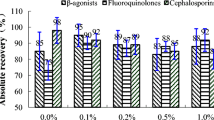
Abstract
In this work, two sample treatment procedures have been evaluated for the determination of veterinary drug residues in milk. In order to cover a wide range of polarities, a total of 66 veterinary drugs with log Kow ranging from − 1 to 5 were selected. Two sample cleanup steps, (i) dispersive solid-phase extraction (dSPE) using enhanced matrix removal lipid as sorbent and (ii) solid-phase extraction (in pass-through mode) using Oasis HLB PRiME cartridges, were critically assessed in terms of sample throughput, recovery, matrix effect, cleanliness of extracts, limit of quantification, and repeatability. The veterinary drugs tested (viz. benzimidazoles, cephalosporins, imidazothiazoles, macrolides, NSAIDs, penicillins, quinolones, steroids, sulfonamides, and β-agonists) were analyzed by ultra-high-performance liquid chromatography tandem mass spectrometry. According to the results, both methods exhibited similar recovery rates between 70 and 120% for most of compounds tested. Matrix effects were satisfactory for both methodologies, although the tolerance to matrix effects was slightly higher with HLB PRiME with nearly negligible matrix effects in most cases. Limits of quantitation were also well below the current maximum residue levels established by the European Union. Notably, sample throughput was higher in the case of HLB PRiME, since this pass-through SPE cleanup approach involved fewer steps than the EMR-Lipid dSPE approach. The results in terms of analysis time, sensitivity, precision, cleanliness of extracts, and matrix effect showed the suitability of both procedures for the monitoring of veterinary drugs residues in milk samples in a single run.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dairy products can be regarded as one of the main markets in terms of global economic importance, due to its high consumption and the fact that milk is one of the most complete animal origin food to maintain a healthy and balanced diet [1, 2]. On the other hand, the use of veterinary drugs in livestock farming and bovine milk production has contributed to improve animal health and well-being [3]. However, a misuse of these compounds in veterinary practice may yield residues in foodstuffs that may originate adverse effects in humans, as allergic reactions or antibiotic resistance [4]. In fact, the World Health Organization warns that antibiotic resistance is one of the biggest threats to global health and food security nowadays [5]. According to this, the European Union has set maximum residue limits (MRLs) of antibiotics in foodstuffs of animal origin by means of Commission Regulation 37/2010. These MRLs are lower than 100 μg kg−1 in the case of milk [6].
Consequently, the development of sensitive and rugged analytical methods to address the current framework is of utmost importance. In order to fulfill the EU requirements in terms of sensitivity and specificity, reported methods so far make use of high-performance liquid chromatography (HPLC) or ultra-high-performance liquid chromatography (UHPLC) coupled with tandem mass spectrometry (MS/MS) [7,8,9,10,11]. Despite countless advances in detection techniques and separation such as development of new stationary phases and more sensitive mass spectrometers, sample treatment step remains a mayor bottleneck in food analysis [12], involving typically more than 70% of the total analysis time.
Several procedures have been proposed for the determination of veterinary drugs in milk involving solid-phase extraction (SPE) [13, 14], protein precipitation [15], simple solvent extraction [16], or QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) [17, 18]. Due to the high concentration of proteins and lipids in milk, procedures should be conceived to diminish their content or even completely remove them from the final extract. The target is, thus, to minimize matrix effects while achieving appropriate recoveries without time-consuming steps. To accomplish this, the coextracted lipids (e.g., fatty acids and triglycerides) obtained when applying the mentioned solvent-based generic sample treatment methods (e.g., QuEChERS) in fatty matrices should be subjected to an additional cleanup step. A new generation of sample preparation sorbents has been recently developed [19,20,21,22,23,24,25], including HLB PRiME (process, robustness, improvements, matrix effects, ease of use), used in pass-through SPE mode, and the so-called Enhanced Matrix Removal-Lipid (EMR-Lipid) sorbent, used as dispersive SPE (dSPE) agent. EMR-Lipid has been proposed for the determination pesticides in fatty vegetable matrices [19, 20]. Meanwhile, HLB PRiME has been successfully applied for the determination of antibiotics in milk samples [21, 22]. Satisfactory extraction has been reported for the multiresidue determination of veterinary drugs with HLB PRiME [23] and EMR-Lipid [24] in milk and bovine liver, respectively. It should be noted that, Wittenberg et al. developed a method for the determination of 40 veterinary drugs in various milk-based powders using HLB PRiME [25]. In this work, the use of EMR-Lipid was also tested. Unfortunately, an in-depth comparison of both procedures has not been addressed hitherto.
With this in mind, a thorough comparison between these two sample treatment approaches has been accomplished in this work in terms of sample throughput, matrix effect, recovery, and precision. A set of 66 veterinary drugs, which covered a wide ranges of polarities, were selected and determined by UHPLC-MS/MS, in order to critically assess these two cleanup procedures in milk samples.
Experimental
Chemicals, reagents, and apparatus
HPLC grade methanol (MeOH), acetonitrile (MeCN), and formic acid were supplied from Sigma-Aldrich (St. Louis, MO, USA). Ultra-pure water was obtained from a Milli-QPlus system (Millipore, Milford, MA, USA).
Sixty-six veterinary drugs including benzimidazoles, cephalosporins, imidazothiazoles, macrolides, NSAIDs, penicillins, quinolones, steroids, sulfonamides, and β-agonist were selected so that a wide range of polarities between log Kow − 1 and + 5 was covered (see Electronic Supplementary Material (ESM) Table S1). Notably, the list of selected compounds studied in this work is essentially different and more thorough than those included in related studies [19, 21]. Analytical standards of veterinary drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA). Each veterinary drug solution (500 mg L−1) was prepared in MeCN and stored at − 18 °C. After that, a working solution which contained a mixture of all the standards (10 mg L−1) was prepared in H2O:MeOH (80:20) and also stored at − 18 °C.
For sample treatment procedures, centrifuge tubes obtained from Deltalab (Barcelona, Spain) were required. Oasis HLB PRiME 3-cc cartridges (Waters, Milford, MA, USA) were used for the first sample treatment. For the second one, EMR-Lipid dSPE tubes and EMR-Lipid Polish tubes were supplied by Agilent Technologies (Santa Clara, CA, USA).
A centrifuge (Sartorius 2-16P from Sigma, St. Louis, MO, USA), an evaporator (TurboVap LV, Caliper Life Sciences, Waltham, MA, USA), and a vortex (Vortex-Genie 2 from Scientific Industries, NY, USA) were also used.
Sample treatment
Procedure I: Solvent extraction followed by pass-through SPE cleanup using HLB PRiME
The proposed method was adapted from a previous study [23] with slight changes. The first step for milk is often a liquid-liquid extraction with an organic solvent with few percent of formic acid to prompt the removal of proteins by its precipitation. It was made by adding 1 g of cow milk to a 15-mL centrifuge tube; then, 4 mL of MeCN (2% formic acid) was added to the same tube and the mixture was vortexed for 30 s. After centrifugation at 6460g for 5 min, 4 mL of the organic layer were pippeted and passed through the HLB PRiME cartridge, which was previously conditioned with 3 mL of MeCN (2% formic acid). One hundred microliters of the extract eluted from the cartridge was diluted with 100 μL of MeCN and 800 μL of H2O, so that the final composition was 80:20 (v/v) aqueous:organic, the same organic content of the initial mobile phase.
Procedure II: Solvent extraction followed by EMR-Lipid dSPE cleanup
The procedure was adapted from a previous method for the analysis of veterinary drugs in bovine liver [24]. The procedure is as follows: 2 g of cow milk was pipetted into a 50-mL centrifuge tube and 10 mL of MeCN (5% formic acid) for the initial extraction step. This tube was vortexed for 2 min and centrifuged at 2650g for 5 min. Prior to its use, the EMR sorbent must be activated/conditioned. For this, 5 mL of ammonium acetate buffer solution (5 mM) was added to an EMR-Lipid dSPE tube. After that, 5 mL of the organic layer—resulting from the centrifugation—were added to the EMR-Lipid dSPE tube, and it was manually shaken for 1 min and vortexed for another minute. The tube was centrifuged at 2650g for 3 min; at this stage, no phase separation was observed because H2O and MeCN are miscible solvents. Then, 5 mL of the upper layer of the tube was transferred to an EMR-Lipid Polish tube (containing 1.6 g of MgSO4 + 0.4 g NaCl). The mixture was then vigorously shaken for 2 min. After this, the tube was centrifuged at 2650g for 3 min, and subsequently two different liquid phases are generated, being the organic phase extract collected. A 1:10 final dilution was made using a 100-μL aliquot of the extract, yielding a final composition of 80:20 (H2O/MeCN, v/v).
LC-MS analysis and equipment
Separation was performed on a Dionex Ultimate 3000 ultra-high-performance liquid chromatography (UHPLC) instrument (Thermo Fisher Scientific, Waltham, MA, USA), using a Zorbax Rapid Resolution High Definition (RRHD) Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8-μm particle size) supplied by Agilent Technologies (Santa Clara, MA, USA). The UHPLC system was coupled to a triple quadrupole analyzer (TSQ Quantiva, Thermo Fisher Scientific, San José, CA, USA) using a heated electrospray ionization source ((HESI-II, Thermo Fisher Scientific, San José, CA, USA). Mobile phases were water (solvent A) and MeCN (solvent B) both of them with 1% formic acid. The following gradient program was used: an initial composition of 10% B was kept constant for 4 min; it increased to 30% B in 1 min and then a linear gradient to 95% B for 3 min, being then held for 2.5 additional minutes. Finally, the initial mobile composition was reached after 0.5 min followed by a re-equilibration time of 4 min. The injection volume was 10 μL and the flow rate was 0.4 mL min−1 during all the analysis. Column temperature was kept constant at 25 °C throughout the analysis. Veterinary drugs were detected in the positive ionization mode using multiple reaction monitoring (MRM) MS acquisition mode. Ion source parameters were as follows: spray voltage, 3500 V; sheath gas, 35 arbitrary units; aux gas, 15 arbitrary units; sweep gas, 1 arbitrary unit; ion transfer tube temp, 250 °C; vaporizer temp, 300 °C; collision gas (CID), 2 mTorr.
The cleanliness of extracts was assessed using UHPLC-TOFMS analysis with full-scan acquisition so that all the ionizable species present in the extract were accounted. It was performed with an UHPLC system (Agilent Series 1290 Infinity, Agilent Technologies, Santa Clara, CA, USA), coupled to a time-of-flight (TOF) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) using electrospray ionization with default parameters (capillary voltage, 4000 V; fragmentor voltage, 190 V; drying gas, 9 L/min; drying gas temperature, 325 °C). The full-scan acquisition was made in the range from m/z 50 to 1000 in the positive ionization mode and with the same optimized chromatographic conditions (e.g., column, mobile phases, flow rate, injected volume) described previously for UHPLC-MS/MS analysis.
Results and discussion
Optimization of UHPLC-MS/MS method
The mass spectrometer parameters were optimized to obtain the best response for the quantification of each individual veterinary drug using an MRM MS/MS method. Two products ions were selected, the most abundant was used for the quantification and the second one was used as confirmatory MS/MS transition. MRM transitions, optimized collision energy, and lens voltage of each veterinary drugs are shown in the ESM (Table S2). These two precursor-product ion transitions together with the corresponding retention time of each compound were used for the identification of each targeted analyte. Precursor ion was in all cases the protonated molecule [M-H]+.
Two C18 columns were tested: Accure aQ 2.1 mm × 100 mm, 2.6 μm (Thermo Fisher Scientific, Waltham, MA, USA) and Zorbax RRHD Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 μm). Better overall results in terms of or selectivity, ruggedness, and reproducibility were obtained using Zorbax RRHD Eclipse Plus C18 column. Different organic solvents such as MeCN and methanol as organic mobile phases were then studied. The use of MeCN improved analyte peak shape and separation in terms of peak resolution for all analytes. In order to enhance analyte signal, the concentration of formic acid was tested between 0.1 and 1.5%. The ionization efficiency was improved for several compounds with 1% of formic acid and no ammonium formate was used in both phases. Finally, other parameters of the UHPLC-ESI method including mobile phase flow rate, column temperature, injection volume, spray voltage, and vaporizer temperature were also optimized, being the optimum values detailed in the “Experimental” section.
Evaluation of the sample treatment procedures
The determination of veterinary drugs in milk samples is challenging due to the inherent complexity of the matrix. Milk contains about 4–5% sugar (lactose), 2–5% fat, and 4–5% proteins (caseins and whey proteins), as well as calcium, potassium, and B vitamins [26]. Milk fat is composed primarily of triglycerides (95% of the total milk fat). Other milk lipids are diacylglycerides (0.25–0.48%), monoacylglycerides (0.02–0.04%), phospholipids (0.6–1.0%), cholesterol (0.2–0.4%), glycolipids (0.006%), and free fatty acids (0.1–0.4%) [27]. In particular, the high concentration of proteins and lipids in milk adds on complexity to the sample treatment leading to final extracts rich in potentially interfering substances. Thus, it is central to reduce the number of coeluting interfering species to obtain clean extracts, keeping also recovery rates and precision at appropriate levels. All these aspects together with sample throughput were considered for the comparison between HLB PRiME SPE pass-through and EMR-Lipid dSPE cleanup procedures.
Extraction efficiency
Ten blank samples of whole cow milk were spiked with the mixture of analytes at 50 μg kg−1 and subjected to both HLB PRiME and EMR-Lipid procedures, being subsequently injected by triplicate in the UHPLC-MS/MS (n = 30). Recovery rates were calculated for each compound by means of interpolation of the areas integrated in the quantification peaks with the matrix-matched calibration curves. These average recovery rates for each compound (Table 1) are plotted against the corresponding octanol/water partition coefficient (log KOW) in Fig. 1. Notably, both sample treatments led to outstanding recovery rates between 70 and 120%, thus yielding a similar extraction efficiency. In addition, no significant patterns were noticed for increasing low Kow values, nor for family classes. The repeatability was also satisfactory (RSD ≤ 19.4%), thus fulfilling the current regulatory requirements [28].
Extract cleanliness and matrix effects
In order to estimate the effectiveness of both cleanup procedures, blank matrix extracts of whole milk obtained from the two tested sample treatment approaches and sample solvent (80:20 H2O/MeCN) were injected in a UHPLC-TOF-MS instrument operated in high-resolution full-scan (FS) acquisition mode. As shown in the overlapped FS total ion chromatograms (TICs) (Fig. 2), the amount of coextracted species in the case of EMR-Lipid is higher, particularly in the time segment between 5.0 and 8.0 min. To examine whether this fact could have an impact on the analytical performance, matrix effect for all veterinary drugs proposed in this work was assessed for both sample treatment methods. Thus, external (solvent) standard and matrix-matched calibration curves were evaluated at six levels of concentration (0.001, 0.01, 0.1, 1, 10, and 100 μg kg−1).
Matrix effect is a key factor in LC-ESI-MS analysis. It describes the deviation between the slope of matrix-matched calibration curves and the slope of external standard calibration curves, using the following equation [(calibration curve slope in matrix ∕ calibration curve slope in solvent) − 1] × 100 [29]. Matrix effect classification was accomplished according to the following criteria: negligible ([0%] − [± 10%]), soft ([± 10%] − [± 20%]), and medium ([± 20%] − [± 50%]) [30]. The full results are provided in ESM (Table S3) and illustrated as a 2D plot versus retention time for each sorbent in Fig. 3. The best results were obtained with HLB PRiME, achieving negligible matrix effect for the 65% of compounds, whereas EMR-lipid showed that 43% of compounds exhibited soft matrix effect and 23% medium matrix effect. In the case of EMR-Lipid, a relevant matrix effect was observed in the late eluting compounds (e.g., retention times > 5.5 min). Thus, these results in combination with TICs showed that HLB PRiME is more effective to remove coextracted lipids. According to the literature, HLB PRiME can remove about 90% fat from milk samples, it involves that 45 mg of lipids could have been retained in the cartridge with the procedure applied. This amount can be higher because in other matrix with higher fat concentration, like meat, the cartridge can retain 135 mg of lipids [31], which implies that it could be possible to use more amount of milk sample for the extraction. On the other hand, EMR-Lipid sorbent can remove 56% of matrix co-extractive compounds present in the bovine liver [24], which contains the same percent of fat as whole cow milk. This suggest that in other fatty animal origin matrix with similar percent of lipids, like milk, the removal could be equated. An example of extracted ion chromatograms corresponding to selected compounds (mebendazole and colchicine) at 1 μg L−1 in solvent and whole milk sample extracts spiked at 50 μg kg−1 is displayed in Fig. 4, showing the absolute absence of matrix effects observed.
Linearity, detection, and quantification limits and precision
Appropriate linearity was achieved with both sample treatment methods with regression coefficients (R2) higher than 0.998 in all cases. Limits of quantitation (LOQs) were established using signal-to-noise criterion (S/N = 10) and were calculated using the less abundant (confirmatory) MS/MS transition for each compound from the lower level tested in recovery studies. As could be observed in Table S4 (see ESM), these values ranged from 0.02 to 18.25 μg kg−1. CCα and CCβ (ESM Table S5) were also calculated for all compounds examined following Commission Decision 2002/657/EC guidelines [28]. For compounds with established MRLs, CCα was calculated as the MRL plus 1.64 times the standard deviation of the interday precision at the MRL level. For compounds with no set MRL, CCα was calculated as the concentration at the y-intercept plus 2.33 times the standard deviation of the reproducibility at the lowest concentration level. CCβ was estimated as the decision limit plus 1.64 times the standard deviation of the reproducibility at the corresponding concentrations.
Repeatability (intraday precision) and intermediate precision (interday precision) were also examined. Repeatability was assessed by means of repetitive application of the entire procedure to five whole milk samples (experimental replicates) spiked at 50 μg kg−1 for each veterinary drug. Each sample was injected by triplicate (instrumental replicates) on the same day. Intermediate precision was assessed with a similar procedure, but the samples were analyzed in five different days. Appropriate results—detailed in Table 1 as RSD (%) of peak areas—were obtained in all cases.
Sample throughput
In light of the results collected, both methodologies provide satisfactory results in terms of matrix effect, sensitivity, recoveries, and precision and being environmentally friendly. Interestingly, the HLB PRiME procedure is faster than EMR-Lipid, as EMR-Lipid dSPE procedure implies a step of sorbent activation, along with centrifugation and a final salting out step. In contrast, HLB PRiME reduces the number of processing steps, which turns out in an average increase of sample throughput. In addition, the tolerance to matrix effect was superior with the pass-through SPE cleanup.
Analysis of market samples
Twenty-four cow milk samples obtained from local supermarkets were analyzed with the HLB PRiME procedure in order to determine the veterinary drugs chosen in this study. This procedure was chosen because it takes less manipulation steps. It was tried to have different types of milk as whole milk, semi-skim, and skim, with or without lactose and enriched with calcium and omega-3 fatty acids. All the milk samples were kept under recommended conditions in their original packaging before using them. The veterinary drugs concentration was calculated from the corresponding calibration curve and is presented as the mean ± standard deviation of four determinations. Traces of danofloxacin were found in two whole cow milk, which are in the range of 0.7–1.5 μg kg−1. However, these concentrations were below the current MRL established [6].
Conclusions
In this study, two different sample treatment approaches using HLB PRiME SPE and EMR-Lipid have been evaluated in terms of recovery, cleanliness precision, tolerance to matrix effects, and sample throughput. Both approaches have shown to be convenient strategies for the cleanup of veterinary drugs from milk, providing satisfactory recoveries, matrix effects, and precision. However, HLB PRiME involved fewer manipulation steps, which makes it the more straightforward and suitable option. Consequently, taking into account these facts, it can be concluded that the SPE pass-through cleanup with HLB PRiME is the more convenient sample treatment approach for the determination of the selected compounds.
The analysis of 24 milk samples was carried out to show the applicability of the method, detecting some findings, although at lower levels than MRLs established by the European Union.
References
Pereira PC. Milk nutritional composition and its role in human health. Nutrition. 2014;30:619–27.
Milk and Dairy Products in Human Nutrition, in: Muehlhoff E. Bennett A. MacMahon D. (Eds.), Food and Agriculture Organization of the United Nations, Rome, 2013 http://www.fao.org/docrep/018/i3396e/i3396e.pdf. Accessed 30 Oct 2018.
Oliver SP, Murinda SE, Jayarao BM. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog Dis. 2011;8.
Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41:120–6.
World Health Organization (WHO). Antibiotic resistence, 2018, http://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. accessed 30 October 2018.
European Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin Off. J. Eur Communities 2010; L15:1–72.
Li J, Ren X, Diao Y, Chen Y, Wang Q, Jin W, et al. Multiclass analysis of 25 veterinary drugs in milk by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2018;257:259–64.
Dasenaki ME, Thomaidis NS. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography—tandem mass spectrometry. Anal Chim Acta. 2015;880:103–21.
Wang J, Leung D, Chow W, Chang J, Wong JW. Development and validation of a multiclass method for analysis of veterinary drug residues in milk using ultrahigh performance liquid chromatography electrospray ionization quadrupole orbitrap mass spectrometry. J Agric Food Chem. 2015;63:9175–87.
Kaufmann A, Butcher P, Maden K, Walker S, Widmer M. Multi-residue quantification of veterinary drugs in milk with a novel extraction and cleanup technique: salting out supported liquid extraction (SOSLE). Anal Chim Acta. 2014;820:56–68.
Masiá A, Morales Suarez-Varela M, Agustin Llopis-Gonzalez A, Picó Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: a review. Anal Chim Acta. 2016;936:40–61.
Rossi R, Saluti G, Moretti S, Diamanti I, Giusepponi D, Galarini R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: a review. Food Addit Contam A. 2018;35:241–57.
Junza A, Amatya R, Barron D, Barbosa J. Comparative study of the LC- MS/MS and UPLC-MS/MS for the multi-residue analysis of quinolones, penicillinsand cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J Chromatogr B. 2011;879:2601–10.
Schwaiger B, König J, Lesueur C. Development and validation of a multi-class UHPLC-MS/MS method for determination of antibiotic residues in dairy products. Food Anal Methods. 2018;11:1417–34.
Bohm DA, Stachel CS, Gowik P. Multi-method for the determination of antibiotics of different substance groups in milk and validation in accordance with Commission Decision 2002/657/EC. J Chromatogr A. 2009;1216:8217–23.
Chico J, Rúbies A, Centrich F, Companyó R, Prat MD, Granados M. High-throughput multiclass method for antibiotic residue analysis by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2008;1213:189–99.
Zhang Y, Li X, Liu X, Zhang J, Cao Y, Shi Z, et al. Multi-class, multi-residue analysis of trace veterinary drugs in milk by rapid screening and quantification using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J Dairy Sci. 2015;98:8433–44.
Zhou J, Xua JJ, Cong JM, Cai ZX, Zhang JS, Wang JL, et al. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. J Chromatogr A. 2018;1532:20–9.
López-Blanco R, Nortes-Méndez R, Robles-Molina J, Moreno-González D, Gilbert-López B, García-Reyes JF, et al. Evaluation of different cleanup sorbents for multiresidue pesticide analysis in fatty vegetable matrices by liquid chromatography tandem mass spectrometry. J Chromatogr A. 2016;1456:89–104.
Parrilla Vázquez Hakme PE, Uclés S, Cutillas V, Martínez Galera M, Mughari AR, Fernández-Alba AR. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography–tandem mass spectrometry. J Chromatogr A. 2016;1463:20–31.
Moreno-González D, Hamed AM, Gilbert-López B, Gámiz-Gracia L, García-Campaña AM. Evaluation of a multiresidue capillary electrophoresis-quadrupole-time-of-flight mass spectrometry method for the determination of antibiotics in milk sample. J Chromatogr A. 2017;1510:100–7.
Wang J, Fan X, Liu YDZ, Feng Y, Jia L, Zhang J. Extraction optimization of sixteen cephalosporins in milk by filtered solid phase extraction and ultra high pressure liquid chromatography coupled to tandem mass spectrometry. Anal Methods. 2017;91:282–1289.
Huang D. Tran KV. Young MS. A simple cleanup protocol using a novel SPE device for UPLC-MS/MS analysis of multi-residue veterinary drugs in milk. Waters Application Note. 2015.
Zhao L. Lukas D. Multiresidue analysis of veterinary drugs in bovine liver by LC/MS/MS Agilent Bond Elut Enhanced Matrix Removal—Lipid, Agilent Technologies Application Note. 2015.
Wittenberg JB, Simon KA, Wong JW. Targeted multiresidue analysis of veterinary drugs in milk-based powders using liquid chromatography−tandem mass spectrometry (LC-MS/MS). J Agric Food Chem. 2017;65:7288–93.
U.S. Department of Agriculture Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 26, http://ndb.nal.usda.gov 2011. Accessed 30 Oct 2018.
Jensen RG. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. 2002;85:295–350.
European Commission decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, Commission Decision 2002/657/EEC. Off. J. Eur. Communities 2002; L221: 1–36.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30.
Ferrer-Amate C, Unterluggauer H, Fischer RJ, Fernández-Alba AR, Masselter S. Development and validation of a LC–MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal Bional Chem. 2010;397:93–107.
Young MS, Shia J, Shah D, Tran K, Huang D. Oasis PRiME HLB Food Applications Notebook. Agilent Technologies 2017.
Acknowledgments
D.C.F. thanks the Andalusian government and European Union for Youth Guarantee research contract (SNGJ-JINV-P-010). D.M.G. thanks the Spanish Ministerio de Economía y Competitividad (MINECO) for a Juan de la Cierva Formación postdoctoral fellowship (Ref. FJCI-2014-19573).
Funding
This study received funding from Ministerio de Economía y Competitividad (MINECO) (Ref. CTQ-2015-71321-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 201 kb)
Rights and permissions
About this article
Cite this article
Castilla-Fernández, D., Moreno-González, D., Beneito-Cambra, M. et al. Critical assessment of two sample treatment methods for multiresidue determination of veterinary drugs in milk by UHPLC-MS/MS. Anal Bioanal Chem 411, 1433–1442 (2019). https://doi.org/10.1007/s00216-019-01582-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01582-y