Abstract
Herein, a dual-emission metal-organic framework based ratiometric fluorescence nanoprobe was reported for detecting copper(II) ions. In particular, carbon dots (CDs) and gold nanoclusters (AuNCs) were embedded into ZIF-8 (one of the classical metal-organic frameworks) to form CDs/AuNCs@ZIF-8 nanocomposites, which exhibited dual-emission peaks at UV excitation. In the presence of Cu2+, the fluorescence attributed to AuNCs can be rapidly quenched, while the fluorescence of CDs serves as reference with undetectable changes. Therefore, the CDs/AuNCs@ZIF-8 nanocomposites were utilized as a ratiometric fluorescence nanoprobe for sensitive and selective detection of Cu2+. A good linear relationship between the ratiometric fluorescence signal of CDs/AuNCs@ZIF-8 and Cu2+ concentration was obtained in the range of 10−3–103 μM, and the detection limit was as low as 0.3324 nM. The current ratiometric fluorescence nanoprobe showed promising prospects in cost-effective and rapid determination of Cu2+ ions with good sensitivity and selectivity. Furthermore, this nanoprobe has been successfully applied for the quantitative detection of Cu2+ in serum samples, indicating its value of practical application.

Carbon dots (CDs) and gold nanoclusters (AuNCs) were embedded into metal-organic frameworks (ZIF-8) to form CDs/AuNCs@ZIF-8 nanocomposites, which exhibited dual-emission peaks at 365 nm excitation. In the presence of Cu2+, the fluorescence emission peak at 574 nm can rapidly respond by quenching, while the fluorescence at 462 nm serves as reference with undetectable changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper is one of important elements for human health, which plays pivotal functions in multiple physiological processes dependent on the copper homeostasis in the human body [1]. Excessive copper ions in the body would disturb the cellular homeostasis that causes Alzheimer’s, Wilson, and Menkes diseases [2,3,4]. Consequently, the accurate detection of copper is of great importance in disease diagnosis. Up to now, a number of methods have been developed for the detection of Cu2+, such as colorimetric [5, 6], fluorescence [7, 8], electrochemistry [9], chemiluminescence [10], and Raman spectroscopy [11]. Among them, the fluorescence assay may be a good alternative due to the advantages of high precision and repeatability. So far, some fluorescent probes have been used for detecting Cu2+, but the most among that have single-wavelength emission which is the only detection signal [12,13,14,15]. In this case, factors such as probe concentration, environmental conditions, and instrumental efficiency would cause negative effects for the output of detection signal [16]. Ratiometric fluorescent probes are of particular interest since they could provide intrinsic reference to eliminate most or all interferences by self-calibration of two emission bands [17, 18].
Recently, various ratiometric fluorescent probes have been designed and fabricated [19,20,21,22,23]. Among them, nanohybrids have attracted more attentions compared to organic dyes, owing to their better stability with respect to photobleaching, higher fluorescence quantum yield, and easier preparation [24, 25]. Generally, these nanohybrids were assembled by combining two different fluorescent nanoparticles, in which one fluorophore as reference and another as a signal report unit. In present, the nanohybrids based on metal-organic framework materials (MOFs) exhibit attractive properties, which have outstanding benefits that derive from the incorporated foreign nanomaterials [26,27,28,29]. Several reports have demonstrated introducing guest nanomaterials with versatile shapes and functions into ZIF-8 is an important way to synthesize ideal luminescence-functionalized MOFs for chemical sensing [30,31,32,33]. Moreover, the accumulation effect of MOFs can greatly amplify the signal of incorporated nanomaterials [27]. For example, Chen et al. reported encapsulating fluorescent carbon quantum dots (CDs) into ZIF-8 to form CDs@ZIF-8 for ultrasensitive chemical sensing of Cu2+ in environmental water samples [34]. However, the fluorescence probes with single “turn-off” mode usually generate false-positive results, which restrict their practical applications.
Herein, we reported a dual-emission fluorescent nanoprobe CDs/AuNCs@ZIF-8 for the detection of Cu2+. The sensitivity and accuracy for detecting Cu2+ were greatly improved by the self-calibration ability of dual-emission fluorescent nanoprobe. The CDs/AuNCs@ZIF-8 nanocomposites with dual-emission peaks were constructed by embedding carbon dots (CDs) and gold nanoclusters (AuNCs) into ZIF-8. Interestingly, in the presence of Cu2+, the fluorescence which belonged to AuNCs could be responded rapidly by quenching, while the fluorescence of CDs served as reference with negligible change. Furthermore, the proposed ratiometric fluorescence nanoprobe could rapidly detect Cu2+ in cost-effective and showed good selectivity and anti-interference ability.
Experimental section
Reagents and apparatus
Glutathione (GSH), 2-methylimidazole, cysteine (Cys), glycine (Gly), phenylalanine (Phe), tyrosine (Tyr), glutamate (Glu), and aspartic acid (Asp) were purchased from Aladdin Chemical (Shanghai, China). Tripolycyanamide, sodium citrate, zinc nitrate hexahydrate (Zn(NO3)2·6H2O), chloroauric acid (HAuCl4), sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O), sodium monohydrogen heptahydrate phosphate (Na2HPO4·7H2O), copper nitrate (Cu(NO3)2), zinc chloride (ZnCl2), sodium chloride (NaCl), magnesium chloride hexahydrate (MgCl2·6H2O), iron sulfate hydrate (FeSO4·7H2O), lead nitrate (Pb(NO3)2), and alchlor (AlCl3) were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All the reagents were of analytical grade and used without further purification. All the solutions were prepared using phosphate buffer solution (PBS).
Transmission electron microscopy (TEM) were carried out with a JEM-2100 PLUS microscope (Japan). Fourier transform infrared spectrometry (FTIR) spectrum was recorded with a Beifen-Ruili WQF-520A FTIR spectrometer (China). Ultraviolet-visible (UV-vis) absorption spectra were recorded using a Varian Cary-300 bio UV/vis spectrophotometer (USA). The fluorescence lifetime was studied by a Fluorolog-3-21 spectrometer (USA). All fluorescence measurements were carried out with a Hitachi F-7000 spectrometer (Japan). The instrument parameters were set as follows: λex = 365 nm (slit = 10 nm), λem1 = 462 nm (slit = 10 nm), λem2 = 574 nm (slit = 10 nm), PMT detector voltage = 400 V.
Synthesis of CDs
The CDs was prepared via the method documented in previous report with minor modification [35]. Specifically, 0.24 g of tripolycyanamide, 0.58 g of sodium citrate, and 25 mL of distilled water were mixed and sonicated for 5 min to form homogeneous mixture solution. The above solution was then transferred to a 50 mL Teflon-lined autoclave. After hydrothermal reaction proceeded at 180 °C for 12 h, the mixture was filtered with a cylinder membrane filter (0.22 μm) to remove the large particles, and dialyzed against ultrapure water through a 500 D of dialysis membrane for 48 h. The as-prepared CDs were stored at 4 °C for further use.
Synthesis of luminescent AuNCs
The luminescent AuNCs were synthesized according to the literature [36]. In brief, HAuCl4 (20 mM, 0.50 mL) and GSH (100 mM, 0.15 mL) were mixed with 4.35 mL ultrapure water at 25 °C. The reaction mixture was gently stirring (500 rpm) for 24 h at 70 °C. The resultant orange-emitting AuNCs solution was stored at 4 °C for further use.
Synthesis of CDs/AuNCs@ZIF-8
The preparation of CDs/AuNCs@ZIF-8 nanocomposites was carried out by a one-pot method with minor modification [31, 37]. Typically, a certain volume of CDs and AuNCs mixture was added into Zn(NO3)2 aqueous solution, after ultrasonic dispersion for 30 min, rapidly injecting the mixture into 2-methylimidazole aqueous solution with molar ratio of Zn2+:2-methylimidazole: H2O = 1:70:1238 under vigorous stirring. After a 10-min reaction, the resultant precipitate was centrifuged, washed, and vacuum-dried at 50 °C to get the powder. The CDs/AuNCs@ZIF-8 nanoprobe was obtained by dissolving the powder with buffer solution. The fluorescence intensity of CDs/AuNCs@ZIF-8 could be regulated by varying the volume of AuNCs and CDs solution.
General procedure for detecting Cu2+
In a typical process, the CDs/AuNCs@ZIF-8 nanocomposites solutions and Cu2+ solutions were prepared with buffer solution (PBS = 7.4, 10 mM). Nine hundred microliters of CDs/AuNCs@ZIF-8 nanocomposites solution and 100 μL of Cu2+ solutions with different concentrations (1 nM–1 mM) were incubated for 10 min at room temperature; then, the fluorescence intensity of the mixture at 462 nm and 574 nm was measured at excitation wavelength of 365 nm.
Results and discussion
Possible mechanism of Cu2+ determination
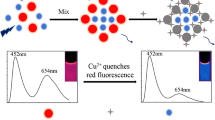
The possible principle of CDs/AuNCs@ZIF-8 nanoprobe for detecting Cu2+ is illustrated in Scheme 1. The CDs/AuNCs@ZIF-8 nanocomposites were successfully synthesized by mixing CDs and AuNCs in the presence of Zn(NO3)2 and 2-methylimidazole (the precursors of ZIF-8). The CDs/AuNCs@ZIF-8 nanocomposites exhibited dual-emission peaks at 365 nm excitation. The fluorescence peak at 462 nm was attributed to the transition from LUMO to HOMO orbitals of the CDs [38], and the remarkable luminescence of AuNCs (574 nm) was attributed to the electronic interband transitions in occupied “sp” and “d” bands (LUMO-HOMO) [39]. After the addition of Cu2+, the 574 nm emission peak was quenched drastically, while the fluorescence at 462 nm remained almost unchanged. The phenomenon could be explained by that Cu2+ could coordinate with -COOH of GSH on the surface of AuNCs, leading to the quench of AuNCs fluorescence.
To explore whether the quenching process was static or dynamic, fluorescence lifetimes of AuNCs in the absence or presence of Cu2+ were investigated (Fig. 1a). As shown in Table S1 (see Electronic Supplementary Material, ESM), fluorescence lifetimes of AuNCs and AuNCs + Cu2+ were 581.18 ns and 56.69 ns, respectively. The fluorescence lifetime of AuNCs was obviously shorten by adding Cu2+, revealing that Cu2+-triggered quenching process could be attributed to dynamic quenching [40]. This result was consistent with the fluorescence spectra of AuNCs + Cu2+ (Fig. 1b). Besides, the fluorescence spectra of CDs in the absence or presence of Cu2+ were also explored (Fig. 1c), the difference of the fluorescence intensity of CDs with and without Cu2+ is negligible. That could be for the interaction between Cu2+ and CDs was too weak to affect the fluorescence of CDs. The above mechanism was confirmed again by fluorescence spectra of CDs/AuNCs@ZIF-8 + Cu2+ (Fig. 1d).
Characterization of CDs/AuNCs@ZIF-8
The morphology and size of synthesized CDs/AuNCs@ZIF-8 nanocomposites were observed by the transmission electron microscopy (TEM). As illustrated in Fig. 2a, the prepared CDs with an average size of 4–6 nm were uniform and monodispersed with a quasi-spherical shape. The high-resolution TEM (HRTEM) image of CDs (the inset in Fig. 2a) showed the spacing of the adjacent lattice planes for random nanoparticle were 0.25 nm, which agrees well with the (100) diffraction plane of graphite. That indicated the prepared CDs possess a graphite-like structure. Figure. 2b revealed the typical TEM image of AuNCs; the size of AuNCs was about 2 nm. The inset in Fig. 2b demonstrated the HRTEM image of AuNCs, the spacing of the adjacent lattice planes for AuNCs were 0.235 nm, which were in accord with the previous reports [23]. Figure 2c and d manifested the pure ZIF-8 and CDs/AuNCs@ZIF-8 nanocomposites, respectively. The CDs/AuNCs@ZIF-8 exhibited a hexagonal shape with the size of approximately 175 nm (see Fig. S1 in the ESM). The difference of between CDs/AuNCs@ZIF-8 nanocomposites and pure ZIF-8 was negligible, which was well coincide with the previous studies [27, 31, 32]. The consistency in size and shape between the CDs/AuNCs@ZIF-8 and pure ZIF-8 proved that the incorporation of CDs and AuNCs would not affect the morphology and size of ZIF-8. We consider that the formation of CDs/AuNCs@ZIF-8 nanocomposites may result from the following three coordination interactions: (1) the coordinative interaction between Zn2+ and the imidazole molecules in ZnN4 tetrahedron of ZIF-8 [41], (2) the coordinative interaction between Zn2+ and -NH on the CDs surface [42], (3) the coordinative interaction between Zn2+ and -COOH in GSH of AuNCs [31]. These interactions led to the formation of CDs/AuNCs@ZIF-8.
In order to deeply confirm the forming process of CDs/AuNCs@ZIF-8 nano composites, the FTIR spectra measurement was performed. As shown in Fig. 3, the FTIR spectrum for the CDs was dominated by bands at ~ 3130, 1580, and 1420 cm−1, which were attributed to amine and hydroxyl groups, C=O and C=C stretching vibrations and C–N bending vibrations, respectively [35]. The FTIR spectrum for the AuNCs showed a distinct absorption peak at ~ 3400 cm−1, which could be assigned to -OH stretching vibrations. It could be seen that the absorption peaks at 1580 and 1420 cm−1 of CDs and 3400 cm−1 of AuNCs were also observed in the spectrum of the CDs/AuNCs@ZIF-8 nanocomposites while that was not found in the spectrum of pure ZIF-8. On the basis of these results, we concluded that the CDs/AuNCs@ZIF-8 composites were prepared successfully.
The optical properties of CDs/AuNCs@ZIF-8 were investigated by UV-vis absorption spectra and fluorescence spectra. Figure 4 showed UV-vis absorption spectra of the CDs (red), AuNCs (black), pure ZIF-8 (pink), and CDs/AuNCs@ZIF-8 nanocomposites (blue). In the range of 300–600 nm, the CDs exhibited an absorption peak at 345 nm and the AuNCs displayed an absorption peak at 365 nm, which matched well with results in literatures [35, 36]. The pure ZIF-8 had a very weak absorption band while the CDs/AuNCs@ZIF-8 nanocomposites had a strong absorption band in UV-vis range. These results indicated that CDs and AuNCs were embedded deeply into the dense ZIF-8 matrix. In addition, the fluorescence spectrum of CDs/AuNCs@ZIF-8 nanocomposites with different volume AuNCs and CDs were recorded by fluorescence spectrometer. The obtained results (see Fig. S2 in the ESM) proved the fluorescence intensity of CDs/AuNCs@ZIF-8 could be regulated by varying the volume of AuNCs and CDs solution.
Optimization of experimental conditions
To get the excellent detection performance, the experimental conditions such as pH, reaction temperature, and the type of buffer solution were optimized. Considering that the pH may affect the structure of ZIF-8, it is optimized firstly. It can be seen from Fig. S3a (see the ESM) that the pH had remarkable influence on the change of fluorescence intensity ratio (△F574/F462). (△F574/F462 means the changed value of fluorescence intensity ratio of the CDs/AuNCs@ZIF-8 in the absence and presence of Cu2+.) At pH 7.4, the △F574/F462 reached a maximum; therefore, pH 7.4 was chosen as the optimal pH for Cu2+ detection. In addition, the influence of the reaction temperature on the △F574/F462 was also investigated (see Fig. S3b in the ESM). The results showed that the △F574/F462 reached the maximum at 37 °C. Thus, we chose 37 °C as the optimal reaction temperature for Cu2+ detection in the further experiments. Lastly, taking into account that different buffer solutions contain different components, some of components may interact with Cu2+ to affect the experimental results. Therefore, the types of buffer solutions were optimized. The results indicated that the △F574/F462 reached a maximum in PBS buffer (see Fig. S3c in the ESM); therefore, next experiments were completed in PBS buffer.
Performance for Cu2+ detection
The CDs/AuNCs@ZIF-8 nanoprobe was applied to detect Cu2+. As shown in Fig. 5a, with the increase of Cu2+ concentrations, the fluorescence peak of CDs/AuNCs@ZIF-8 located at 462 nm remained almost unchanged, while the fluorescence peak at 574 nm was quenched obviously. A good fitted regression line between the fluorescence intensity ratio F574/F462 and Cu2+ concentrations was obtained in the range of 10−3 to 103 μM (Fig. 5b), with a linear regression equation as Y = 0.9732–0.1595 log C (C was the concentration of Cu2+ (μM), R2 = 0.9928). The detection limit of Cu2+ is estimated to be 0.3324 nM according to the 3σ rule. These results demonstrated that the current strategy was more sensitive compared to most of the previous reported methods for Cu2+ determination (see Table S2 in the ESM).
a Fluorescence emission spectra of CDs/AuNCs@ZIF-8 in the presence of various Cu2+ concentrations (1 nM–1 mM). b Relationship between the fluorescence intensity ratio F574/F462 and Cu2+ concentrations. (F574 and F462 are the fluorescence intensities of CDs/AuNCs@ZIF-8 at 574 nm and 462 nm, respectively)
Selectivity and interference studies
In addition to the sensitivity, selectivity is another significant factor to evaluate the potential applications of a newly designed detection system in a real sample. To investigate the selectivity of the CDs/AuNCs@ZIF-8 nanoprobe for Cu2+, some potential interferents, such as inorganic ions (Na+, Fe3+, Zn2+, Mg2+, Fe2+, Pb2+, Al3+, Ca2+), amino acids (Cys, Gly, Phe, Tyr, Glu, and Asp), and other biological molecules (GSH), were tested under the same conditions. As shown in Fig. 6a, the fluorescence response (F574/F462) of this nanoprobe was immensely decreased by Cu2+. However, no obvious interferences were observed for other substance including inorganic ion, glutathione, and some amino acids. The result indicated that this designed fluorescence detection system possessed a high selectivity toward Cu2+. Besides, interference experiments were also performed and the results were showed in Fig. 6b. Interfering substances had no significant impact on ratio fluorescence intensity F574/F462 of CDs/AuNCs@ZIF-8 containing Cu2+. All the above results manifested that this method had high selectivity and anti-interference ability, which may be directly applied to detect Cu2+ in real samples.
a Fluorescence intensity ratio F574/F462 of CDs/AuNCs@ZIF-8 for Cu2+ over other potential interferences. b Fluorescence intensity ratio F574/F462 of CDs/AuNCs@ZIF-8 for Cu2+ with other potential interferences. (The concentration of all metal ions is 10 μM; the concentration of amino acids and GSH is 1 μM). The error bars were derived from the standard deviation of three measurements. Error bar = SD (n = 3)
Determination of Cu2+ in real samples
To explore the practical application of the developed method, we applied this nanoprobe to analyze Cu2+ in serum samples using the standard addition method. As listed in Table 1, the original Cu2+ concentrations in the three human serum samples (1%) were 0.13, 0.18, and 0.16 μM, respectively, which were in the normal range of 11.00–24.39 μM without dilution. Next, Cu2+ with different concentrations (20 μM, 50 μM, 100 μM) was added into each serum samples, the recovery of Cu2+ was ranged from 95.49 to 104.4%, and the RSD was ranged from 2.1 to 4.5%. That indicated the precision and accuracy of the method for Cu2+ determination in real samples.
Conclusion
In summary, a metal-organic framework–based dual-emission fluorescent nanoprobe had been prepared for the detection of Cu2+. The nanohybrids CDs/AuNCs@ZIF-8 were used as a novel sensing nanoprobe for label-free and sensitive detection of Cu2+ with the detection limit of 0.3324 nM. Moreover, the practical application of the nanoprobe was validated through the detection of Cu2+ in serum samples with satisfactory results. The present study offered a ratiometric fluorescent nanoprobe for detecting Cu2+ and opened a new direction for the development of ratiometric fluorescent methods based on nanomaterials embedded into ZIF-8 nanostructures for practical applications.
References
Cotruvo JJ, Aron AT, Ramos-Torres KM, Chang CJ. Synthetic fluorescent probes for studying copper in biological systems. Chem Soc Rev. 2015;44:4400–14.
Andrási E, Farkas É, Scheibler H, Réffy A, Bezúr L. Al, Zn, Cu, Mn and Fe levels in brain in Alzheimer’s disease. Arch Gerontol Geriatr. 1995;21:89–97.
Yatsunyk LA, Rosenzweig AC. Cu (I) binding and transfer by the N terminus of the Wilson disease protein. J Biol Chem. 2007;282:8622–31.
Viles JH. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord Chem Rev. 2012;256:2271–84.
Shen QP, Li WH, Tang SY, Hu YF, Nie Z, Huang Y, et al. A simple “clickable” biosensor for colorimetric detection of copper (II) ions based on unmodified gold nanoparticles. Biosens Bioelectron. 2013;41:663–8.
Park Y, Lee CY, Park KS, Park HG. Enzyme-free colorimetric detection of Cu2+ by utilizing target-triggered DNAzymes and toehold-mediated DNA strand displacement events. Chem Eur J. 2017;23:17379–83.
Zhao L, Li HY, Xu Y, Liu HC, Zhou TY, Huang N, et al. Selective detection of copper ion in complex real samples based on nitrogen-doped carbon quantum dots. Anal Bioanal Chem. 2018;410:4301–9.
Wang CX, Yang F, Tang YS, Yang W, Zhong HL, Yu CC, et al. Graphene quantum dots nanosensor derived from 3D nanomesh graphene frameworks and its application for fluorescent sensing of Cu2+ in rat brain. Sensors Actuators B Chem. 2018;258:672–81.
Zhou M, Han L, Deng DM, Zhang Z, He HB, Zhang L, et al. 4-mercaptobenzoic acid modified silver nanoparticles-enhanced electrochemical sensor for highly sensitive detection of Cu2+. Sensors Actuators B Chem. 2019;291:164–9.
Cheng CM, Huang Y, Tian XQ, Zheng BZ, Li Y, Yuan HY, et al. Electrogenerated chemiluminescence behavior of graphite-like carbon nitride and its application in selective sensing Cu2+. Anal Chem. 2012;84:4754–9.
Liu Y, Wu Y, Guo X, Wen Y, Yang H. Rapid and selective detection of trace Cu2+ by accumulation-reaction-based Raman spectroscopy. Sensors Actuators B Chem. 2019;283:278–83.
Ding H, Liang CS, Sun KB, Wang H, Hiltunen JK, Chen ZJ, et al. Dithiothreitol-capped fluorescent gold nanoclusters: an efficient probe for detection of copper (II) ions in aqueous solution. Biosens Bioelectron. 2014;59:216–20.
Wang J, Li RS, Zhang HZ, Wang N, Zhang Z, Huang CZ. Highly fluorescent carbon dots as selective and visual probes for sensing copper ions in living cells via an electron transfer process. Biosens Bioelectron. 2017;97:157–63.
Zhang XL, Jing X, Liu T, Han G, Li HQ, Duan CY. Dual-functional gadolinium-based copper (II) probe for selective magnetic resonance imaging and fluorescence sensing. Inorg Chem. 2012;51:2325–31.
Wu XM, Wang H, Yang SX, Tian HY, Liu YG, Sun BG. A novel coumarin-based fluorescent probe for sensitive detection of copper (II) in wine. Food Chem. 2019;284:23–7.
Doussineau T, Schulz A, Lapresta-Fernandez A, Moro A, Körsten S, Trupp S, et al. On the design of fluorescent ratiometric nanosensors. Chem Eur J. 2010;16:10290–9.
Lee MH, Kim JS, Sessler JL. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev. 2015;44:4185–91.
Xiao HB, Li P, Zhang W, Tang B. An ultrasensitive near-infrared ratiometric fluorescent probe for imaging mitochondrial polarity in live cells and in vivo. Chem Sci. 2016;7:1588–93.
Yuan L, Lin WY, Cao ZM, Wang JL, Chen B. Development of FRET-based dual-excitation ratiometric fluorescent pH probes and their photocaged derivatives. Chem Eur J. 2012;18:1247–55.
Niu WJ, Shan D, Zhu RH, Deng SY, Cosnier S, Zhang XJ. Dumbbell-shaped carbon quantum dots/AuNCs nanohybrid as an efficient ratiometric fluorescent probe for sensing cadmium (II) ions and l-ascorbic acid. Carbon. 2016;96:1034–42.
Rao HB, Liu W, Lu ZW, Wang YY, Ge HW, Zou P, et al. Silica-coated carbon dots conjugated to CdTe quantum dots: a ratiometric fluorescent probe for copper (II). Microchim Acta. 2016;183:581–8.
Yang WQ, Ni JC, Luo F, Weng W, Wei QH, Lin ZY, et al. Cationic carbon dots for modification-free detection of hyaluronidase via an electrostatic-controlled ratiometric fluorescence assay. Anal Chem. 2017;89:8384–90.
Su DD, Wang MK, Liu Q, Chen JY, Su XG. Dual-emission ratio fluorescence detection of bleomycin based on nitrogen doped graphene quantum dots@gold nanoclusters assembly. Sensors Actuators B Chem. 2019;290:163–9.
Zhang LB, Wang EK. Metal nanoclusters: new fluorescent probes for sensors and bioimaging. Nano Today. 2014;9:132–57.
Lu WJ, Gao YF, Jiao Y, Shuang SM, Li CZ, Dong C. Carbon nano-dots as a fluorescent and colorimetric dual-readout probe for the detection of arginine and Cu2+ and its logic gate operation. Nanoscale. 2017;9:11545–52.
Aguilera-Sigalat J, Bradshaw D. Synthesis and applications of metal-organic framework-quantum dot (QD@MOF) composites. Coord Chem Rev. 2016;307:267–91.
Gao Q, Xu SY, Guo C, Chen YG, Wang LY. Embedding nanocluster in MOF via crystalline ion-triggered growth strategy for improved emission and selective sensing. ACS Appl Mater Interfaces. 2018;10:16059–65.
Sun HZ, Cong S, Zheng ZH, Wang Z, Chen ZG, Zhao ZG. Metal–organic frameworks as surface enhanced Raman scattering substrates with high tailorability. J Am Chem Soc. 2019;141:870–8.
Chang JF, Wang X, Wang J, Li HY, Li F. Nucleic acid functionalized metal-organic frameworks-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal Chem. 2019;91:3604–10.
Zheng G, de Marchi S, López-Puente V, Sentosun K, Polavarapu L, Pérez-Juste I, et al. Encapsulation of single plasmonic nanoparticles within ZIF-8 and SERS analysis of the MOF flexibility. Small. 2016;12:3935–43.
Luo YC, Fan SY, Yu WQ, Wu ZL, Cullen DA, Liang CL, et al. Fabrication of Au25 (SG)18-ZIF-8 nanocomposites: a facile strategy to position Au25 (SG)18 nanoclusters inside and outside ZIF-8. Adv Mater. 2018;30:1704576–84.
Ma YJ, Xu GH, Wei FD, Cen Y, Ma YS, Song YY, et al. A dual-emissive fluorescent sensor fabricated by encapsulating quantum dots and carbon dots into metal–organic frameworks for the ratiometric detection of Cu2+ in tap water. J Mater Chem C. 2017;5:8566–71.
Wang J, Chen HY, Ru F, Zhang Z, Mao X, Shan DL, et al. Encapsulation of dual-emitting fluorescent magnetic nanoprobe in metal-organic frameworks for ultrasensitive ratiometric detection of Cu2+. Chem Eur J. 2018;24:3499–505.
Lin XM, Gao GM, Zheng LY, Chi YW, Chen GN. Encapsulation of strongly fluorescent carbon quantum dots in metal–organic frameworks for enhancing chemical sensing. Anal Chem. 2013;86:1223–8.
Kong WH, Wu D, Li GL, Chen XF, Gong PW, Sun ZW, et al. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta. 2017;165:677–84.
Luo ZT, Yuan X, Yu Y, Zhang QB, Leong DT, Lee JY, et al. From aggregation-induced emission of Au (I)-thiolate complexes to ultrabright Au (0)@ Au (I)-thiolate core-shell nanoclusters. J Am Chem Soc. 2012;134:16662–70.
Pan YC, Liu YY, Zeng GF, Zhao L, Lai ZP. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem Commun. 2011;47:2071–3.
Yuan YH, Liu ZX, Li RS, Zou HY, Lin M, Liu H, et al. Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale. 2016;8:6770–6.
Qian H, Zhu M, Wu Z, Jin R. Quantum sized gold nanoclusters with atomic precision. Acc Chem Res. 2012;45:1470–9.
Ding SN, Li CM, Bao N. Off-on phosphorescence assay of heparin via gold nanoclusters modulated with protamine. Biosens Bioelectron. 2015;64:333–7.
Li Z, Zeng HC. Surface and bulk integrations of single-layered Au or Ag nanoparticles onto designated crystal planes {110} or {100} of ZIF-8. Chem Mater. 2013;25:1761–8.
He L, Wang TT, An JP, Li XM, Zhang LY, Li L, et al. Carbon nanodots@ zeolitic imidazolate framework-8 nanoparticles for simultaneous pH-responsive drug delivery and fluorescence imaging. CrystEngComm. 2014;16:3259–63.
Funding
This work was supported by the National Natural Science Foundation of China (21775089, 51862014, 21665010, 21705151), Outstanding Youth Foundation of Shandong Province (ZR2017JL010), the Key Research and Development Program of Jining City (2018ZDGH032), Taishan scholar of Shandong Province, Shandong Provincial Key Laboratory of Clean Production of Fine Chemicals (2019FCCEKL07), and the open foundation of Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University (No. 2018KLMNP06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical committee approval
The human serum sample experiments were performed in strict accordance with the guidelines from the Ethical Committee, Qufu Normal University. All serum samples were obtained from healthy college student volunteers with their informed consent. All studies were approved by the Ethical Committee of Qufu Normal University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 364 kb)
Rights and permissions
About this article
Cite this article
Tan, Q., Zhang, R., Zhang, G. et al. Embedding carbon dots and gold nanoclusters in metal-organic frameworks for ratiometric fluorescence detection of Cu2+. Anal Bioanal Chem 412, 1317–1324 (2020). https://doi.org/10.1007/s00216-019-02353-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02353-5