Abstract
Metal–organic frameworks (MOFs) have emerged as one of the most fascinating libraries of porous materials with a huge potential in very diverse application areas. In particular, the bioanalytical and biomedical fields have evolved tremendously due to the emergence of these hybrid inorganic–organic MOF-based materials. This is because these materials possess a series of key properties essential for bioapplications, such as minimal toxicity to living cells, intrinsic biodegradability, and possibility of synthesizing with nanoscale sizes. Additional properties of MOFs such as ultra-large surface-to-volume ratios, tunable pore size, high drug loading capacity, tunable structure and chemical composition, and potential for multiple postsynthetic modification make them ideal candidates for drug delivery. This review highlights recent research progress on MOF-based drug delivery systems (DDS), pointing out the evolution of these systems toward the development of theranostic nanoplatforms. Rather than a comprehensive review, representative recent examples are selected to illustrate such an evolution, and a critical discussion of the advantages and limitations of the different DDS types is given. Finally, the remaining challenges and future opportunities in this field are presented, highlighting that overcoming the current issues will pave the way toward the elusive dream of “personalized medicine.”

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
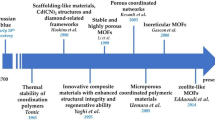
The involvement of metal–organic frameworks (MOFs) in a myriad of diverse fields has brought forward a true revolution, and it is therefore not by chance that MOF-based materials are currently one of the hot topics in the field of hybrid porous solids [1,2,3]. MOFs, also known as porous coordination polymers (PCPs) or coordination networks, are a class of hybrid porous materials formed by the self-assembly of polydentate organic ligands (carboxylates, imidazolates, or phosphonates) and metal-containing nodes (isolated cations, clusters, or chains) linked through coordination bonds and resulting in networks with potential voids [4,5,6,7]. The acronym “MOF” was first introduced by Yaghi and coworkers in 1999 [8], and nowadays, they are a huge family with about 20,000 different structures (as reported in the Cambridge database [7]) and with quite diverse properties. Regardless of the MOF type, all of them present high porosities (with pore diameter up to 9.8 nm; pore volume up to 4.4 cm3 g−1) [7, 9, 10], which is ideal for capture, storage, separation, and/or delivery applications. Owing to the modular nature, the specific physical and chemical properties (e.g., pore size, hydrophilic or hydrophobic nature within the pores or the surface, catalytic sites) of MOF materials can be systematically tuned by judicious selection of building blocks [11], and a variety of functional moieties can be introduced into metal containing nodes, organic ligands, or pore spaces through predesigning or postsynthetic approaches [12,13,14]. It is important to note that the synthetic conditions (e.g., counter ions, solvent, pH, metal–ligand ratio, temperature, and reaction time) also have a strong influence on the structure of the MOFs, as well as on their physicochemical properties [15, 16]. These mutable properties have expanded the versatility of these materials, making them attractive alternatives to the traditional porous materials [1, 17, 18].
While the first applications (named as “classical applications”) of MOFs were mainly focused on gas adsorption (storage and selective separation) [19,20,21], catalysis [1, 22, 23], and sensing [24], the implementation of MOFs in green applications (e.g., solar energy conversion, energy storage, air/water pollution remediation) has aroused much interest and a bright future is expected [25, 26]. Analytical chemistry has also benefited from the potential of MOFs [27, 28], which are involved as sorbent materials in analytical sample preparation methods [29,30,31,32], as stationary phases in analytical separation techniques [31, 33, 34], and as sensors in spectroscopy and/or electroanalytical methods [35, 36]. On the other hand, the development of synthetic methods for the preparation of MOFs at the nanoscale [13, 37] (i.e., nanoscale MOFs, nanosized MOFs, or nMOFs) was a major breakthrough which opened the door to explore their potential in bioapplications. Nowadays, there are numerous synthetic methods for scaling down the MOFs to nanometer sizes [13, 37,38,39,40], but all of them can be grouped into two main approaches: (i) confinement of the self-assembly of metal ions and organic linkers to force the MOF formation at nanoscopic locations, for example by using emulsions, templates, or surfactants; and (ii) favoring nucleation versus crystal growth, for example by using fast precipitation methods, or microwave and ultrasound synthesis. In addition, many kinds of nMOFs have showed low cytotoxicity, which guarantees eventual in vivo applications, and nMOFs are also intrinsically biodegradable in view of the relatively labile metal–ligand bonds [41], making it possible to rapidly degrade and clear away after the intended mission is accomplished. These specific features of the nMOFs have enabled them to serve as platforms for biomedical applications, such as sensing and imaging for diagnostic purposes, and drug delivery for therapeutic applications [40,41,42,43,44,45].
In the field of drug delivery, one of the still remaining challenge is to develop new controllable drug delivery systems (DDS) with enhanced therapeutic efficiency by overcoming the typical associated drawbacks, i.e., low drug loading, rapid biodegradation, instability, low specificity of the treatment, systemic side effects, and toxicities. In spite of the efforts in exploring different host porous materials for drugs, either organic (such as polymeric nanoparticles, liposomes, dendrimers) or inorganic (such as mesoporous silica, zeolites), and each one with its own strengths and drawbacks [46], the ideal candidate for their clinical translation has not been found yet [47]. Even if there are several systems currently under preclinical development or in clinical trials, and four nanosystems already approved by FDA as nanomedicines, i.e., liposomal formulations of doxorubicin (Doxil® or Caelex®), daunorubicin (DaunoXome®), and albumin-bound paclitaxel (Abraxane®), early clinical data demonstrated that while drug encapsulation into these nanocarriers alleviated some dose-limiting toxicities, it did not significantly improve treatment efficacy [48, 49].
To overcome the issue of low efficacy, nMOFs are demonstrating to have a promising future thanks to their “hybrid” organic–inorganic nature, bringing the gap of purely inorganic and organic nanocarriers. They present high and well-defined porosity typical of inorganic porous materials that is a key feature to achieve a controlled release, together with the presence of tunable organic groups within the framework that allows for encapsulating a wide diversity of drugs and large amounts of drug molecules by modulating the drug–framework interactions [17, 46]. Significant progress has been achieved in the development of MOF-based DDS, but most attempts still remain in the proof-of-concept stage. This is because a precise spatiotemporal control and dosage control of the drug release under in vitro and in vivo settings, while maintaining minimal side effects and biocompatibility, is not an easy task to achieve. The recent advances are focused on designing stimuli-responsive MOF-based DDS [50, 51], as well as combining therapeutic and diagnostic capabilities (i.e., theranostics) simultaneously within the same nMOF [45, 52,53,54].
Several excellent reviews related to the potential of MOFs in drug delivery have been published within the period 2017–2018, but each one approached from a different perspective [55,56,57,58,59]. Some reviews discuss the bioapplications of MOFs from a broad perspective, ranging from the synthetic methods and drug-loading strategies [55,56,57], to a wide variety of applications which cover not only drug delivery but also nondrug delivery therapeutics such as photothermal therapy, photodynamic therapy, heavy metal adsorption, and antimicrobial activity, among others [58, 59]. On the other hand, Wuttke et al. addressed the use of MOFs as drug delivery systems by conducting a comprehensive comparison with other nanocarriers such as dendrimers and mesoporous silica nanoparticles [18]. In contrast, this review differs in that it will focus on highlighting the latest progress in the design of MOFs as DDS and proving a rational classification of the MOF-based DDS types depending on the complexity and control level. Rather than presenting a comprehensive overview, the aim of this review is to provide a critical perspective on the role of MOFs as key players in the context of drug delivery and theranostics. For that reason, several key publications within the period 2016–2018 are selected to illustrate the progress in MOF-based DDS and to identify the advantages and limitations of the different proposed DDS types, with the ultimate goal of motivating future research to advance in this field. Thus, it is organized in four sections: first, the key properties of MOFs in the context of drug delivery are briefly presented. Second, the different types of MOF-based DDS developed so far are classified depending on the complexity level, control level, and the stimuli-responsive characteristics, and the strengths and weaknesses of each type are pointed out. In a third section, the significance of MOFs in the development of promising theranostic nanoplatforms is discussed. Finally, the open issues and challenges for the future perspectives of MOF in the field of drug delivery and theranostics are presented.
This review hopefully can stimulate interdisciplinary research and effective collaboration between chemists, pharmacologists, and clinicians, since it may be key to converting research-developed DDS into clinical applications.
Key features of MOFs for drug delivery
The vast majority of MOFs possess a number of unique characteristics which are key requirements for their potential application as DDS, as well as in other bioapplications. There are a few strict requirements that one should keep in mind when it comes to designing a MOF-based material for its application in drug delivery. These key features responsible for its success as DDS are the following:
- 1.
Nanoscale materials. Nowadays, there are diverse synthetic methods for the preparation of MOFs at the nanoscale [13, 37,38,39,40], and these nanosized MOFs (below 200 nm) are superior as DDS due to their higher cellular uptake (internalization rates), longer blood circulation times, and enhanced permeability and retention (EPR) effect to increase the drug concentration at tumor regions [43, 52, 60]. Moreover, the smaller the size (i.e., shorter diffusion paths [61, 62]), the faster the delivery kinetics are.
- 2.
Nontoxic nature. They can be built up from “biologically benevolent” metals and organic linkers. The metals more appropriate due to their low toxicity are Fe, Zn, Zr, Mn, Mg, and Cu, having the following oral lethal dose 50 (LD50): 30 g kg–1 for Fe, 350 μg kg–1 for Zn, 4.1 g kg–1 for Zr, 1.5 g kg–1 for Mn, 8.1 g kg–1 for Mg, and 25 g kg–1 for Cu. As for exogenous organic building blocks, terephthalic acid (LD50 = 5 g kg–1), trimesic acid (LD50 = 8.4 g kg–1), 1-methylimidazole (LD50 = 1.13 g kg–1), and 2-methylimidazole (LD50 = 1.4 g kg–1) are generally used [40, 52]. Moreover some MOFs, termed as bioMOFs, can be prepared from components that are endogenous and, therefore, the body can metabolize them reducing side effects [63]. Another type of bioMOF involves the introduction of a therapeutic molecule directly as an organic constitutive part of the MOF framework itself (i.e., bioactive MOFs) [30]. Even though their individual components are biocompatible and endogenous, and the particle size is below 200 nm, sometimes these materials are still toxic. This is because parameters like cellular uptake, biodistribution, translocation, and excretion from the body are also highly affected by degradation rate, shape, nature of functionalized surface, as well as the use of single or repetitive dose, among others [64, 65].
- 3.
Large surfaces and pore volumes associated with high drug loading capacity. Most of the nanocarriers show poor drug loading (generally less than 5 wt% of the transported drug versus the carrier material), whereas MOFs present much higher loadings. For example, the antiviral drug azidothymidine triphosphate (AZT-TP) was loaded in the MIL-101-NH2 in an extent as high as 42 wt% [52].
- 4.
High, regular, and permanent porosity. The characteristic well-defined porosity of the MOFs together with the presence of organic groups within the framework allows a controlled and progressive release of the drug avoiding the undesirable “burst effect” (i.e., the rapid release of much of the drug) [52, 66].
- 5.
Chemical and structural tunability. The ability to tune their structures and porosities depending on the size and nature of the drug is a significant advantage of MOFs, which allows better drug interactions and higher loadings [52]. Moreover, some MOFs present a high structural flexibility that enables the adaptation of their porosity to the shape of the drug/guest molecule [67].
- 6.
Biodegradability. Most MOFs display intrinsic biodegradability, at least to some degree, upon exposure to aqueous medium because of having relatively labile metal–ligand bonds in the structure [41]. This makes them possible to rapidly degrade after the intended task is completed and be eliminated from the body preventing endogenous accumulation and associated toxicity effects. For example, in the case of MIL-88A, a major degradation occurs after 7 days at 37 °C of in vitro incubation under physiological conditions. Moreover, its degradation products, iron and fumaric acid, are endogenous and showed low toxicity values [52].
- 7.
Versatility. The amenity of both the inner and outer surfaces of MOFs to functionalization (i.e., postsynthetic modification) [68,69,70] is especially relevant for drug delivery. The incorporation of functional groups with different polarities allows to modulate the hydrophilic–hydrophobic internal microenvironment, fine-tuning of pore size, improving the drug interactions with the framework (e.g., hydrophilic MOF pores for drugs with charges opposite to the MOF backbone’s charge), and changing the structure of the solid (interconnectivity, pore size, flexibility) to control diffusion through the porous structure [71]. Moreover, the incorporation of targeting ligands to bind specific receptors and control in vivo fate, and the functionalization with polymers to improve their in vivo stability or even circumvent the immune system are also commonly used strategies [72, 73].
Types of MOF-based drug delivery systems
One of the major challenges of the current nanosystem-based drug delivery that has yet to address is how to achieve controlled drug release to the target site without compromising the therapeutic efficacy and bioavailability of the drug. In this context, MOF-based DDS seems to be a promising approach for addressing this challenge due to their unique properties discussed above.
The past few years has witnessed a substantial rise in the number of nanoscale MOFs with specific features and functionalities and with diverse responsiveness, which is greatly contributing to the progress of the drug delivery field for achieving a controllable drug release. A rational classification of those MOF-based DDS may help us to get a better understanding on the drug release mechanism involved in each case and ultimately improve the design for optimized performance of the DDS. The different systems developed until now can be classified according to different criteria (cf. Fig. 1):
- 1.
Depending on the control over its performance. This allows to classify these DDS into two main groups: non-controllable DDS and controllable ones. The noncontrollable DDS are characterized by their simplicity, but the low control over the dug release limits their use to some very specific applications. Note that even if they are termed as non-controllable systems, some control over the release kinetic is possible by modifying the nature of the MOF as it will be explained later. In contrast, the controllable DDS, known as stimuli-responsive MOF-based DDS or on-demand MOF-based DDS, are endowed with controlled release functions. The accurate design of a controlled release behavior should be based on a full understanding of the relevant stimuli signals and release mechanisms. These DDS can provide spatial and temporal control over drug release, at least to some degree, as well as dosage control in some cases, and the increase in the level of control over the drug release gave rise to different generations of DDS.
- 2.
Depending on the stimulus type. Various stimuli signals, either endogenous (e.g., pH, redox activities, biomolecules, etc.) or exogenous (e.g., light, magnetic/electric field, temperature, ultrasounds, etc.), can be selected to trigger localized drug release and facilitate its therapeutic action. The selection of one or another will depend mainly on the intrinsic properties of the target site (for example the tumor phenotype and anatomical location in the case of cancer therapy), as well as on the specific requirements for the intended use. Note that most of the endogenous stimuli are chemical stimuli, whereas the exogenous ones are generally physical stimuli.
- 3.
Depending on the complexity level. Due to the wide tunability in structural design and multifunctionality potential of MOFs, a high level of complexity can be achieved by using a careful design of the MOF components and further postsynthetic modifications, opening up many possibilities in terms of versatility. Exploiting this, MOF-based DDS can be designed to respond to a single stimulus or to multiple stimuli. Normally, a higher level of complexity implies greater control of the system, although this is not always the case. Thus, instead of complicating the DDS unnecessarily, the rule that should be followed is “to be as simple as possible as long as it meets the necessary requirements.”
Non-controllable MOF-based DDS
The first type of MOF-based DDS was developed by Férey and co-workers in 2006 [46]; they were the first to envisage the potential of MOFs in drug delivery, owing to their considerable loading capacities and controlled release behavior. They synthesized the first two Cr-based MOFs, named MIL-100(Cr) and MIL-101(Cr) [46], and the latter MIL-53(Cr) and its much less toxic analog MIL-53(Fe) [74], all of them exhibiting remarkable loading capacities of ibuprofen as a model drug (up to an unprecedented 1.4 g of drug per gram of porous solid in the case of MIL-101). Studies of the release kinetics in simulated body fluid at 37 °C showed that the complete release of ibuprofen from MIL-100(Cr) and MIL-101(Cr) occurred after 3 and 6 days, respectively, whereas the complete release from MIL-53(Cr) and MIL-53(Fe) took around 21 days. Importantly, these differences demonstrated that the release kinetics could be modified considerably by playing with the pore size and optimizing the drug–framework interactions. Such possibility of having a sustained release during days is owing to the well-defined porosity of the framework, which is a clear advantage of MOFs over other traditional nanocarriers. However, other MOF-based DDS showed a fast release due to the instability of the MOF under specific conditions; this is the case of MIL-100(Fe) and MIL-101(Fe), which disintegrate in phosphate solutions because of the strong interactions between phosphates and iron, and this degradation was well correlated to drug release [75]. Moreover, nanoscale MOFs are more prone to faster degradation because of larger surface areas [76].
This kind of DDS are known as the first generation of MOF-based DDS, in which the system does not respond to any stimulus, and the drug release mechanisms can be merely diffusion, ion exchange, or dissolution. Depending on the specific release mechanism, the release profile will be different. For example, there will be a sustained release over a period of time in the case that the drug is released by merely diffusion or slow degradation, whereas a burst release will be driven by the fast dissolution (i.e., dissociation or disintegration) of the MOF under specific medium conditions. In principle, one could think that a sustained release is desirable as it could lead to a stable blood concentration, as well as a minimization of the toxicity effects. Moreover, the slow release may protect the drug from degradation processes by increasing its plasmatic half-life and bioavailability and, therefore, its efficiency. However, this behavior is not always the desired one. Since the drug efficacy remains the same as long as the drug concentration is between the minimum effective level and the maximum safe concentration, maintaining the constant drug concentration in the blood is not really required. For some drugs, such as insulin, the constant blood level may not be even desired. In this case, insulin has to be delivered at the right time, i.e., when the blood glucose level increases, in an accurate amount just enough to reduce the glucose level in blood. This clearly points out that the desired release profile (i.e., release kinetics) will depend entirely on the intended application, and thus, the MOF type will have to be chosen accordingly. To avoid the premature release issue, that is the leakage of the drug before reaching the target site, coatings of silica [77], lipid bilayers [78, 79], and exosomes [80] onto the MOF surface were reported to enhance the stability of the nMOF under physiological conditions.
Single stimulus-responsive MOF-based DDS
A stimuli-responsive MOF, also termed as smart MOF, is defined as a MOF-based material, including pristine MOF, MOF composite, and their derivative, that can sense a stimulus, such as changes in their local environment or exogenous signals, and respond in a predictable and controlled manner by altering their inherent physical and/or chemical properties. The overall structure may change (dynamic) or remain the same (rigid), and the change in the properties of the MOFs can be reversible (if the change is reversed by stopping the stimulus or by applying an antagonistic stimulus) or irreversible (if the change is permanent and cannot be reversed). The emergence of numerous stimuli-responsive MOF-based DDS gave rise to the second generation of MOF-based DDS. There are two main groups of stimuli: endogenous (e.g., pH, redox activities, biomolecules, etc.) or exogenous (e.g., light, magnetic/electric field, temperature, ultrasounds, etc.). Every stimulus has advantages and disadvantages, as reflected in the selected examples discussed below. Table 1 summarizes the representative examples of smart MOF-based DDS commented along the manuscript.
Endogenous stimuli
These are very suitable when the intrinsic properties of the target site are rather different from the surrounding environment. In the case of cancer, the tremendous intracellular environment differences between the tumor tissues and the normal tissues cause heterogeneities in pH value, redox state, and types and amounts of biomolecules [99]. These natural gradients make internal stimuli an ideal trigger for controlled release and enhanced specificity against tumor cells.
Among the possible endogenous stimuli, pH is one of the most widely investigated for controlled delivery of anticancer drugs because of the acidic tumor extracellular microenvironment and intracellular organelles (endosomes and lysosomes). ZIF-8 is a good candidate for the preparation of a pH-responsive DDS due to its pH sensitivity; ZIF-8 is stable at pH 7.4 and decomposes under acidic conditions, since the coordination between the zinc and imidazolate ions dissociates at pH 5.0–6.0 [100]. As example of these DDS, Zheng et al. reported a one-pot method for the synthesis of nanosized ZIF-8 to encapsulate the anticancer drug doxorubicin (DOX), obtaining a high loading (20 wt%) and a homogeneous distribution of the DOX within the mesopores of the ZIF-8 [81]. Results demonstrated that these DOX@ZIF-8 nanoparticles could be used as an efficient pH-responsive DDS, leading to the DOX release in a controlled manner at low pH (5.0–6.5) due to the instability of ZIF-8 under acidic conditions. The good chemical stability of the ZIF-8 under physiological conditions (PBS, pH 7.4) is advantageous as it avoids the release of the drug in the systemic circulation. Thus, the release only takes place when the system reaches selected cells, such as cancer cells, upon activation by the low pH. Alternatively, DDS can be designed as a core–shell system with ZIF-8 being a pH-sensitive shell [82, 83, 101]. By following this approach, poly(acrylic acid)-Zn nanoparticles were used as a template and further overcoated with a shell of ZIF-8 to give PAA@ZIF-8 nanoparticles [82]. These nanoparticles were able to load a quite higher amount of DOX (1.9 g DOX g−1 NPs) than the previous DDS example, which may be attributed to electrostatic interactions between the negatively charged PAA and positively charged DOX, as well as the coordination interaction of Zn(II)-DOX. Confocal laser scanning microscopy (CLSM) analysis showed that DOX was released in a very slow fashion at pH = 7.4, with the cumulative release of DOX being only about 35% after 60 h. In contrast, a faster drug release rate was observed at pH = 5.5 (76% of DOX after 60 h). Studies in breast cancer cell line MCF-7 revealed the pH-sensitive intracellular behavior of this DDS, observing that the cytotoxicity of PAA@ZIF-8 particles was negligible in the absence of DOX loaded into the particles. More recently, Liang et al. synthesized a protein@ZIF-8 as an efficient DDS for DOX using bovine serum albumin (BSA) [83]. BSA/DOX nanoparticles were first prepared in a one-pot method via a desolvation process, followed by the growth of a ZIF-8 shell. This porous shell ensures the accessibility of water but prevents the leaching of drugs under physiological conditions due to the ultra-small window (3.4 Å). Under acidic conditions, the ZIF-8 was gradually decomposed leading to the release of the DOX, and this DDS presented a much higher efficiency against MCF-7 cells than the use of free DOX.
The significant difference in redox potentials between intracellular and extracellular environments is also commonly used as stimulus for DDS. This difference is even more pronounced in tumor tissues. For example, the concentration of the reducing agent glutathione (GSH) within the intracellular cytosol of tumor cells is about 100-fold higher than in the extracellular environment [102, 103]. The disulfide bond (S−S), a redox-responsive group, is stable in mildly oxidative extracellular milieu while it undergoes rapid cleavage upon exposure to the high levels of intracellular reducing agents such as GSH [104]. Thus, the introduction of disulfide bonds in the construction of the MOF is the most straightforward approach for the preparation of GSH-responsive DDS. Lei et al. were the first to incorporate organic ligands containing disulfide bonds as linkers within the structure of a MOF. Particularly, they prepared MOFs using zirconium (Zr) as metal centers and 4,4′-dithiobisbenzoic acid (4,4′-DTBA) as a GSH-sensitive organic linker ligand [84]. This Zr-DTBA MOF was loaded with the natural polyphenol anticancer drug curcumin (CCM), and the release profiles were studied in the absence and presence of GSH. The disulfide bond in the 4,4′-DTBA was efficiently cleaved by GSH, leading to the MOF framework’s dissociation and the subsequent CCM release. In vitro and in vivo experiments indicated the superior anticancer efficacy of CCM@MOF-Zr(DTBA) over that of free CCM.
By exploiting the presence of specific ions (e.g., phosphate anions) as stimulus to trigger the drug release, an interesting MOF-based DDS was developed by Chen et al. for the oral delivery of insulin, which is the most important protein drug for the treatment of type I diabetes [85]. Advancements in developing an oral insulin delivery agent have been hindered by challenges arising from the instability of insulin in the stomach. However, when using Zr-MOF (NU-1000) as a carrier for oral delivery of insulin, these acid-stable MOF has a 2-fold function: (i) maintain the integrity of insulin in the stomach acid environment and (ii) exclude pepsin (digestive enzyme) from getting access to the insulin and thus limiting its proteolysis. However, under simulated physiological conditions (e.g., in the bloodstream), the insulin is released from NU-1000 owing to the disassembly of this MOF in the presence of phosphate ions, which competitively binds to Zr clusters. This example shows clearly the dual function of the MOF which not only carries the drug to the target site but also protects it from decomposing during transportation.
Adenosine triphosphate (ATP) is a very important complex organic chemical that provides energy to drive many processes in living cells. ATP is present in low concentrations (< 0.4 mM) in the extracellular environment but concentrated in the cytosol (1–10 mM), and it is also overexpressed in cancer cells. Thus, ATP can be used as a trigger for the controlled release of anticancer drugs or other functional cargoes such as proteins [86, 105, 106]. By this approach, Yang et al. developed very recently an ATP-responsive ZIF-90 platform for the delivery of CRISPR/Cas9 (i.e., cytosolic protein and clustered regularly interspaced short palindromic repeats-associated protein 9 [74]). The protein CRISPR/Cas9 could be successfully encapsulated into ZIF-90 without affecting its intrinsic function. A high concentration of intracellular ATP promoted the disassembly of ZIF-90/protein due to the competitive coordination between the metal node of ZIF-90 (Zn+2) and ATP, thus releasing the CRISPR/Ca9. The feasibility of this DDS was demonstrated by knocking out the expression of the green fluorescent protein in HeLa cells as a consequence of the effective release of CRISPR/Cas9. Another demonstration was performed by encapsulating the cytotoxic RNase A into ZIF-90 and observing the inhibition of cancer cell growth. It is interesting to note that this work demonstrates that MOFs are capable not only of carrying proteins into cells, but also transporting them to the cytosol (i.e., efficient endosomal escape) in response to the intracellular environment.
The major disadvantages of these intrinsic stimuli-responsive systems are the low flexibility and low level of control. Temporal control of the drug release is not possible, as this will occur as soon as the DDS reaches a site containing the specific stimulus component. In addition, some of these DDS lack selectivity and, thus, may respond to other non-intended stimulus leading to the drug release in a non-desired location. On the other hand, in case that the release mechanism is the degradation/disassembly of the MOF, the kinetic of the drug release is dictated by the intrinsic instability of the MOF under specific conditions. As demonstrated in the discussed works, the degradation rate can be tuned by playing with the nature and size of the MOF, and therefore, the drug release can vary from hours to days, but an initial delay is not possible. Importantly, the postsynthetic modification of the MOF surface with polymers is an alternative way of achieving prolonged release times. In this manner, MIL-101-Fe MOF particles loaded with ibuprofen were coated with a polyethyleneglycol (PEG) layer for extending the drug release time by controlling the fast pH-mediated MOF degradation in biological buffers [107]. Moreover, the fast degradation of this kind of MOFs in the presence of phosphate ions (in PBS buffer) was overcome because the phosphate anions had to diffuse through the PEG layer prior to reaching the MOF particles, and thus, the burst effect was avoided.
It is important to highlight that the drug release mechanism involved in the above presented examples is the disassembly or degradation of the MOF triggered by a specific stimulus once the DDS reaches the desired location. However, the drug release can also occur through a gate mode mechanism, where “gates” are modified with capping units (e.g., molecular or supramolecular switches or chemically modified MOF) as a “lock” to regulate the flow of the drug molecules, and thus control the drug release. An interesting approach for the design of MOF-based DDS based on this release mechanism is the preparation of MOFs loaded with a drug and further functionalized with stimuli-responsive DNA capping units. These DNA caps could be unlocked by different triggers, such as pH [87], cleavage of the caps by the metal-ion–driven activation of a DNAzyme [87], or formation of ATP–aptamer complexes [88]. These DNA-gated nMOFs were prepared by covalent attachment of the nucleic acids to the nMOF surface via “click-chemistry.” In the presence of the appropriate trigger (pH = 5, metal ions, or ATP) depending on each DDS example, the nMOFs were unlocked, leading to the release of the loaded drug. All these DDS revealed selective cytotoxicity toward MDA-MB-231 breast cancer cells as compared to MCF-10A normal epithelial breast cells, owing to the successful release of DOX.
Exogenous stimuli
Light and magnetic fields are the most commonly used and promising exogenous stimuli due to their particular properties, and for that reason, the selected examples discussed in the following are light- or magnetic-responsive MOF-based DDS. Importantly, exogenous stimuli allow more accurate control over the DDS than endogenous ones, particularly with regard to temporal and dosage control. That is the reason why they have emerged as the third generation of MOF-based DDS. On the one hand, the amount of drug delivered can sometimes be controlled by the intensity/duration of the stimulus. On the other hand, the possibility of stopping the drug release by switching off the stimulus, and activating later the release again (i.e., operating in on/off cycles), is very appealing. Furthermore, these stimuli are independent of the biological environment conditions, enabling a precise and explicit triggering of the drug release and avoiding individual variability. These are the clear advantages of exogenous stimuli-responsive DDS over the endogenous ones. However, they lack the autonomy associated with endogenous stimuli.
Light as an exogenous stimulus for controlled drug delivery is advantageous for a number of reasons including its noninvasive nature, high spatial resolution and temporal control, and convenience and ease of use. Additionally, a broad range of parameters (wavelength, light intensity, duration of exposure, and beam diameter) can be adjusted to modulate release profiles. In particular, near-infrared (NIR) light (650–900 nm) is preferred because it can penetrate deeper than visible light. Moreover, NIR light is innocuous, that is, it does not cause any significant damage in the area of its application. Therefore, NIR light has been successfully used for triggering drug release on demand at well-delimited sites of the body. There are five types of mechanisms for light-triggered drug delivery, but they all have in common materials that absorb electromagnetic radiation and convert it to various forms of energy, which is ultimately responsible for the release [108]. These different mechanisms are as follows: (1) photochemically triggered, where the absorbed light energy is sufficient to break covalent bonds directly or by a photochemical reaction; (2) photoisomerization, where the excess energy causes structural changes; (3) photothermal, where the absorbed photon energy is dissipated via vibrational motion and thus generating heat; (4) two-photon absorption, where two photons are adsorbed and the resulting energy activates a photosensitive material; and (5) photon upconversion, where the absorbed NIR light is converted into high energy UV/visible photons to activate a photosensitive material. Depending on the mechanism involved, some light-responsive DDS are of a single use (i.e., the light triggers an irreversible structural change that provokes the delivery of the entire dose), while others are able to undergo reversible changes when on/off cycles are applied, releasing then the drug in a pulsatile manner.
As an example of the single-use type, Stefaniak et al. developed a light-responsive UiO-type MOF incorporating photoisomerizable azobenzenedicarboxylate (AZB) linkers [89]. Such nanosized UiO-AZB degrades upon irradiation with UV light due to the photoisomerization of the AZB linker, while no degradation is observed in the dark over time [109]. 5-Fluorouracil (5-Fu) was loaded into the MOF, and an aminated polyethylene glycol (PEG-NH2) coating was postsynthetically attached to the MOF surface to increase the aqueous stability and biocompatibility. The irradiation with UV light triggered the degradation of the PEGNH2@5-FuUiO-AZB, leading to the 5-Fu release and the subsequent concentration-dependent cell death. Based on a similar release mechanism, i.e., photoisomerization, but without causing degradation of the MOF, Wang et al. reported a light-responsive DDS consisting of a UiO-68 MOF modified first with azobenzene stalks on the surface and then capped with β-cyclodextrin (β-CD) onto the azobenzene by host–guest interactions [90]. UV light irradiation induced the isomerization of azobenzene from trans to cis, and this structural change promoted the dethreading of the gating β-CD. This in turn exposed MOF pores to the solvent, facilitating thus the cargo release (rhodamine B used as model) from the MOF. A clear drawback of these two DDS is that they require the use of UV light, which is not compatible with living cells, and thus, the application under real conditions for intracellular drug release is hampered.
To overcome this issue, NIR light-responsive nanosized DDS are nowadays preferred and have a brighter future [91, 110, 111]. Most of them are designed as core–shell nanoparticle@MOF structures and the drug release mechanism relies on photothermal effect. In this direction, a plasmonic core–shell nanocomposite comprising gold nanostars coated with ZIF-8 and stabilized with an amphiphilic polymer was developed for light-triggered release of encapsulated cargo inside cells [91]. The postsynthetic functionalization with an amphiphilic polymer prevented ZIF-8 degradation and cargo (bisbenzimide as model) leaking in aqueous media and inside living cells (cf. Fig. 2b). The release mechanism involved here was a photothermal effect, that is, NIR light irradiation coupled to the plasmonic absorption of the core gold nanostars created local temperature gradients and, thus, cargo thermodiffusion. The successful release of the cargo inside the cells was easily confirmed by visualizing the location of the fluorescence from the cargo: first as blue fluorescent dots at the endosomes/lysosomes before release and bright nuclei staining after NIR irradiation as a consequence of the cargo release.
Selected examples of stimuli-responsive MOF-based DDS. a Scheme of ATP-triggered cytosolic protein (CRISPR/Cas9) released from ZIF-90 nanoparticle and genome editing. Adapted with permission from [86]. Copyright 2019, American Chemical Society. b Au nanostar/ZIF-8 nanocomposite (NC) for light-triggered release of encapsulated cargo inside cells. Confocal microscopy images of HeLa cells incubated with the nanocomposite loaded with the Hoechst (cargo molecule) before and after near-IR irradiation are shown. Blue and orange colors represent Hoechst and cell membrane staining (CellMask™ Deep Red), respectively. Adapted with permission from [91]. Copyright 2018, John Wiley & Sons, Inc. c Triply responsive (pH, Ca+2, and temperature) Zr-MOF, which is modified with positively charged A stalks encircled by carboxylatopillar[5]arene (CP[5]A) rings on the surfaces, for on-command release of drug (5-Fu). Adapted with permission from [92]. Copyright 2016, Royal Society of Chemistry. d Scheme of the insulin/GOx-loaded ZIF-8 nMOFs and the pH-induced degradation of the nMOFs through the GOx-catalyzed oxidation of glucose. Adapted with permission from [93]. Copyright 2018, American Chemical Society
The magnetic field represents also an attractive tool for a wide variety of uses in biomedical applications, such as magnetic separation, magnetic targeting, magnetic resonance imaging (MRI), and magnetic hyperthermia [112,113,114]. The major advantage of using a magnetic field as external stimulus is that it has a dual function in DDS: (1) it allows the directivity toward the targeting area (i.e., magnetically guided DDS), which is a unique strategy to concentrate the DDS in tumor sites leading to improved therapeutic efficacy; and (2) it provides a controlled release of drugs through the conversion of magnetic energy into thermal energy. Generally, magnetic field-responsive MOF-based DDS are core–shell systems containing magnetite (Fe3O4) or maghemite (Fe2O3) nanoparticles in the core [115,116,117]. These nanoparticles with an average diameter of about 20 nm exhibit superparamagnetic property and possess considerable saturation magnetization to be directed to specific parts of the body using a weak external magnetic field [118]. Furthermore, these magnetic nanoparticles can generate heat under an alternate current (AC) magnetic field due to the relaxation of its magnetic moment. Interestingly, by modulating the strength of the magnetic field and/or size of the nanoparticles, the amount of heat generated can be controlled. For these reasons, the employment of magnetic nanoparticles to design magnetic field-responsive MOF-based composites with high relaxivity, large drug payload, and good biocompatibility is appealing. More importantly and to exploit the full potential of magnetic MOFs, they are nowadays being widely studied as theranostic systems for MRI-guided therapy as will be discussed in a succeeding section with some recent examples.
Despite the clear advantages of external stimuli-responsive DDS, they also have drawbacks. For instance, the light-responsive modality suffers from shallow penetration, and the magnetic-responsive modality needs a large amount of sample. Therefore, multiple stimuli-responsive modality is being developed to overcome these limitations.
Multiple stimuli-responsive MOF-based DDS
The individual drawbacks of each stimulus, together with the complexity of the human body environment, make that the delivery of drugs or functional cargoes in a precise and accurate manner is not a straightforward task. These issues may be overcome by designing DDS responsive to multiple triggers rather than a single stimulus. Thus, multiple stimuli-responsive MOF-based DDS are being developed to improve the overall performance of DDS and, consequently, its therapeutic efficacy. Table 1 summarizes the representative examples discussed below.
Among the developed multiple-stimuli MOF-based DDS, increasing attention is given recently to the combination of MOFs with supramolecular chemistry for getting a better control of the drug release by exploiting the unique properties of supramolecular materials such as their special structure, facile functionalization, and desirable host–guest performance [90, 119, 120]. MOF surfaces have been successfully functionalized with supramolecular host molecules such as pillararenes and cyclodextrins [92, 121,122,123,124,125]. These macrocycles can act as supramolecular gates or nanovalves, allowing the formation of host–guest complexes in a manner that is sensitive to, or can be governed by, either externally applied triggers, disease-specific analytes, or the presence of a competing guest. Recently, a triply responsive (pH, Ca2+, and temperature) DDS based on Zr-MOFs functionalized with carboxylatopillar[5]arenes CP[5]A as supramolecular switches was developed by Tan et al. [87]. Positively charged quaternary ammonium salt stalks were tethered on UiO-66-NH2 via postsynthetic modification, and then negatively charged CP[5]A was encircled on the stalks sitting on the surface of the MOF through host–guest complexation (cf. Fig. 2c). CP[5]A act as gating nanovalves to control the release of the therapeutic molecule 5-Fu previously loaded within the pores of Zr-MOF. Low pH, increasing temperature, and competitive binding agents could weaken the supramolecular host–guest interactions between the MOF scaffold and the (CP[5]A) gating nanovalves, leading thus to the release of the drug. Controlled release experiments revealed that lower pH and higher concentration of Ca2+ in osteoclasts and tumor cells could stimulate the release of the drug via pH responsiveness and Ca2+-competitive responsiveness, having thus the potential application in bone regeneration and bone cancer therapy. Moreover, high temperatures could also weaken host–guest interactions to prompt gradually drug release.
Within multiple stimuli-responsive DDS, self-regulated systems are a particularly interesting type of MOF-based DDS. They can detect certain changes in biological variables (e.g., pH or concentration of some substances) by activating or modulating the response, that is, by switching drug release on and off or automatically adjusting the release rate. Recently, a smart sense-and-treat MOF carrier was developed by encapsulating both insulin and glucose oxidase into ZIF-8 nanoparticles [93]. Insulin is commonly used in the treatment of type I diabetes because it can lower the blood glucose level; however, insulin may lead to hypoglycemia when not used properly. To overcome this issue, glucose oxidase (GOx) was in this system used as a sensor of glucose, with insulin release being controlled by the concentration of glucose. In the presence of high levels of glucose, GOx converted glucose to gluconic acid and lowered the pH of the local microenvironment (cf. Fig. 2d). Due to the pH sensitivity of ZIF-8, the low pH led to the degradation of ZIF-8, releasing thus the loaded insulin to balance the blood glucose level. On the other hand, the lower blood glucose level balanced by insulin could also balance the pH of the local microenvironment, thus regulating the release of insulin. Therefore, this smart glucose-responsive insulin release displayed the potential to decrease the risk of hypoglycemia.
Multifunctional MOF-based theranostic platforms
Over the years, there has been a gradual paradigm shift from traditional medicine, which has been based on identifying therapies which target an entire population, to the concept of “personalized medicine” [126]. This means a shift from the mindset of “one-drug-fits-all” to “the right drug for the right patient at the right dose and time” [127]. This latter approach relies on tailoring of treatment to the unique molecular or genetic mapping of individual patients and how these unique features contribute to the occurrence of certain disease pattern and progression [128, 129]. In line with this change, the drug delivery field is currently evolving toward the development of theranostic nanosystems, which are able to combine therapy and diagnosis in one integrated system [130,131,132]. Due to the rapid advances in the design of customized MOFs and its tremendous potential of being endowed with multifunctionality, MOF-based theranostic platforms are developing quickly and will surely play a key role in the development of personalized medicine [45, 52]. The integration of favorable biocompatibility, high drug loading ability, active tumor-targeting ability, and imaging-guided smart drug delivery is the key to taking full advantage of nanoscale MOFs for tumor theranostics. These platforms could allow monitoring the drug release, its biodistribution and accumulation at the target site, dose adjustment to individual patients, and eventually, monitoring the course of a disease. Herewith, the potential of MOFs as theranostic platforms is highlighted by means of some selected examples, summarized in Table 1.
The rational structural design of nMOFs for imaging-guided therapy will be different depending on the proper imaging modality for the intended application. For example, the incorporation of paramagnetic metal ions (such as Fe2+ [52], Gd3+ [54], and Mn2+ [94]) in the nMOF or the use of core–shell magnetic nanoparticle@MOF [53, 133] has been proposed as imaging contrast agents for MRI. Otherwise, using luminescent building blocks for the construction of nMOF [134], encapsulating fluorescent organic dyes into the nMOF [98], or designing core–shell luminescent nanoparticle@MOF [135] allows for optical imaging modality.
Zhang et al. have recently developed an effective theranostic system based on a Mn-porphyrin nMOF for MR imaging-guided controllable NO release and photothermal synergetic therapy under a single NIR irradiation [94]. nMOFs were prepared with biocompatible Zr+4 ions and Mn-porphyrin as a bridging ligand, and further functionalized with S-nitrosothiol (SNO) on the surface for heat-sensitive NO generation. The insertion of paramagnetic Mn ions into porphyrin rings provides strong T1-weighted MR contrast capacity and high photothermal conversion for efficient photothermal therapy (PTT) without increasing their complexity. Irradiation with NIR light triggered the controllable NO release for NO therapy and PTT simultaneously with one-step operation. The system was validated with cells and mice as models, confirming the efficiency of in vitro and in vivo NO and photothermal synergistic therapy. However, NO treatment is still not well understood, and thus, the biological effects of NO therapy should be evaluated with further research.
By taking advantage of nanoparticle@MOF (NP@MOF) core–shell structures, which encompass the benefits of the porous structure of the MOF and the unique properties of NPs, Deng et al. developed a core–shell NP@MOF platform for fluorescent imaging-guided therapy, allowing simultaneous targeted drug delivery and cell imaging of cancer cells [95]. This theranostic platform consisted of up-conversion luminescent NaYF4:Yb3+/Er3+ nanoparticle (UCNP) core, which could emit strong green emission under 980 nm laser, and mesoporous MIL-100(Fe) as shell for loading the anticancer drug DOX. Moreover, the AS1411 aptamer was covalently conjugated to the MOF surface for targeting cancer cells and enhancing intracellular uptake, since it can bind to nucleolin (a receptor for AS1411 and overexpressed on the plasma membrane of tumor cells). The UCNPs@MOF-DOX-AS1411 exhibited a pH-sensitive release of the DOX that lasted as long as 35 days. These nanocomposites could specifically recognize cancer cells, go across the cell membrane by receptor-mediated endocytosis, exhibit up-conversion fluorescence imaging under 980 nm laser, and ultimately release DOX leading to high cytotoxicity to MCF-7 cells. Thus, this platform showed great promise for the simultaneous targeted labeling and therapy of cancer cells.
Exploiting not only the benefits of NP@MOF structures but also the potential of supramolecular chemistry discussed above, Wu et al. reported the construction of a theranostic core–shell nanoplatform comprising of Fe3O4 magnetic nanoparticles as core, UiO-66 MOF as shell allowing a high loading of the drug (5-Fu), and CP[6]A-based pseudorotaxanes (WP6) anchored on the surface as the gatekeepers [96]. Remarkably, this system, 5-Fu-loaded Fe3O4@UiO-66@WP6, integrated multifunctions including tumor microenvironment-responsive drug release (triple responsiveness toward pH, temperature, and competitive binding agents), effective chemotherapy, sustained release ascribed to the tight host–guest interactions, and superior abilities of MRI, which eventually resulted in an enhanced in vitro therapeutic effect. Importantly, they also demonstrated the relevance of the proper selection of the macrocyclic molecules as gatekeepers or nanovalves in the precise control of the drug release. Compared with the carboxylatopillar[5]arene (WP5)-based nanovalve system with less gate tightness as a control, the WP6-based one exhibited a prolonged drug release owing to the stronger binding capacity of WP6 toward the stalks on the MOF surface.
Although optical and magnetic resonance imaging are the imaging modalities most commonly used, there are a few examples of theranostic MOF-based platform based on other imaging techniques such as photoacoustic (PA) imaging, which is a noninvasive imaging modality that detects the pressure wave caused by the photoacoustic effect. Wang et al. combined the merits of nMOF and polydopamine (PDA) to design a multifunctional nanoplatform for photoacoustic imaging (PAI)-guided chemo-/photothermal combinational tumor therapy [97]. MIL-100 MOF was utilized to load curcumin as chemotherapeutic drug and was further coated with polydopamine-modified hyaluronic acid (HA-PDA) with the aim of improving the stability in the systemic circulation in vivo of nMOFs, as well as providing tumor-targeting ability. Moreover, PDA was selected due to the already proven high photothermal conversion efficiency and also its potential as a PA contrast agent. The good efficiency of the curcumin-loaded MIL-100@HA-PDA nanoplatform was demonstrated in vitro and in vivo. After being intravenously injected into xenograft HeLa tumor-bearing mice, the nanoplatform was preferably accumulated at the tumor site and achieved photoacoustic imaging-guided chemo-/photothermal tumor therapy, generating nearly complete tumor ablation.
Taking into account that each imaging modality has its advantages and limitations (e.g., high sensitivity but poor resolution and shallow tissue penetration in the case of optical imaging, whereas MRI possesses high resolution and good soft tissue contrast but low sensitivity) [136], the development of nMOFs endowed with dual MR/optical imaging potential is desirable for the ultimate goal of in vivo application [98, 137,138,139]. Going a step further, the possibility of integrating three imaging modalities (fluorescence, MR, and PA) together with PTT of cancer under NIR laser irradiation into a single MOF-based platform has been recently demonstrated [98]. Cai et al. developed a multifunctional ICG-engineered MOF coated with hyaluronic acid (HA) as nanotheranostic agent for anticancer treatment (cf. Fig. 3). The incorporation of photoresponsive indocyanine green (ICG; the only NIR organic dye for clinical applications approved by the FDA) into the MOF MIL-100(Fe) enhanced ICG’s tumor accumulation and endowed the MOF with strong NIR fluorescence, which allowed not only for optical imaging but also for PA imaging. Fe3+ ions in the MOF NPs were used for T2-weighted MRI, and the functionalization of the MOF surface with HA mediated the targeting recognition of CD44 overexpressing cancer cells. Importantly, this MOF@HA@ICG nanoplatform exhibited good NIR absorbance, low cytotoxicity, great cellular uptake in CD44-positive MCF-7 cancer cells and tumor accumulation in xenograft tumors, and good in vitro and in vivo capabilities in fluorescence imaging, PAI, T2-weighted MRI, and PTT treatment under NIR irradiation. Significant MCF-7 cell death in vitro and efficient suppression of MCF-7 tumor growth in vivo were observed. Therefore, this theranostic MOF@HA@ICG nanoplatform is very promising for imaging-guided PTT cancer therapy of solid tumors, contributing significantly toward the clinical translation of MOF-based hybrid nanocomposites.
a Schematic representation of the preparation of the theranostic MIL-100(Fe) MOF@HA@ICG nanoplatform and its multimodal imaging-guided PTT performance. b FL images of MCF-7 tumor-bearing mice injected with free ICG, MOF@ICG, or MOF@HA@ICG solutions (each with 170 μg/mL of ICG). c In vivo PA images. d T2-weighted MR images with MOF@HA@ICG treatment. The tumors are highlighted by red circles. Adapted with permission from [98]. Copyright 2016, American Chemical Society
Challenges and perspectives
Despite the rapid and incredible progress made in recent years, further research studies are required to fully understand the interactions and mechanism involved in those MOF-based nanoplatforms, and thus, be able to exploit the entire potential of these materials in drug delivery and specially in theranostics. Researchers will have to address the following issues and challenges before the translation of these MOF-based nanoplatforms into clinical practice in the future.
- 1.
Refinement of the MOF design and synthetic methods. More precise optimization of the MOF-based material in terms of size, morphology, surface properties, and incorporation of additional functionalities should be made in order to ensure the prolonged blood circulation, stability under physiological condition at least until reaching the target site, controllable cargo release, enhanced cell uptake, and selective targeting after systemic in vivo administration. In spite of the great advances in engineering the MOF surface (e.g., silica and polymer coatings), many MOF types have still limited stability in aqueous and buffer solutions which hampers their application as DDS. Importantly, the administration route of the DSS, which will depend on the intended application, must be taken into account for the proper selection of the desirable physicochemical properties and, thus, design the MOF accordingly. For example, the parenteral route requires particles with size less than 200 nm to freely circulate in the smallest capillaries [43]. Regarding the MOF preparation, the synthesis of MOFs is largely performed under solvothermal conditions in high boiling point and polar solvents such as dimethylformamide and diethylformamide [140]. Faced with growing environmental issues and the increasing demand for sustainable products and processes, the development of greener methods (i.e., reducing waste production, minimizing the use of organic solvents, reducing the use of hazardous reagents or by-products, maximizing yields, etc.) is of utmost importance [141,142,143]; however, this is a major challenge for synthetic material chemists. Nowadays, there are significant research efforts dedicated to finding suitable replacement solvents for the synthesis of MOFs [144,145,146]. The fact that some MOFs are already commercially available has been probably a driving force for the emerging interest in upscaling of MOF synthesis and the adjustment of synthetic conditions toward sustainable industrial processes [143]. On the other hand, the formulation of MOFs in a suitable form (patches, pellets, tablets, creams, etc) is also an important aspect, not studied much to date, that will depend strongly on the administration route (oral, intravenous, intranasal, cutaneous, etc.) [42, 43, 147, 148]. More research in this direction is needed for the practical application of MOFs.
- 2.
Simplicity and reproducibility of preparation. There is a generalized trend of designing increasingly sophisticated and complex DDS, having long synthetic procedures and sometimes high cost preparation because of the complicated organic ligands involved. On the one hand, the complexity of the preparation procedure may lead to lack of reproducibility from batch to batch, which ultimately results in differences in the DDS performance in vitro and in vivo. On the other hand, the high cost of complicated organic ligands will finally restrict the practical application of these DDS. Therefore, the golden rule to follow should be “the simpler, the better,” as long as the DDS meets the essential requirements for the intended application.
- 3.
Biosafety. This is a major concern for the clinical application of nanomedicine; therefore, more systematic in vitro and in vivo studies for investigating the toxicity of MOF-based nanomaterials are mandatory. Currently, only very few studies on the in vivo toxicity of MOFs have been performed [149,150,151,152]. Careful selection of the inorganic and organic building blocks for constructing the MOF is the first step in ensuring biosafety. They must be nontoxic or low toxic to ensure the safety of decomposition products in the body and further to process through the body’s metabolic system. The controlled biodegradability of the MOF, that is its decomposition at a desired region, is recommended to avoid endogenous accumulation. Furthermore, the “ADME” (“absorption–distribution–metabolism–excretion”) mechanism of MOF-based DDS needs to be fully evaluated after in vivo administration. It is worth mentioning that small animal models commonly used for in vivo studies have not been a good predictor of the drug efficacy and DDS performance in humans. Therefore, innovative in vitro models should be developed to accurately predict the in vivo pharmacokinetic profiles in humans. This will mean a huge step toward the successful clinical translation of these DDS.
- 4.
Regulatory issues. Ultimately, any MOFs destined for the clinic must first meet stringent safety and efficacy standards set by regulatory agencies, such as the Food and Drug Administration (FDA) in the USA, before being approved for use on patients. However, conducting clinical trials is expensive, and pharmaceutical companies and drug manufacturers usually prefer spending their legal and lobbying resources on modifications of existing technology (already earned FDA approval) instead of novel therapies. Therefore, a change of mindset is of utmost importance for the future of MOFs and whichever nanomaterial in biomedicine. Currently, there is only one MOF-based system for the treatment of cancer under a phase 1 clinical trial (NCT03444714). This MOF system, developed by Lin’s group at the University of Chicago and further exploited by his company RiMO Therapeutics, enables synergistic radiotherapy–radiodynamic therapy and immunotherapy using extremely low doses of X-rays [153]. On the other hand, Morris’s group is embedding nitric oxide (NO)-filled MOFs in bandages to promote wound healing and coating stents and catheters with the material to prevent blood clots and reduce infection risk [154]. His spin-off company (MOFgen) is currently raising funds to registering NO-filled MOFs for use in medical devices.
These weakness and open issues point out the opportunities and challenges for the future perspectives in the progress of MOF-based materials as DDS and theranostic platforms. By resolving these issues, researchers will yield significant advances paving the way toward personalized medicine.
Conclusions
The development of MOF-based nanomaterials as drug nanocarriers and theranostic systems has attracted much attention from the scientific community in the last decade (2008−2018). This is mainly due to their unique properties such as large surface area, high drug loading capacity, tailorable composition and structure, versatile functionality, and biodegradability, which are far superior to those of any other nanocarrier material. The examples discussed along the review are illustrative of the continually expanding interest and bright future for MOFs in this field. However, more effort should be devoted to the development of MOF-based DDS with better therapeutic efficacy not only for cancer but also for other diseases. Most importantly, more systematic investigations on their toxicity after long-term use and in vivo studies with these systems should be conducted to evaluate possible side effects before their clinical translation.
Going forward, research should focus on the development of multimodal MOF-based theranostic nanoplatforms combined with different mechanisms to benefit from the full potential of this relatively new class of porous materials. Although theranostic MOFs for clinical applications are still a long-standing challenge, great achievements have been made in such a short period of time.
Finally, it must keep in mind that developing a MOF-based DDS or theranostic MOF-based platform from the structural design to the preclinical evaluation is an interdisciplinary task, and thus, the involvement of chemists, pharmacologists, and clinicians at an early stage would be the most appropriate way to eventually achieve success in translating research outcome into clinical practice.
References
Férey G. Hybrid porous solids: past, present, future. Chem Soc Rev. 2008;37(1):191–214.
Férey G. Some suggested perspectives for multifunctional hybrid porous solids. Dalton Trans. 2009;23:4400–15.
Maurin G, Serre C, Cooper A, Férey G. The new age of MOFs and of their porous-related solids. Chem Soc Rev. 2017;46(11):3104–7.
Zhou H-C, Long JR, Yaghi OM. Introduction to metal–organic frameworks. Chem Rev. 2012;112(2):673–4.
Kitagawa S. Metal–organic frameworks (MOFs). Chem Soc Rev. 2014;43(16):5415–8.
Batten SR, Champness NR, Chen XM, Garcia-Martinez J, Kitagawa S, Öhrström L, et al. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl Chem. 2013;85(8):1715–24.
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341(6149):1230444.
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature. 1999;402(6759):276–9.
Park YK, Choi SB, Kim H, Kim K, Won BH, Choi K, et al. Crystal structure and guest uptake of a mesoporous metal–organic framework containing cages of 3.9 and 4.7 nm in diameter. Angew Chem. 2007;46(43):8230–3.
Deng H, Grunder S, Cordova KE, Valente C, Furukawa H, Hmadeh M, et al. Large-pore apertures in a series of metal-organic frameworks. Science. 2012;336(6084):1018–23.
Spokoyny AM, Kim D, Sumrein A, Mirkin CA. Infinite coordination polymer nano- and microparticle structures. Chem Soc Rev. 2009;38(5):1218–27.
Tanabe KK, Cohen SM. Postsynthetic modification of metal–organic frameworks—a progress report. Chem Soc Rev. 2011;40(2):498–519.
Wang S, McGuirk CM, d’Aquino A, Mason JA, Mirkin CA. Metal–organic framework nanoparticles. Adv Mater. 2018;30(37):1800202.
Yin Z, Wan S, Yang J, Kurmoo M, Zeng M-H. Recent advances in post-synthetic modification of metal–organic frameworks: new types and tandem reactions. Coord Chem Rev. 2019;378:500–12.
Stock N, Biswas S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev. 2011;112(2):933–69.
Liu B, Vellingiri K, Jo S-H, Kumar P, Ok YS, Kim K-H. Recent advances in controlled modification of the size and morphology of metal-organic frameworks. Nano Res. 2018;11(9):4441–67.
Baeza A, Ruiz-Molina D, Vallet-Regí M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin Drug Deliv. 2017;14(6):783–96.
Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ. Positioning metal-organic framework nanoparticles within the context of drug delivery—a comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials. 2017;123:172–83.
Dincă M, Long JR. Hydrogen storage in microporous metal–organic frameworks with exposed metal sites. Angew Chem. 2008;47(36):6766–79.
Li J-R, Kuppler RJ, Zhou H-C. Selective gas adsorption and separation in metal–organic frameworks. Chem Soc Rev. 2009;38(5):1477–504.
Li B, Wen H-M, Zhou W, Chen B. Porous metal–organic frameworks for gas storage and separation: what, how, and why? J Phys Chem Lett. 2014;5(20):3468–79.
Kuppler RJ, Timmons DJ, Fang Q-R, Li J-R, Makal TA, Young MD, et al. Potential applications of metal-organic frameworks. Coord Chem Rev. 2009;253(23-24):3042–66.
Li G, Zhao S, Zhang Y, Tang Z. Metal–organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: recent progress and perspectives. Adv Mater. 2018;30(51):1800702.
Kumar P, Deep A, Kim K-H. Metal organic frameworks for sensing applications. Trends Anal Chem. 2015;73:39–53.
Ajoyan Z, Marino P, Howarth AJ. (2018). Green applications of metal–organic frameworks. CrystEngComm. 2018;20(39):5899–912.
Wang B, Xie LH, Wang X, Liu XM, Li J, Li JR. Applications of metal–organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal. Green Energy & Environment. 2018;3(3):191–228.
Gu ZY, Yang CX, Chang NA, Yan XP. Metal–organic frameworks for analytical chemistry: from sample collection to chromatographic separation. Acc Chem Res. 2012;45(5):734–45.
Rocío-Bautista P, Taima-Mancera I, Pasán J, Pino V. Metal-organic frameworks in green analytical chemistry. Separations. 2019;6(3):33.
Rocío-Bautista P, Pacheco-Fernández I, Pasán J, Pino V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—a review. Anal Chim Acta. 2016;939:26–41.
Rocío-Bautista P, González-Hernández P, Pino V, Pasán J, Afonso AM. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. Trends Anal Chem. 2017;90:114–34.
Wang X, Ye N. Recent advances in metal-organic frameworks and covalent organic frameworks for sample preparation and chromatographic analysis. Electrophoresis. 2017;38:3059–78.
Yang SS, Shi MY, Tao ZR. Wang, C, Gu ZY. (2019). Recent applications of metal–organic frameworks in matrix-assisted laser desorption/ionization mass spectrometry. Anal. Bioanal. Chem. 2019;411(19):4509–22.
Pacheco-Fernández I, González-Hernández P, Pasán J, Ayala JH, Pino V. The rise of metal-organic frameworks in analytical chemistry. In: De la Guardia M, Esteve-Turrillas FA, editors. Handbook of smart materials in analytical chemistry. Hoboken: Wiley; 2016. p. 463–502.
Zhang J, Chen Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J Chromatogr A. 2017;1530:1–18.
Li A, Liu X, Chai H, Huang Y. Recent advances in the construction and analytical applications of metal-organic frameworks-based nanozymes. Trends Anal Chem. 2018;105:391–403.
Yadav DK, Ganesan V, Marken F, Gupta R, Sonkar PK. Metal@MOF materials in electroanalysis: silver-enhanced oxidation reactivity towards nitrophenols adsorbed into a zinc metal organic framework—Ag@MOF-5(Zn). Electrochim Acta. 2016;219:482–91.
Lin W, Rieter WJ, Taylor KM. Modular synthesis of functional nanoscale coordination polymers. Angew Chem. 2009;48(4):650–8.
Carne A, Carbonell C, Imaz I, Maspoch D. Nanoscale metal–organic materials. Chem Soc Rev. 2011;40(1):291–305.
He C, Liu D, Lin W. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem Rev. 2015;115(19):11079–108.
Giménez-Marqués M, Hidalgo T, Serre C, Horcajada P. Nanostructured metal–organic frameworks and their bio-related applications. Coord Chem Rev. 2016;307:342–60.
Morris RE, Brammer L. Coordination change, lability and hemilability in metal–organic frameworks. Chem Soc Rev. 2017;46(17):5444–62.
McKinlay AC, Morris RE, Horcajada P, Férey G, Gref R, Couvreur P, et al. BioMOFs: metal–organic frameworks for biological and medical applications. Angew Chem. 2010;49(36):6260–6.
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, et al. Metal–organic frameworks in biomedicine. Chem Rev. 2012;112(2):1232–68.
Keskin S, Kızılel S. Biomedical applications of metal organic frameworks. Ind Eng Chem Res. 2011;50(4):1799–812.
Cai W, Chu CC, Liu G, Wáng YXJ. Metal–organic framework-based nanomedicine platforms for drug delivery and molecular imaging. Small. 2015;11(37):4806–22.
Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G. Metal–organic frameworks as efficient materials for drug delivery. Angew Chem. 2006;45(36):5974–8.
Hua S, De Matos MB, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790.
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445–51.
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9.
Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal–organic framework-based stimuli-responsive systems for drug delivery. Adv Sci. 2019;6(1):1801526.
Feng J, Xu Z, Dong P, Yu W, Liu F, Jiang Q, et al. Stimuli-responsive multifunctional metal–organic framework nanoparticles for enhanced chemo-photothermal therapy. J Mater Chem B. 2019;7(6):994–1004.
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9(2):172.
Zhao H-X, Zou Q, Sun S-K, Yu C, Zhang X, Li R-J, et al. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem Sci. 2016;7(8):5294–301.
Zhang H, Shang Y, Li Y-H, Sun S-K, Yin X-B. Smart metal–organic framework-based nanoplatforms for imaging-guided precise chemotherapy. ACS Appl Mater Interfaces. 2019;11(2):1886–95.
Lu K, Aung T, Guo N, Weichselbaum R, Lin W. Nanoscale metal–organic frameworks for therapeutic, imaging, and sensing applications. Adv Mater. 2018;30(37):1707634.
Wang L, Zheng M, Xie Z. Nanoscale metal–organic frameworks for drug delivery: a conventional platform with new promise. J Mater Chem B. 2018;6(5):707–17.
Ibrahim M, Sabouni R, Husseini A, G. Anti-cancer drug delivery using metal organic frameworks (MOFs). Curr Med Chem. 2017;24(2):193–214.
Chedid G, Yassin A. Recent trends in covalent and metal organic frameworks for biomedical applications. Nanomaterials. 2018;8(11):916.
Liu Y, Zhao Y, Chen X. Bioengineering of metal-organic frameworks for nanomedicine. Theranostics. 2019;9(11):3122–33.
Della Rocca J, Liu D, Lin W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44(10):957–68.
Diring S, Carné-Sánchez A, Zhang J, Ikemura S, Kim C, Inaba H, et al. Light responsive metal–organic frameworks as controllable CO-releasing cell culture substrates. Chem Sci. 2017;8(3):2381–6.
Gao W-Y, Cardenal AD, Wang C-H, Powers DC. In operando analysis of diffusion in porous metal-organic framework catalysts. Chem Eur J. 2019;25(14):3465–76.
An J, Geib SJ, Rosi NL. Cation-triggered drug release from a porous zinc–adeninate metal–organic framework. J Am Chem Soc. 2009;131(24):8376–7.
Sajid M. Toxicity of nanoscale metal organic frameworks: a perspective. Environ Sci Pollut Res. 2016;23(15):14805–7.
Orellana-Tavra C, Haddad S, Marshall RJ, Abánades Lázaro I, Boix G, Imaz I, et al. Tuning the endocytosis mechanism of Zr-based metal–organic frameworks through linker functionalization. ACS Appl Mater Interfaces. 2017;9(41):35516–25.
Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2-3):121–36.
Férey G, Serre C. Large breathing effects in three-dimensional porous hybrid matter: facts, analyses, rules and consequences. Chem Soc Rev. 2009;38(5):1380–99.
Cohen SM. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem Rev. 2011;112(2):970–1000.
Cohen SM. The postsynthetic renaissance in porous solids. J Am Chem Soc. 2017;139(8):2855–63.
Islamoglu T, Goswami S, Li Z, Howarth AJ, Farha OK, Hupp JT. Postsynthetic tuning of metal–organic frameworks for targeted applications. Acc Chem Res. 2017;50(4):805–13.
Deria P, Mondloch JE, Karagiaridi O, Bury W, Hupp JT, Farha OK. Beyond post-synthesis modification: evolution of metal–organic frameworks via building block replacement. Chem Soc Rev. 2014;43(16):5896–912.
Bellido E, Hidalgo T, Lozano MV, Guillevic M, Simón-Vázquez R, Santander-Ortega MJ, et al. Heparin-engineered mesoporous iron metal-organic framework nanoparticles: toward stealth drug nanocarriers. Adv Healthc Mater. 2015;4(8):1246–57.
Ning W, Di Z, Yu Y, Zeng P, Di C, Chen D, et al. Imparting designer biorecognition functionality to metal–organic frameworks by a DNA-mediated surface engineering strategy. Small. 2018;14(11):1703812.
Horcajada P, Serre C, Maurin G, Ramsahye NA, Balas F, Vallet-Regi M, et al. Flexible porous metal-organic frameworks for a controlled drug delivery. J Am Chem Soc. 2008;130(21):6774–80.
Li X, Lachmanski L, Safi S, Sene S, Serre C, Grenèche J-M, et al. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci Rep. 2017;7(1):13142.
Agostoni V, Horcajada P, Noiray M, Malanga M, Aykaç A, Jicsinszky L, et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci Rep. 2015;5:7925.
Taylor-Pashow KM, Della Rocca J, Xie Z, Tran S, Lin W. Postsynthetic modifications of iron-carboxylate nanoscale metal–organic frameworks for imaging and drug delivery. J Am Chem Soc. 2009;131(40):14261–3.
Wuttke S, Braig S, Preiß T, Zimpel A, Sicklinger J, Bellomo C, et al. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem Commun. 2015;51(87):15752–5.
Yang J, Chen X, Li Y, Zhuang Q, Liu P, Gu J. Zr-based MOFs shielded with phospholipid bilayers: improved biostability and cell uptake for biological applications. Chem Mater. 2017;29(10):4580–9.
Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Exosome-coated metal–organic framework nanoparticles: an efficient drug delivery platform. Chem Mater. 2017;29(19):8042–6.
Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nyström AM, et al. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138(3):962–8.
Ren H, Zhang L, An J, Wang T, Li L, Si X, et al. Polyacrylic acid@ zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem Commun. 2014;50(8):1000–2.
Liang Z, Yang Z, Yuan H, Wang C, Qi J, Liu K, et al. A protein@ metal–organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018;47(30):10223–8.
Lei B, Wang M, Jiang Z, Qi W, Su R, He Z. Constructing redox-responsive metal–organic framework nanocarriers for anticancer drug delivery. ACS Appl Mater Interfaces. 2018;10(19):16698–706.
Chen Y, Li P, Modica JA, Drout RJ, Farha OK. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: protein encapsulation, protection, and release. J Am Chem Soc. 2018;140(17):5678–81.
Yang X, Tang Q, Jiang Y, Zhang M, Wang M, Mao L. Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J Am Chem Soc. 2019;141(9):3782–6.
Chen W-H, Yu X, Cecconello A, Sohn YS, Nechushtai R, Willner I. Stimuli-responsive nucleic acid-functionalized metal–organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem Sci. 2017;8(8):5769–80.
Chen WH, Yu X, Liao WC, Sohn YS, Cecconello A, Kozell A, et al. ATP-responsive aptamer-based metal–organic framework nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv Funct Mater. 2017;27(37):1702102.
Roth Stefaniak K, Epley CC, Novak JJ, McAndrew ML, Cornell HD, Zhu J, et al. Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle. Chem Commun. 2018;54(55):7617–20.
Meng X, Gui B, Yuan D, Zeller M, Wang C. Mechanized azobenzene-functionalized zirconium metal-organic framework for on-command cargo release. Sci Adv. 2016;2(8):e1600480.
Carrillo-Carrión C, Martínez R, Navarro Poupard MF, Pelaz B, Polo E, Arenas-Vivo A, et al. Aqueous stable gold nanostar/ZIF-8 nanocomposites for light-triggered release of active cargo inside living cells. Angew Chem. 2019;58(21):7078–82.
Tan L-L, Song N, Zhang SX-A, Li H, Wang B, Yang Y-W. Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J Mater Chem B. 2016;4(1):135–40.
Chen W-H, Luo G-F, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R, et al. Glucose-responsive metal–organic-framework nanoparticles act as “smart” sense-and-treat carriers. ACS Nano. 2018;12(8):7538–45.
Zhang H, Tian X-T, Shang Y, Li Y-H, Yin X-B. Theranostic Mn-porphyrin metal–organic frameworks for magnetic resonance imaging-guided nitric oxide and photothermal synergistic therapy. ACS Appl Mater Interfaces. 2018;10(34):28390–8.
Deng K, Hou Z, Li X, Li C, Zhang Y, Deng X, et al. Aptamer-mediated up-conversion core/MOF shell nanocomposites for targeted drug delivery and cell imaging. Sci Rep. 2015;5:7851.
Wu M-X, Gao J, Wang F, Yang J, Song N, Jin X, et al. Multistimuli responsive core–shell nanoplatform constructed from Fe3O4@MOF equipped with pillar[6]arene nanovalves. Small. 2018;14(17):1704440.
Zhang Y, Wang L, Liu L, Lin L, Liu F, Xie Z, et al. Engineering metal–organic frameworks for photoacoustic imaging-guided chemo-/photothermal combinational tumor therapy. ACS Appl Mater Interfaces. 2018;10(48):41035–45.
Cai W, Gao H, Chu C, Wang X, Wang J, Zhang P, et al. Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl Mater Interfaces. 2017;9(3):2040–51.
Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46(12):3830–52.
Zhuang J, Kuo C-H, Chou L-Y, Liu D-Y, Weerapana E, Tsung C-K. Optimized metal–organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–9.
Jia X, Yang Z, Wang Y, Chen Y, Yuan H, Chen H, et al. Hollow mesoporous silica@ metal–organic framework and applications for pH-responsive drug delivery. ChemMedChem. 2018;13(5):400–5.
Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92.
Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–98.
Xiao D, Jia H-Z, Ma N, Zhuo R-X, Zhang X-Z. A redox-responsive mesoporous silica nanoparticle capped with amphiphilic peptides by self-assembly for cancer targeting drug delivery. Nanoscale. 2015;7(22):10071–7.
Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364.
Sun W, Gu Z. ATP-responsive drug delivery systems. Expert Opin Drug Deliv. 2016;13(3):311–4.
Gupta V, Tyagi S, Paul A. Development of biocompatible iron-carboxylate metal organic frameworks for pH-responsive drug delivery application. J Nanosci Nanotechnol. 2019;19(2):646–54.
Rwei AY, Wang W, Kohane DS. Photoresponsive nanoparticles for drug delivery. Nano Today. 2015;10(4):451–67.
Epley CC, Roth KL, Lin S, Ahrenholtz SR, Grove TZ, Morris AJ. Cargo delivery on demand from photodegradable MOF nano-cages. Dalton Trans. 2017;46(15):4917–22.
Tian Z, Yao X, Ma K, Niu X, Grothe J, Xu Q, et al. Metal–organic framework/graphene quantum dot nanoparticles used for synergistic chemo- and photothermal therapy. ACS Omega. 2017;2(3):1249–58.
Zeng J-Y, Zhang M-K, Peng M-Y, Gong D, Zhang X-Z. Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic/photothermal/chemotherapy of tumor. Adv Funct Mater. 2018;28(8):1705451.
Grillone A, Ciofani G. Magnetic nanotransducers in biomedicine. Chem Eur J. 2017;23(64):16109–14.
Adedoyin AA, Ekenseair AK. Biomedical applications of magneto-responsive scaffolds. Nano Res. 2018;11(10):5049–64.
Sahle FF, Gulfam M, Lowe TL. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov Today. 2018;23(5):992–1006.
Wu Y-n, Zhou M, Li S, Li Z, Li J, Wu B, et al. Magnetic metal–organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small. 2014;10(14):2927–36.
Wang D, Zhou J, Chen R, Shi R, Xia G, Zhou S, et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials. 2016;107:88–101.
Li S, Bi K, Xiao L, Shi X. Facile preparation of magnetic metal organic frameworks core–shell nanoparticles for stimuli-responsive drug carrier. Nanotechnology. 2017;28(49):495601.
Kolosnjaj-Tabi J, Di Corato R, Lartigue L, Marangon I, Guardia P, Silva AKA, et al. Heat-generating iron oxide nanocubes: subtle “destructurators” of the tumoral microenvironment. ACS Nano. 2014;8(5):4268–83.
Webber MJ, Langer R. Drug delivery by supramolecular design. Chem Soc Rev. 2017;46(21):6600–20.
Braegelman AS, Webber MJ. Integrating stimuli-responsive properties in host-guest supramolecular drug delivery systems. Theranostics. 2019;9(11):3017–40.
Tan L-L, Li H, Qiu Y-C, Chen D-X, Wang X, Pan R-Y, et al. Stimuli-responsive metal–organic frameworks gated by pillar[5]arene supramolecular switches. Chem Sci. 2015;6(3):1640–4.
Tan L-L, Li H, Zhou Y, Zhang Y, Feng X, Wang B, et al. Zn2+-triggered drug release from biocompatible zirconium MOFs equipped with supramolecular gates. Small. 2015;11(31):3807–13.
Abuçafy MP, Caetano BL, Chiari-Andréo BG, Fonseca-Santos B, do Santos AM, Chorilli M, et al. Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs. Eur J Pharm Biopharm. 2018;127:112–9.
Yang K, Yang K, Chao S, Wen J, Pei Y, Pei Z. A supramolecular hybrid material constructed from pillar[6]arene-based host–guest complexation and ZIF-8 for targeted drug delivery. Chem Commun. 2018;54(70):9817–20.
Chen Y, Yu B, Xu S, Ma F, Gong J. Core–shell-structured cyclodextrin metal–organic frameworks for programmable cargo release. ACS Appl Mater Interfaces. 2019;11(18):16280–4.
La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nat Rev Clin Oncol. 2011;8:587.
Sadée W, Dai Z. Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet. 2005;14(suppl_2):R207–R14.
Diamandis M, White NMA, Yousef GM. Personalized medicine: marking a new epoch in cancer patient management. Mol Cancer Res. 2010;8(9):1175–87.
Kim TH, Lee S, Chen X. Nanotheranostics for personalized medicine. Expert Rev Mol Diagn. 2013;13(3):257–69.
Lee D-E, Koo H, Sun I-C, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41(7):2656–72.
Ryu JH, Lee S, Son S, Kim SH, Leary JF, Choi K, et al. Theranostic nanoparticles for future personalized medicine. J Control Release. 2014;190:477–84.
Jo SD, Ku SH, Won Y-Y, Kim SH, Kwon IC. Targeted nanotheranostics for future personalized medicine: recent progress in cancer therapy. Theranostics. 2016;6(9):1362–77.
Yang J-C, Chen Y, Li Y-H, Yin X-B. Magnetic resonance imaging-guided multi-drug chemotherapy and photothermal synergistic therapy with pH and NIR-stimulation release. ACS Appl Mater Interfaces. 2017;9(27):22278–88.
Liu W, Wang Y-M, Li Y-H, Cai S-J, Yin X-B, He X-W, et al. Fluorescent imaging-guided chemotherapy-and-photodynamic dual therapy with nanoscale porphyrin metal–organic framework. Small. 2017;13(17):1603459.
He L, Wang T, An J, Li X, Zhang L, Li L, et al. Carbon nanodots@zeolitic imidazolate framework-8 nanoparticles for simultaneous pH-responsive drug delivery and fluorescence imaging. CrystEngComm. 2014;16(16):3259–63.
Penet M-F, Mikhaylova M, Li C, Krishnamachary B, Glunde K, Pathak AP, et al. Applications of molecular MRI and optical imaging in cancer. Future Med Chem. 2010;2(6):975–88.
Li Y, Tang J, He L, Liu Y, Liu Y, Chen C, et al. Core–shell upconversion nanoparticle@metal–organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv Mater. 2015;27(27):4075–80.
Wang D, Zhou J, Shi R, Wu H, Chen R, Duan B, et al. Biodegradable core-shell dual-metal-organic-frameworks nanotheranostic agent for multiple imaging guided combination cancer therapy. Theranostics. 2017;7(18):4605–17.
Li B, Wang X, Chen L, Zhou Y, Dang W, Chang J, et al. Ultrathin Cu-TCPP MOF nanosheets: a new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics. 2018;8(15):4086–96.
Howarth AJ, Peters AW, Vermeulen NA, Wang TC, Hupp JT, Farha OK. Best practices for the synthesis, activation, and characterization of metal–organic frameworks. Chem Mater. 2016;29(1):26–39.
Chen J, Shen K, Li Y. Greening the processes of metal–organic framework synthesis and their use in sustainable catalysis. ChemSusChem. 2017;10(16):3165–87.
Reinsch H. “Green” synthesis of metal-organic frameworks. Eur J Inorg Chem. 2016;27:4290–9.
Julien PA, Mottillo C, Friščić T. Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017;19(12):2729–47.
Reinsch H, Bueken B, Vermoortele F, Stassen I, Lieb A, Lillerud KP, et al. Green synthesis of zirconium-MOFs. CrystEngComm. 2015;17(22):4070–4.
Li F, Duan C, Zhang H, Yan X, Li J, Xi H. Hierarchically porous metal–organic frameworks: green synthesis and high space-time yield. Ind Eng Chem Res. 2018;57(28):9136–43.
Karadeniz B, Howarth AJ, Stolar T, Islamoglu T, Dejanović I, Tireli M, et al. Benign by design: green and scalable synthesis of zirconium UiO-metal–organic frameworks by water-assisted mechanochemistry. ACS Sustain Chem Eng. 2018;6(11):15841–9.
Rojas S, Colinet I, Cunha D, Hidalgo T, Salles F, Serre C, et al. Toward understanding drug incorporation and delivery from biocompatible metal–organic frameworks in view of cutaneous administration. ACS Omega. 2018;3(3):2994–3003.
Márquez AG, Hidalgo T, Lana H, Cunha D, Blanco-Prieto MJ, Álvarez-Lorenzo C, et al. Biocompatible polymer–metal–organic framework composite patches for cutaneous administration of cosmetic molecules. J Mater Chem B. 2016;4(43):7031–40.
Baati T, Njim L, Neffati F, Kerkeni A, Bouttemi M, Gref R, et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(iii) metal–organic frameworks. Chem Sci. 2013;4(4):1597–607.
Kundu T, Mitra S, Patra P, Goswami A, Díaz Díaz D, Banerjee R. Mechanical downsizing of a gadolinium(III)-based metal–organic framework for anticancer drug delivery. Chem Eur J. 2014;20(33):10514–8.
Ruyra À, Yazdi A, Espín J, Carné-Sánchez A, Roher N, Lorenzo J, et al. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal–organic framework nanoparticles. Chem Eur J. 2015;21(6):2508–18.
Mohamed NA, Davies RP, Lickiss PD, Ahmetaj-Shala B, Reed DM, Gashaw HH, et al. Chemical and biological assessment of metal organic frameworks (MOFs) in pulmonary cells and in an acute in vivo model: relevance to pulmonary arterial hypertension therapy. Pulm Circ. 2017;7(3):643–53.
Lu K, He C, Guo N, Chan C, Ni K, Lan G, et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2(8):600–10.
Gregg ST, Yuan Q, Morris RE, Xiao B. Functionalised solids delivering bioactive nitric oxide gas for therapeutic applications. Mater Today Commun. 2017;12:95–105.
Conflict of interest
The author declares that there is no conflict of interest.
Funding
C.C.C. thanks the financial support from the European Union under the H2020-MSCA-IF-2016 program (MSCA-IF-EF-ST Grant Agreement No. 749667).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ABC Highlights: authored by Rising Stars and Top Experts.
Rights and permissions
About this article
Cite this article
Carrillo-Carrión, C. Nanoscale metal–organic frameworks as key players in the context of drug delivery: evolution toward theranostic platforms. Anal Bioanal Chem 412, 37–54 (2020). https://doi.org/10.1007/s00216-019-02217-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02217-y