester River Basin with the Black Sea
The findings from the Dniester River Basin well correspond with the findings from the Joint Black Sea Surveys (JBSS) that took place in 2016 and 2017 [41]. The Dniester River flows into the Black Sea and carries CECs and their TPs. Emerging substances enter the marine environment, where they are diluted, sorbed in the marine particulate matter, further degraded or uptaken by the marine organisms.
In the context of the presented study, it was evaluated whether the detected contaminants in the Dniester River were also detected in the Black Sea close to the estuary. Many contaminants belonging to various classes of emerging substances were also detected in the marine ecosystem. Some of these classes include: pesticides (atrazine, bentazone, carbendazim, DEET, dinoterb, metolachlor, prometryn, simazine) and their TPs (2-hydroxyatrazine, methyldesphenylchloridazone, metolachlor-ESA, desethylterbuthylazine), pharmaceuticals (carbamazepine, ketoprofen, lamotrigine, salicylamide, sulfamethoxazole, telmisartan, valsartan) and their TPs (4-acetamidoantipyrine, 4-formylaminoantipyrine, lidocaine N-oxide), stimulants (caffeine and cotinine), sweeteners (acesulfame, sucralose, saccharine), and industrial chemicals (2,4-dinitrophenol, 1-H-benzotriazole, didecyldimethylammonium, diethyl phthalate, dimethyl phthalate and tolyltriazole).
Tentatively identified emerging organic contaminants
Once all samples from the sampling campaign were uploaded into DSFP, the batch-mode screening module was used to screen SusDat substances with experimental fragment information available. In total, 6152 compounds were screened (5459 in positive ESI and 2352 in negative ESI; 1659 compounds both positively and negatively ionized). After the exclusion of target substances determined in the samples as presented above and naturally occurring compounds, 30 emerging contaminants were tentatively identified in surface water, sediments, and biota. Most of them belonged to the classes of industrial chemicals (including surfactants, plasticizers, food additives) and pharmaceuticals. The detected substances are presented in Fig. 3 and in Table S5 (ESM).
Twenty-one compounds were tentatively identified in surface water samples. Nine of them were detected in at least half of the samples (FoA > 50%): six plasticizers (6-methylbenzotriazole, dimethyl sebacate, glutaric acid, N-butylbenzenesulfonamide, TEBT (tris(2-butoxyethyl) phosphate), tributylphosphate), one additive used in food industry (glycerol monostearate), one surfactant (N,N-bis(2-hydroxyethyl)dodecanamide), and one pharmaceutical (hymecromone, used for bile therapy). The remaining 12 emerging contaminants were detected only in smaller number of samples. Apart from the TP of illicit drug N-formylmethamphetamine, the rest of the substances were industrial chemicals (2-(2-(2-butoxyethoxy)ethoxy)ethanol, bisphenol S, diethanolamine, dimethyl cyclohexane-1,4-dicarboxylate, 2-furoic acid, heptanophenone, lauramine oxide, 4-phenylbutenone, sulfamethoxazole-PtO, tetradecylamine, tiglic acid).
Seven emerging contaminants were tentatively identified in river sediment samples. Except glutaric acid, glycerol monostearate, and N-butylbenzenesulfonamide, which were also detected in surface water samples, the industrial chemicals 12-aminododecanoic acid (additive), anethole (additive) and DDAO (N,N-dimethyldodecylamine N-oxide) (surfactant) exclusively occurred in sediments. Moreover, a drug impurity, gabapentin-related compound E, was tentatively identified.
Finally, eight CECs were tentatively identified in biota samples. From the class of industrial chemicals, 2-amino-4-methylpyrimidine, 8-hydroxyquinoline, and malic acid were detected exclusively in biota, while lauramine oxide and N,N-bis(2-hydroxyethyl)dodecanamide were detected in both surface water and biota samples. Glycerol monostearate was the most frequently detected substance in all investigated matrices. From the class of pharmaceuticals, two compounds from various therapeutic classes, telbivudine (antiviral drug) and viloxazine (antidepressant drug), were detected. These tentative findings supplement the results of target screening and highlight the input of industrial chemicals in the Dniester River Basin.
Risk assessment and prioritization of organic contaminants
Three WFD priority substances were detected in surface water of the Dniester River Basin: atrazine (AA-EQS, 600 ng/L), diuron (AA-EQS, 200 ng/L), and simazine (AA-EQS, 1000 ng/L). Atrazine and simazine were detected at concentrations below the EQS. The concentration of diuron at site 10 (1.2 μg/L) exceeded the EQS value. This is a matter of concern and regular monitoring should reveal if the exceedance is causing the failure to reach good chemical status of the impacted water body.
Four substances (acetamiprid, imidacloprid, thiacloprid, thiamethoxam) that were detected in surface water samples are listed in the EU Watch List. These compounds belong to the class of neonicotinoids, which are neuro-active insecticides chemically similar with nicotine. PNECs proposed by the EC Joint Research Center [42] were used for their risk assessment. The concentrations of imidacloprid and thiamethoxam exceeded the PNECs at sites 6, 9, and 10, which indicates that they should be included in the regional monitoring program.
A risk of the rest of the detected substances was assessed by comparing measured concentrations with PNEC values, which were applied according to the following order of credibility: experimental PNEC (PNEC chronic followed by PNEC acute) and in silico P-PNEC. The NORMAN Ecotoxicology Database [22] contained at the time of the study lowest PNEC values for > 65,000 substances, which were either obtained experimentally or predicted by QSAR [43]. All detected contaminants together with their ecotoxicological threshold values and the PNEC type are included in Tables S6A, S6B, and S6C (ESM).
Overall, sixteen pesticides (acetochlor, carbaryl, carbendazim, dimethenamid, dimethoate, diuron, fipronil, imidacloprid, metazachlor, metolachlor, nicosulfuron, omethoate, 2-hydroxypropazine, terbutylazine, desethylterbutylazine, thiamethoxam) and ten pharmaceuticals (4-acetamidoantipyrine, carbamazepine, 10,11-dihydro-10,11-dihydroxycarbamazepine, fluconazole, ibuprofen, lopinavir, mefenamic acid, sulfamethoxazole, telmisartan, triclosan) exceeded the ecotoxicological thresholds in at least one surface water sample. FoA, FoE, and EoE were calculated for these 26 substances to retrieve a risk score (sum of FoA + FoE + EoE (0–3)) for their prioritization, as presented in Τable 2. The determination of the compounds of national or regional concern (characterized as river basin–specific pollutants) is a requirement by the WFD [44]. The risk scores indicated that pharmaceuticals telmisartan and 4-acetamidoantipyrine, as well as herbicides metolachlor and terbuthylazine, could potentially pose high risk and might be considered good candidates for river basin–specific pollutants. In general, all substances exceeding their EQS or PNEC values should be included into the regional investigative monitoring program to confirm if the exceedance is widespread. If yes, critical mass of the data should be collected allowing for their proper risk assessment, e.g., according to the NORMAN Prioritisation Framework [23], followed by the targeted Programme of Measures.
In sediments, two emerging contaminants, pesticides azoxystrobin and carbendazim, exceeded the PNEC at site 4.
In this study, the used sediment and biota PNECs were calculated from freshwater PNECs and therefore should be used only as an indicative values.
Conclusions
The presented study provided a comprehensive overview of the chemical pollution status of the Dniester River Basin and brought up evidence needed to improve the water quality of the Dniester River. Metal analysis revealed presence of eight metals in surface water and sediments. Systematic wide-scope target screening of 2273 subatances in surface water, sediment, and biota samples revealed presence of 109 organic contaminants from different chemical classes. Suspect screening of 6152 substances resulted in detection of additional 30 emerging contaminants, belonging mostly to the classes of industrial chemicals (including surfactants, plasticizers, food additives) and pharmaceuticals. The samples’ chromatograms were also digitally “frozen” and thus allowing for future retrospective screening of currently unknown or unsuspected compounds. The screening campaign revealed places with significantly higher pollution load, such as the Byk River downstream Chisinau (Moldova), where 69 organic contaminants were determined with a total cumulative concentration of 36.4 μg/L. Among the main polluting sources were traced to be pharmaceutical industry, other industrial plants, and municipal wastewater outlets. The Tysmenytsia River in Ukraine showed a total cumulative concentration of organic contaminants of 7.4 μg/L, mainly due to increased levels of pharmaceuticals. Metals were observed at their highest concentration in the Byk and Tysmenytsia rivers. Significant load of wide range organic pollutants was also observed in Mohyliv-Podilskyi (Ukraine) and in the Reut River (Moldova). In both places, pesticides were the dominating contaminants. The ecotoxicological risk assessment revealed that concentration levels of some detected pesticides, pharmaceuticals, and their TPs exceeded the PNEC values in the tributaries Byk, Reut, Tysmenytsia, and Seret and in the Dniester River close to Rybnitsa/Rezina and Mohyliv-Podilskyi. WFD priority substances (diuron, mercury), WFD Watch List substances (imidacloprid and thiamethoxam) exceeding their EQS, and emerging contaminants (telmisartan, metolachlor, terbuthylazine, and 4-acetamidoantipyrine) exceeding their PNEC values were suggested as candidates for river basin–specific pollutants. Additional 19 pesticides and pharmaceuticals exceeding their ecotoxicological threshold values in at least one surface water sample were suggested to be included in the regional investigative monitoring program. A good match was found between the pollution pattern of the Dniester River and Black Sea close to the estuary, which was investigated within the EU/UNDP EMBLAS project. The obvious adverse impact of the Dniester River on the marine environment was recommended to be studied in more detail. The future monitoring campaigns should be scheduled throughout the year to investigate seasonal variation of the contaminants, mainly of pesticides, because household and industrial discharges have a more stable profile regardless of the season.
References
United Nations Economic Commission for Europe. Transboundary diagnostic study for the Dniester River Basin. 2005. (Accessible at https://www.osce.org/ukraine/104057. Last accessed 4 Feb 2020).
Lebedynets M, Sprynskyy M, Kowalkowski T, Buszewski B. Evaluation of hydrosphere state of the Dniester river catchment. Pol J Environ Stud. 2005;14(1):65–71.
Agreement between the Government of the Republic of Moldova and the Government of Ukraine on the joint management and protection of the cross-border waters. 1994. (Accessible at http://www.cawater-info.net/bk/water_law/pdf/moldova_ukraina_eng.pdf. Last accessed 4 Feb 2020).
Water Code of Ukraine of June 6, 1995 No. 213/95-BP (Accessible at https://zakon.rada.gov.ua/laws/card/ru/213/95-%D0%B2%D1%80?lang=en. Last accessed 4 Feb 2020).
Law of the Republic of Moldova on Water (2011) No. 272 (Accessible at http://www.lawyermoldova.com/2012/10/water-law.html. Last accessed 4 Feb 2020).
Buijs P. Transboundary monitoring of the Dniester River Assessment and evaluation, Action Programme to Improve Transboundary Cooperation and Sustainable Management of the Dniester River Basin (Dniester – III). 2010. (Accessible at http://archive.zoinet.org/web/sites/default/files/publications/transboundary_monitoring_of_the_dniester_river_assessment_and_evaluation-august-2010_0.pdf. Last accessed 4 Feb 2020).
European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Union. 2000;L327:1–72.
Vystavna Y, Cherkashyna M, van der Valk MR. Water laws of Georgia, Moldova and Ukraine: current problems and integration with EU legislation. Water Int. 2018;43(3):424–35. https://doi.org/10.1080/02508060.2018.1447897.
European Commission. DIRECTIVE 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J Eur Union. 2013;L226:1–17.
European Commission (2018) Commission implementing decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495 Official Journal of the European Union L141:9-12.
Dulio V, van Bavel B, Brorstrom-Lunden E, Harmsen J, Hollender J, Schlabach M, et al. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ Sci Eur. 2018;30(1):5. https://doi.org/10.1186/s12302-018-0135-3.
Beretsou VG, Psoma AK, Gago-Ferrero P, Aalizadeh R, Fenner K, Thomaidis NS. Identification of biotransformation products of citalopram formed in activated sludge. Water Res. 2016;103:205–14. https://doi.org/10.1016/j.watres.2016.07.029.
Nika MC, Bletsou AA, Koumaki E, Noutsopoulos C, Mamais D, Stasinakis AS, et al. Chlorination of benzothiazoles and benzotriazoles and transformation products identification by LC-HR-MS/MS. J Hazard Mater. 2017;323(Pt A):400–13. https://doi.org/10.1016/j.jhazmat.2016.03.035.
Sapozhnikova Y, Zubcov N, Hungerford S, Roy LA, Boicenco N, Zubcov E, et al. Evaluation of pesticides and metals in fish of the Dniester River, Moldova. Chemosphere. 2005;60(2):196–205. https://doi.org/10.1016/j.chemosphere.2004.12.061.
Sapozhnikova Y, Zubcov E, Zubcov N, Schlenk D. Occurrence of pesticides, polychlorinated biphenyls (PCBs), and heavy metals in sediments from the Dniester River, Moldova. Arch Environ Contam Toxicol. 2005;49(4):439–48. https://doi.org/10.1007/s00244-005-8011-8.
Munteanu V, Munteanu G. Biomonitoring of mercury pollution: a case study from the Dniester River. Ecol Indic. 2007;7(2):489–96. https://doi.org/10.1016/j.ecolind.2006.01.002.
Zubcov E, Zubcov N, Ene A, Bagrin N, Biletchi L. The dynamics of trace elements in Dniester river ecosystems. J Sci Art. 2010;2(13):281–6.
Maldonado C, Bayona JM, Bodineau L. Sources, distribution, and water column processes of aliphatic and polycyclic aromatic hydrocarbons in the northwestern Black Sea water. Environ Sci Technol. 1999;33(16):2693–702. https://doi.org/10.1021/es9811647.
Gago-Ferrero P, Bletsou AA, Damalas DE, Aalizadeh R, Alygizakis NA, Singer HP, et al. Wide-scope target screening of >2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J Hazard Mater. 2020;387:121712. https://doi.org/10.1016/j.jhazmat.2019.121712.
Alygizakis NA, Oswald P, Thomaidis NS, Schymanski EL, Aalizadeh R, Schulze T, et al. NORMAN digital sample freezing platform: a European virtual platform to exchange liquid chromatography high resolution-mass spectrometry data and screen suspects in “digitally frozen” environmental samples. TrAC Trends Anal Chem. 2019;115:129–37. https://doi.org/10.1016/j.trac.2019.04.008.
European Chemicals Bureau. Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. 2003. (Accessible at https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf. Last accessed 4 Feb 2020).
NORMAN Database System. NORMAN Ecotoxicology Database. 2020. (Accessible at https://www.norman-network.com/nds/ecotox/, Last accessed 4 Feb 2020).
Dulio V, von der Ohe PC (2013) NORMAN Prioritisation framework for emerging substances (Accessible at https://www.norman-network.net/sites/default/files/files/Publications/NORMAN_prioritisation_Manual_15April2013_finalforwebsite-f.pdf. Last accessed 4 Feb 2020).
Alygizakis NA, Besselink H, Paulus GK, Oswald P, Hornstra LM, Oswaldova M, et al. Characterization of wastewater effluents in the Danube River Basin with chemical screening, in vitro bioassays and antibiotic resistant genes analysis. Environ Int. 2019;127:420–9. https://doi.org/10.1016/j.envint.2019.03.060.
Encyclopedia NW. Chisinau. 2017. (Accessible at https://www.newworldencyclopedia.org/entry/Chisinau. Last accessed 4 Feb 2020).
Lin C, He M, Zhou Y, Guo W, Yang Z. Distribution and contamination assessment of heavy metals in sediment of the Second Songhua River, China. Environ Monit Assess. 2008;137(1-3):329–42. https://doi.org/10.1007/s10661-007-9768-1.
Alygizakis NA, Gago-Ferrero P, Borova VL, Pavlidou A, Hatzianestis I, Thomaidis NS. Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci Total Environ. 2016;541:1097–105. https://doi.org/10.1016/j.scitotenv.2015.09.145.
United Nations Environment. Fake pesticides, real problems: addressing Ukraine’s illegal and counterfeit pesticides problem. 2018. (Accessible at https://www.unenvironment.org/news-and-stories/story/fake-pesticides-real-problems-addressing-ukraines-illegal-and-counterfeit, Last accessed 4 February 2020).
Pryde P. Environmental Resources And Constraints In The Former Soviet Republics. 1995. (Accessible at https://books.google.gr/books/about/Environmental_Resources_And_Constraints.html?id=IqubDwAAQBAJ&source=kp_cover&redir_esc=y. Last accessed 4 Feb 2020).
Kimstach V, Maeybeck M, Baroudy E. A Water Quality Assessment of the Former Soviet Union. 1998. (Accessible at https://books.google.gr/books?id=2dk_SC5TIl8C&lpg=PP1&dq=AWaterQualityAssessmentoftheFormerSovietUnion. Last accessed 4 Feb 2020).
Cox L, Hermosin MC, Cornejo J, Mansour M. Photolysis of metamitron in water in the presence of soils and soil components. Chemosphere. 1996;33(10):2057–64. https://doi.org/10.1016/0045-6535(96)00297-4.
Rivard L. Environmental Fate of Metolachlor Linda Rivard Environmental Monitoring Branch Department of Pesticide Regulation. 2003. (Accessible at https://www.semanticscholar.org/paper/Environmental-Fate-of-Metolachlor-Linda-Rivard-of-Rivard/6af152adb607b080f9065eeb22832b9c99027153. Last accessed 4 Feb 2020).
Mudhoo A, Garg VK. Sorption, transport and transformation of atrazine in soils, minerals and composts: a review. Pedosphere. 2011;21(1):11–25. https://doi.org/10.1016/s1002-0160(10)60074-4.
Lucas AD, Bekheit HKM, Goodrow MH, Jones D, Kullman S, Matsumura F, et al. Development of antibodies against hydroxyatrazine and hydroxysimazine: application to environmental samples. J Agric Food Chem. 1993;41:1523–9. https://doi.org/10.1021/jf00041a040.
Guzzella L, Rullo S, Pozzoni F, Giuliano G. Studies on mobility and degradation pathways of tebuthylazine using lysimeters on a field scale. J Environ Qual. 2003;32:1089–98.
Ghosh RK, Singh N. Leaching behaviour of azoxystrobin and metabolites in soil columns. Pest Manag Sci. 2009;65(9):1009–14. https://doi.org/10.1002/ps.1787.
Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, et al. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett. 2016;14(3):317–29. https://doi.org/10.1007/s10311-016-0566-2.
Wiegel S, Aulinger A, Brockmeyer R, Harms H, Loffler J, Reincke H, et al. Pharmaceuticals in the river Elbe and its tributaries. Chemosphere. 2004;57(2):107–26. https://doi.org/10.1016/j.chemosphere.2004.05.017.
Garcia-Martin E, Esguevillas G, Blanca-Lopez N, Garcia-Menaya J, Blanca M, Amo G, et al. Genetic determinants of metamizole metabolism modify the risk of developing anaphylaxis. Pharmacogenet Genomics. 2015;25(9):462–4. https://doi.org/10.1097/FPC.0000000000000157.
Leclercq M, Mathieu O, Gomez E, Casellas C, Fenet H, Hillaire-Buys D. Presence and fate of carbamazepine, oxcarbazepine, and seven of their metabolites at wastewater treatment plants. Arch Environ Contam Toxicol. 2009;56(3):408–15. https://doi.org/10.1007/s00244-008-9202-x.
Slobodnik J, Alexandrov B, Komorin V, Mikaelyan A, Guchmanidze A, Arabidze M, Korshenko A, Moncheva S. National Pilot Monitoring Studies and Joint Open Sea Surveys in Georgia, Russian Federation and Ukraine, 2016. Final Scientific Report, EU/UNDP Project: Improving Environmental Monitoring in the Black Sea e Phase II (EMBLAS-II) ENPI/2013/313-169. 2016. (Accessible at http://emblasproject.org/wp-content/uploads/2017/04/Joint-Black-Sea-Surveys-2016_16.pdf. Last accessed 4 Feb 2020).
Loos R, Marinov D, Sanseverino I, Napierska D, Lettieri T. Review of the 1st Watch List under the Water Framework Directive and recommendations for the 2nd Watch List. 2018. (Accessible at https://publications.jrc.ec.europa.eu/repository/bitstream/JRC111198/wl_report_jrc_2018_04_26_final_online.pdf. Last accessed 4 Feb 2020).
Aalizadeh R, von der Ohe PC, Thomaidis NS. Prediction of acute toxicity of emerging contaminants on the water flea Daphnia magna by Ant Colony Optimization-Support Vector Machine QSTR models. Environ Sci Process Impacts. 2017;19(3):438–48. https://doi.org/10.1039/c6em00679e.
Hollender J, van Bavel B, Dulio V, Farmen E, Furtmann K, Koschorreck J, et al. High resolution mass spectrometry-based non-target screening can support regulatory environmental monitoring and chemicals management. Environ Sci Eur. 2019;31(1). https://doi.org/10.1186/s12302-019-0225-x.
Acknowledgments
The authors would like to acknowledge OSCE Ukraine for financing the study, Ukrainian scientific center of Ecology of Sea in Odessa for support at sampling and sample transport logistics, and NORMAN network (https://www.norman-network.net/) for providing access to its latest version of the Ecotoxicology Database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No vertebrate animals are used in the study. No violation of animal rights occurred during this investigation. The samples were collected after informed consent by the occupants of the premises sampled.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Persistent and Mobile Organic Compounds – An Environmental Challenge with guest editors Torsten C. Schmidt, Thomas P. Knepper, and Thorsten Reemtsma.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 234 kb)
Rights and permissions
About this article
Cite this article
Diamanti, K.S., Alygizakis, N.A., Nika, MC. et al. Assessment of the chemical pollution status of the Dniester River Basin by wide-scope target and suspect screening using mass spectrometric techniques. Anal Bioanal Chem 412, 4893–4907 (2020). https://doi.org/10.1007/s00216-020-02648-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02648-y