Abstract
The aim of this study was to develop an effective and specific visual method to rapidly detect and identify Vibrio parahaemolyticus (V. parahaemolyticus) based on the polymerase spiral reaction (PSR). The method utilized only two pairs of primers designed specifically to target the conserved tlh gene sequence of V. parahaemolyticus. Nucleic acid amplification can be achieved under isothermal conditions using DNA polymerase. The reaction could be accomplished in < 40 min with high specificity and sensitivity. The limits of detection of V. parahaemolyticus in purified genomic DNA and pure culture were 300 fg/μL and 2.4 CFU/mL per reaction, respectively, which were 100-fold more sensitive than with conventional PCR. The model food samples showed consistent specificity and sensitivity to the pure bacterial culture. With these encouraging results, it is expected that the novel, effortless and reliable isothermal nucleic acid testing assay developed in this study has potential to be applied to screening for V. parahaemolyticus in seafood samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vibrio parahaemolyticus is a Gram-negative halophilic bacterium widely found in aquatic environments, including marine and estuarine, especially in subtropical offshore waters [1, 2]. V. parahaemolyticus is recognized as an invertebrate pathogen that can be isolated from coastal waters, marine sediments, shrimps, shellfish, and other soft-shelled crustaceans [3]. Eating raw or undercooked seafood contaminated with this bacterium is likely to cause acute gastrointestinal infection, whose symptoms typically include diarrhea, pain, vomiting, nausea, abdominal cramps, and low fever. It can also cause fatal sepsis in people with underlying diseases or in immunocompromised individuals [4]. Furthermore, food-borne diseases caused by V. parahaemolyticus infection reached 4.95 million person-times each year in China [5].
Since the last decade, researchers have been engaged in the development of efficient and cost-effective techniques for pathogen detection. To date, many investigations for V. parahaemolyticus on-site detection have been established, which include isolated culture, immunological, and molecular biological methods. The national standard developed by the United States Food and Drug Administration takes nearly 1 week at present, which makes it difficult to meet the requirements for prevention and control in public health emergencies. ELISA has poor accuracy for cross-reaction and weak sensitivity. Newly emerging molecular biological methods, such as extreme PCR, portable buoyancy-driven PCR, and integrated continuous-flow PCR, have improved efficiency and resolved the problem of being time consuming, but they require highly skilled workers and precise apparatus [6,7,8,9,10]. Of these methods, loop-mediated isothermal amplification (LAMP) has many advantages, such as high sensitivity, lower limit of detection (LOD), and simple and inexpensive instruments; however, it requires complex primer design and it is difficult to control the false-positive rate. To overcome the above obstacles, the purpose of establishing a simple, economical, and visually identifiable method for V. parahaemolyticus detection is essential.
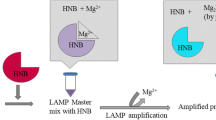
The polymerase spiral reaction (PSR) was developed in 2015 by Liu et al. [11]. This method combines the advantages of LAMP without complicated heterotherm apparatus and simple PCR primer design to realize the rapid and effective detection of V. parahaemolyticus. More specifically, single PCR primers (forward primer F and reverse primer B) clearly do not allow isothermal amplification of the target sequence in a reaction system with only one Bst DNA polymerase, whereas the PSR primer only takes sequence N from the target sequence based on PCR primers and adds this sequence to the 5′ end of the PCR primers (F and B) to form two composite primers (Ft and Bt) (Fig. 1a). Ft or Bt first combines with one of the single-strand DNAs and is extended under the action of DNA polymerase. The newly formed double strands are unchained by betaine and return to single strand. The single strand folds and is re-extended. Another primer then binds to the newly formed single strand, which extends, unchains, and folds again, eventually forming a series of DNA fragments of different molecular sizes (Fig. 1). To speed up the reaction, the accelerating primers LF and LB were designed, which were taken between F and N and between B and N, and the direction of LF and LB from 5′ end to 3′ end is opposite to that of Ft and Bt, respectively. They are also complementary to the DNA single strand and accelerate the extension process, so that the whole reaction can be completed within 40 min. Thus, a simple heating facility such as a water bath is sufficient to implement the PSR requirements, eliminating costly thermal cycle instrumentation. PSR products can quickly be substantiated through visualization of the turbidity change of the mixture, real-time fluorescence monitoring, and hydroxynaphthol blue (HNB) chromogenic dye. Afterwards, these results can be confirmed by 1% agarose gel electrophoresis, which shows a characteristic DNA ladder in positive samples, while the negative controls have an absence of this fragmentation. In terms of sensitivity, specificity, and LOD, our PSR method was found to be 100-fold more sensitive than conventional PCR. PSR conforms to detection technology development criteria put forward by the World Health Organization: ASSURED [12], which includes affordable, sensitive, specific, user-friendly, robust and rapid, equipment-free, deliverable, and they provide strong technical support for real-time detection and on-site diagnostic procedures [13].
Materials and methods
Materials and apparatus
Considering amplification efficiency and cost, Bst 2.0 WarmStart DNA polymerase (New England BioLabs, Ipswich, MA, USA) was selected for PSR. (NH4)2SO4, KCl, MgSO4, and NaCl were purchased from Sinopharm (Beijing, China). HNB and Tween 20 were purchased from Macklin (Shanghai, China) and DingGuo Changsheng (Beijing, China), respectively. Tris HCl and betaine were obtained from Sigma (St. Louis, MO, USA). Tryptone, agar powder, yeast extract, and agarose were procured from Oxoid (Basingstoke, UK). EvaGreen was obtained from YeSen (Shanghai, China). dNTP and DL1,000 DNA Marker were purchased from TAKARA (Kusatsu, Japan). 4S Red Plus, PCR amplification kit, and Ezup Column Bacteria Genomic DNA Purification Kit were obtained from Sangon (Shanghai, China). Washing buffer (0.01 M phosphate-buffered solution (PBS), pH = 7.4) was made in our laboratory.
The PCR instrument used for real-time fluorescence quantification (LightCycler 96) was obtained from Roche (Basel, Switzerland). Electro-heating standing-temperature cultivator and ordinary PCR instruments were purchased from Life Technologies (New York, USA). Electrophoresis apparatus and digital gel imaging system were obtained from Tanon (Shanghai, China). NanoDrop 2000 ultraviolet-visible spectrophotometer was obtained from Thermo Scientific (New York, USA).
Bacterial culture and DNA extraction
The bacteria used in this study were stored in the Department of Hygienic Inspection, School of Public Health, Jilin University (Changchun, China) at − 80 °C. A pure strain of Vibrio parahaemolyticus (ATCC17802) was used for preparation of genomic DNA after incubation in halophilic medium overnight. Non-V. parahaemolyticus organisms including Salmonella typhimurium (S. typhimurium, ATCC13311), Shigella bogdii (S. bogdii, ATCC9207), Escherichia coli O157:H7 (E. coli O157:H7, ATCC25922), and Staphylococcus aureus (S. aureus, ATCC49775) were cultured in Luria-Bertani medium at 37 °C for 12–16 h with shaking at 180 rpm. All of the genomic DNA extractions were performed by a bacterial genomic DNA purification kit purchased from Sangon Biotech (Shanghai, China). The isolated DNA was stored at − 20 °C until further use.
Primer design
Primers were designed within the conserved region of the tlh gene (GenBank Accession No. GU971655.1), which specifically encodes the thermolabile hemolysin in V. parahaemolyticus and has been widely used as the target of many PCR and LAMP protocols [14, 15]. After BLAST sequence alignment in NCBI, four sets of primers specific for V. parahaemolyticus were designed as previously described [11]. The primer sequences were synthesized and purified at Sangon Biotech (Shanghai, China) using HPLC. All nucleotide sequences used in this study are shown in Table 1.
PSR
The optimized reaction was carried out in a final mixture of 25 μL, which consisted of Tris HCl (20.0 mM), (NH4)2SO4 (10.0 mM), KCl (10.0 mM), MgSO4 (8.0 mM), Tween 20 (0.1%), betaine (0.8 M), dNTP (1.4 mM) (each), Bst DNA polymerase (8 U), and 1.6 M Ft and Bt PSR primers. Finally, 2 μL DNA templates were added and the total volume was made up to 25 μL with sterile nuclease-free water (NFW). To determine the analytical sensitivity of PSR, genomic DNA was 10-fold diluted serially (3 × 102 ng/μL, 3 × 101 ng/μL, 3 × 100 ng/μL, 3 × 10−1 ng/μL, 3 × 10−2 ng/μL, 3 × 10−3 ng/μL, 3 × 10−4 ng/μL, 3 × 10−5 ng/μL) by NFW before added. We also diluted the original bacterial solution with NFW by the same multiple (2.4 × 100 CFU/mL, 2.4 × 101 CFU/mL, 2.4 × 102 CFU/mL, 2.4 × 103 CFU/mL, 2.4 × 104 CFU/mL, 2.4 × 105 CFU/mL), and ultraviolet-visible spectrophotometry was used to observe the relationship between the concentration of DNA extraction and the bacterial solution with different dilution ratios. HNB indicator solution (1 μL) with a mass fraction of 0.2% was added to the 25 μL PSR mixture, and the color changes were observed [16]. For comparison, the sensitivity of conventional PCR was also tested as described previously [17]. The PCR mixture contained 12.5 μL master buffer solution, 0.8 μM of each degenerate primer targeting the highly conserved region in the tlh gene, 4.5 μL MgCl2, and 2 μL DNA templates. The reaction program was as follows: initial denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s, and extension at 72 °C for 10 min. Subsequently, the amplified product was electrophoresed in 1% agarose gel with a voltage of 95 V and a reaction time of 45 min. Unless noted otherwise, parallel control and negative controls (no-template control (NC)) were included in each run.
Fabrication and detection of model samples
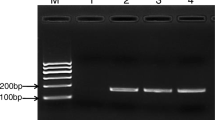
Clam meat (5 g) was weighed and ground thoroughly after shell removal, and 1 ml homogenate was added to 9 ml sterile PBS. To avoid interference by natural accumulation of target bacteria, filtration and sterilization were carried out to obtain the immersion solution. Model samples were prepared by adding V. parahaemolyticus at different concentrations (2.4 × 100 CFU/mL, 2.4 × 101 CFU/mL, 2.4 × 102 CFU/mL, 2.4 × 103 CFU/mL, 2.4 × 104 CFU/mL, 2.4 × 105 CFU/mL) to the sanitized immersion solution. To determine the specificity, the non-target pathogens were also tested in the same matrices. Samples without standard strains were used as the negative control. After that, the amplification products of PSR were separated by 1% agarose gel electrophoresis.
Results
Optimization of PSR
To obtain the optimal performance of the PSR, some important factors, including the best primer, reaction time, and temperature, were optimized using V. parahaemolyticus genomic DNA at 100 ng/μL. First of all, the four groups of primers were reacted for 1 h under the same conditions to select the best one. Through observation, the amplification curves manifested that primer 1 yielded the lowest CT value, so it was selected as the best PSR primer for V. parahaemolyticus detection. The reaction could still proceed for a longer time, even if only one pair of primary primers that specifically targeted the tlh gene was used (see Electronic Supplementary Material (ESM) Fig. S1). According to the optimal primer screening, a series of temperature gradients (63–67 °C) were set to observe the effects of different temperatures on the reaction, with 65 °C being chosen as the optimum temperature of detection because it showed the shortest peak time and the curve gradient rising more apparently compared to other temperatures (Fig. 2).
PSR specificity and sensitivity
Gradient concentrations of extracted genomic DNA and pure culture of V. parahaemolyticus were tested by PSR. Amplification curves were observed in positive samples after < 40 min, whereas no fluorescence changes were seen within 1 h in the negative controls. The LOD of the PSR was 300 fg/μL genomic DNA and 2.4 CFU/mL pure bacterial culture per reaction (Fig. 3). Both were 100 times more sensitive than the conventional PCR, whose detection limits were 30 pg and 2.4 × 102 CFU, respectively, and the target fragment size of PCR was 224 bp (ESM Fig. S2).
Cq values increased with decreasing colony-forming units, and a good linear correlation of the samples with positive amplification was observed between Cq values and CFU number. The regression equation was y = − 2.1057x + 38.293, with a correlation coefficient of 0.9758 (ESM Fig. S3).
To avoid false-positive errors in the PSR, DNA templates of E. coli O157:H7, S aureus, S. bogdii, and S. typhimurium were tested to determine the specificity of the PSR. Non-V. parahaemolyticus bacteria and negative controls negligibly interfered with V. parahaemolyticus detection, as they showed no amplification products. This indicated that the system established in this study had good specificity (Fig. 4).
Detection of model samples
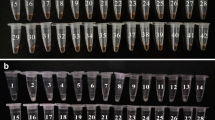
The accuracy and applicability of our method were assessed by conducting replicate analyses of V. parahaemolyticus in clam meat according to the general assay procedures. Despite the protein content being verified in food matrices, the sensitivity of clam samples was not interfered with, which showed good consistency with those of the pure bacterial culture. After adding HNB, the positive sample turned sky blue, while the non-V. parahaemolyticus strains or negative samples remained violet in end-point detection, as well as a distinctive laddering pattern on 1% agarose gel electrophoresis (Fig. 5). In terms of specificity, we observed the same result (Fig. 6). These findings implied that our analytical method had strong selectivity for quantifying V. parahaemolyticus in complex food matrices.
(a) Fluorescence amplification curves of LOD on CFU of model samples of V. parahaemolyticus. (b) 1% agarose gel electrophoresis of PSR-amplified products. Lane M: DL1,000 DNA marker. Lanes 1–6: 2.4 × 100 CFU/mL–2.4 × 105 CFU/mL; lane −: NC. (c) HNB acts as a color indicator for the LOD for CFUs of model samples. Nos. 1–6: 2.4 × 100–2.4 × 105 CFU/mL; −: NC
Discussion
Foodborne diseases caused by pathogenic V. parahaemolyticus have attracted attention in many countries [18, 19]. V. parahaemolyticus has surpassed Salmonella and become the leading cause of human foodborne disease in China [20,21,22] as well as in the USA [23]. It is often difficult to diagnose gastroenteritis merely by observing clinical signs such as vomiting and diarrhea, as these symptoms are also common to other digestive tract diseases. Rapid detection and identification of this pathogen is essential from the perspective of public health. In terms of sensitivity, V. parahaemolyticus detection by PSR is simpler than all of the existing technologies, including the isothermal techniques. PSR can attain an LOD of 300 fg/μL and 2.4 CFU/mL per reaction with excellent specificity, and the entire reaction can be achieved in < 40 min, without the need for sophisticated equipment, as the results can be easily seen by the naked eye.
Aerosol pollution is a difficult problem in the whole field of nucleic acid amplification, since PSR generates so many amplification products. There is a risk of aerosol contamination while opening the reaction tube for subsequent visualization, especially in the field-testing environment. So, before the reaction starts, a drop of transparent paraffin oil is added to the reaction tube to act as a liquid seal. This prevents the reaction liquid from splashing on the wall and reduces pollution of the product aerosol, so that the next step of the experiment can be successfully carried out.
Combined with the advantages of LAMP [24] and PCR, PSR is an isothermal amplification reaction that requires only one pair of primary primers and one enzyme. PSR has been widely used in the detection of bacteria, viruses, and other microorganisms, such as Brucella, porcine epidemic diarrhea virus, and canine parvovirus [25, 26]. Compared to the new detection methods for V. parahaemolyticus developed in recent years such as lateral immunochromatographic, colorimetric, and resistance measurements (Table 2), PSR has demonstrated marked advantages such as requiring fewer operating steps, being less time consuming, and having better LODs. Thus, our present strategy is a powerful tool for the rapid identification of V. parahaemolyticus in food and environmental samples.
Conclusions
We developed a novel approach for rapid detection of foodborne pathogen V. parahaemolyticus. This combined method has sensitivity down to 300 fg/μL and 2.4 CFU/mL in samples and requires < 40 min. Our method is technically simple and has potential as a rapid and accurate way for identification and quantitation of V. parahaemolyticus in seafood. In the future, PSR may demonstrate even greater potency and become a novel method for isothermal nucleic acid testing of other foodborne bacteria.
References
Cruz FR, Mai HN, Dhar AK. Multiplex SYBR Green and duplex TaqMan real-time PCR assays for the detection of Photorhabdus insect-related (Pir) toxin genes pirA and pirB. Mol Cell Probes. 2019;43:20–8.
Twedt RM, Novelli RM. Modified selective and differential isolation medium for Vibrio parahaemolyticus. Appl Microbiol. 1971;22:593–9.
Aguirre GG, Vazquez JR, Ascencio F. Differences in the susceptibility of American white shrimp larval substages (Litopenaeus vannamei) to four Vibrio species. J Invertebr Pathol. 2001;78:215–9.
Fu K, Zheng Y, Li J, Liu Y, Pang B, Song X, et al. Colorimetric immunoassay for rapid detection of Vibrio parahemolyticus based on Mn2+ mediates the assembly of gold nanoparticles. J Agric Food Chem. 2018;66:9516–21.
Zhang Z, Lou Y, Du S, Xiao L, Niu B, Pan Y, et al. Prevalence of Vibrio parahaemolyticus in seafood products from hypermarkets in Shanghai. J Sci Food Agric. 2017;97:705–10.
Banerjee SK, Farber JM. Detection, enumeration, and isolation of Vibrio parahaemolyticus and V. vulnificus from seafood: development of a multidisciplinary protocol. J AOAC Int. 2017;100:445–53.
Zeng J, Wei H, Zhang L, Liu X, Zhang H, Cheng J, et al. Rapid detection of Vibrio parahaemolyticus in raw oysters using immunomagnetic separation combined with loop-mediated isothermal amplification. Int J Food Microbiol. 2014;174:123–8.
Farrar JS, Wittwer CT. Extreme PCR: efficient and specific DNA amplification in 15-60 seconds. Clin Chem. 2015;61:145–53.
Li Z, Ju R, Sekine S, Zhang D, Zhuang S, Yamaguchi Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on an integrated continuous flow PCR and electrophoresis biochip. Lab Chip. 2019;19:2663–8.
Li Z, Zhao Y, Zhang D, Zhuang S, Yamaguchi Y. The development of a portable buoyancy-driven PCR system and its evaluation by capillary electrophoresis. Sens Actuators B: Chem. 2016;230:779–84.
Liu W, Dong D, Yang Z, Zou D, Chen Z, Yuan J, et al. Polymerase spiral reaction (PSR): a novel isothermal nucleic acid amplification method. Sci Rep. 2015;5:12723.
UNICEF/UNDP/World Bank/WHO special program for research and training in tropical diseases. World Health Organization.
Marvin D, Derong Z, Dayang L, Ningwei H, Xiaoming AA, Big Y, et al. Polymerase reaction spiral rapid detection of influenza A (H1N1) virus. Mil Med. 2017;41:449–52.
Taniguchi H. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. Nihon saikingaku zasshi. Jpn J Bacteriol. 1987;42:789–800.
Taniguchi H, Hirano H, Kubomura S, Higashi K, Mizuguchi Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microb Pathog. 1986;1:425–32.
Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–72.
Zhang Z, Xiao L, Lou Y, Jin M, Liao C, Malakar PK, et al. Development of a multiplex real-time PCR method for simultaneous detection of Vibrio parahaemolyticus, Listeria monocytogenes and Salmonella spp. in raw shrimp. Food Control. 2015;51:31–6.
Prompamorn P, Longyant S, Pengsuk C, Sithigorngul P, Chaivisuthangkura P. Rapid identification and differentiation of Vibrio parahaemolyticus from Vibrio spp. in seafood samples using developed monoclonal antibodies. World J Microbiol Biotechnol. 2013;29:721–31.
Li Y, Li Y, Zheng B, Qu L, Li C. Determination of foodborne pathogenic bacteria by multiplex PCR-microchip capillary electrophoresis with genetic algorithm-support vector regression optimization. Anal Chim Acta. 2009;643:100–7.
Wang S, Yang J, Shen Z, et al. Analysis of 766 cases of bacterial food poisoning in China from 1994 to 2003. China Prev Med. 2006;180–184.
Yan C, Yunchang G, Zhutian W, Xm L, Hong L, Month WA, et al. Analysis of surveillance data on foodborne disease outbreaks in China in 2006. Health Res. 2010;39:331–4.
Wang S, Duan H, Zhang W, Li JW. Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005. FEMS Immunol Med Microbiol. 2007;51:8–13.
Taniguchi H, Hirano H, Kubomura S, Higashi K, Mizuguchi Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microb Pathog. 1986;1:425–32.
Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53:1–5.
Gupta V, Chakravarti S, Chander V, Majumder S, Bhat SA, Gupta VK, et al. Polymerase spiral reaction (PSR): a novel, visual isothermal amplification method for detection of canine parvovirus 2 genomic DNA. Arch Virol. 2017;162:1995–2001.
Das A, Kumar B, Chakravarti S, Prakash C, Singh RP, Gupta V, et al. Rapid visual isothermal nucleic acid-based detection assay of Brucella species by polymerase spiral reaction. J Appl Microbiol. 2018;125:646–54.
Liu Y, Zhao C, Song X, Xu K, Wang J, Li J. Colorimetric immunoassay for rapid detection of Vibrio parahaemolyticus. Microchim Acta. 2017;184:4785–92.
Xiang G, Pu X, Jiang D, Liu L, Liu C, Liu X. Development of a real-time resistance measurement for Vibrio parahaemolyticus detection by the lecithin-dependent hemolysin gene. PLoS One. 2013;8:e72342.
Liu X, Guan Y, Cheng S, Huang Y, Yan Q, Zhang J, et al. Development of a highly sensitive lateral immunochromatographic assay for rapid detection of Vibrio parahaemolyticus. J Microbiol Methods. 2016;131:78–84.
Yamazaki W, Ishibashi M, Kawahara R, Inoue K. Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of Vibrio parahaemolyticus. BMC Microbiol. 2008;8:163.
Funding
This study was funded by the National Natural Science Foundation of China (Grant numbers 81502849 and 81872668), the Bethune Medical Scientific Research Fund Project of Jilin University (Grant number 2018B20), the Scientific and Technological Research Project of Jilin Province (Grant numbers 20170204003SF and 20180101095JC), Health science and technology capacity improvement project of Jilin Province (2019Q011) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
(PDF 311 kb)
Rights and permissions
About this article
Cite this article
He, S., Jang, H., Zhao, C. et al. Rapid visualized isothermal nucleic acid testing of Vibrio parahaemolyticus by polymerase spiral reaction. Anal Bioanal Chem 412, 93–101 (2020). https://doi.org/10.1007/s00216-019-02209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02209-y