Abstract
Capillary electrophoresis (CE) is excellent at separating all the ions in a sample but is rarely used as a result of its detection issue and easy loss of very fast ions by common one-end injection methods. Herein we propose a newly developed method aimed at simultaneous determination of positive and negative ions with a home-made CE device, featuring bi-end injection and contactless conductivity detection at the middle. By simply using 2.5 M acetic acid as a running buffer, the method can separate 37 ions (3 inorganic anions, 8 inorganic cations, 10 biogenic amines, and 16 amino acids) per run, with linearity between 10 and 2000 μM (R2 > 0.99), limit of detection of 1.0–16.6 μM, and limit of quantification of 2.3–31.7 μM. The recovery measured by spiking standards into samples at high, middle, and low levels was between 73% and 110%. The intra- and interday repeatability of the 37 analytes ranged from 0.69% to 8.97% and from 0.68% to 11.04%, respectively. The proposed method was evaluated by analysis of 21 beers and, in addition to acquiring the concentration information, the brands of the tested beers were distinguished. This method is of high throughput, fast, and cost-effective. It could be a promising tool for ionomic analysis.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Beers are popular fermented beverages throughout the world. Hence, their safety and authenticity are of global concern and quality assurance requires a thorough analysis of both their nutrient content and potentially toxic substances. Unfortunately, full and accurate determination of beer composition remains a challenging task. In addition to their major constituents of alcohol and water, beers are rich in minor components such as amino acids, vitamins, sugars, inorganic salts, enzymes, etc. [1]. They have a significant effect on beer quality and can be beneficial or harmful to human health. For example, chloride, sulfate, carbonate, and magnesium may affect the taste and flavor of a beer, while iron, lead, copper, zinc, and tin can haze a beer [2]. More importantly, some inorganic salts such as nitrate or nitrite may be carcinogenic to humans through ingestion-induced endogenous nitrosation (Group 2A) [3, 4]. Free amino acids in wort are metabolized by yeast during fermentation and participate in biosynthetic pathways leading to the formation of important flavor components, such as higher alcohols, esters, and sulfur compounds [1]. However, amino acids may also be converted to biogenic amines (BAs) through decarboxylation catalyzed by microbial enzymes (e.g., lysine changes to cadaverine after decarboxylation) [5, 6]. Excessive BAs can cause nausea, headache, nervous system disorders, and so forth [7, 8]. For example, histamine (HA) has been implicated as the causative agent in several outbreaks of food poisoning. HA intake can cause mild, moderate, and severe poisoning in the range of 8–40 mg, 40–100 mg, and above 100 mg, respectively [9]. Putrescine (Put), spermine (Spm), spermidine (Spd), and cadaverine (Cad) have no adverse effects on health, but they may react with nitrite to form carcinogenic nitrosoamines and may also serve as indicators of corruption [10, 11]. These harmful substances should thus be well regulated. Their analytical challenges lie in the effective separation and accurate determination of all these complex components at a concentrations ranging from several percent down to below ppt level.
At present, these components, especially the inorganic ions, can be analyzed by colorimetry [12], electrochemistry [13], atomic absorption spectrum [14], emission spectrum [15], ion chromatography [16,17,18], and capillary electrophoresis (CE) [19]. In terms of ions, ion chromatography is a top choice; however, special instruments and a set of columns are needed to enable measurement of both negative and positive ions. Its separation performance [17, 18] and peak capacity may not be sufficient to elucidate all the concerned ions. Thus, high-performance liquid chromatography (HPLC) [5,6,7,8, 20] tends to be the first choice, which is particularly favorable for the analysis of amino acids, BAs, and other neutral constituents, but for ions, very hydrophilic modes should be considered. An easy alternative is CE, which is known to have fast speed, high separation performance, extremely low running costs, and easy manipulation. In fact, CE could separate dozens of ions at once by indirect UV absorption [21]. Unfortunately, it is not very repeatable and hardly applicable to quantification. Direct detection with contactless conductivity detection (C4D) [22,23,24] has been subsequently exploited and a commercialized detector is now available. However, widespread use of CE remains rare because normal CE easily loses fast ions and has resolution problems, thus it is hardly applicable to the analysis of all ions.
To make CE more powerful in the separation of all ions, we have tried to increase the flow of electroosmosis (EOF), reduce the mobility of fast ions, label neutral composition with charges, and inject samples from both ends of a separation capillary (bi-end injection). Herein, we present a novel methodology using bi-end injection CE coupled with “at-middle” detection via C4D. With it, a newly developed CE method was obtained and applied to the determination of all ions in one sample. Its performance was tested with known ions and its applicability was validated by determining 37 ions in 21 beers. These acquired data demonstrated that it is possible distinguish beer brands on the basis of ionic profiles. This method shows high potential to be exploited as a promising tool for ionomic studies and is therefore worthy of further exploitation.
Materials and methods
Reagents and solutions
Aniline, sodium chloride (NaCl), ammonium nitrate (NaNO3), magnesium sulfate (MgSO4), ammonium chloride (NH4Cl), potassium chloride (KCl), calcium chloride (CaCl2), zinc sulfate (ZnSO4), lithium chloride (LiCl), and copper sulfate (CuSO4) were purchased from Aladdin Ltd. (Shanghai, China). Lysine (Lys), arginine (Arg), histidine (His), glycine (Gly), alanine (Ala), valine (Val), isoleucine (Ile), leucine (Leu), threonine (Thr), methionine (Met), glutamic acid (Glu), phenylalanine (Phe), proline (Pro), tyrosine (Tyr), cysteine (Cys), aspartic acid (Asp), Spm, Spd, Put, Cad, HA, tryptamine (Try), ethanolamine (EA), dopamine (DA), epinephrine (E), and norepinephrine (NE) were from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, sodium hydroxide (NaOH), and other chemicals were obtained from Beijing Chemical Works (Beijing, China). Stock solutions of individual standards were separately prepared at 10 mM in water and stored in the refrigerator at 4 °C before use. The water used for preparation of all solutions was purified with a Millipore Milli-Q academic system (Billerica, MA, USA).
Capillary electrophoresis
All CE separations were performed on a home-made CE device comprising critically a ± 50 kV high voltage power supply (Dongwen High Voltage Power Supply Co., Ltd., Tianjin, China) and a commercial C4D detector (model ER225) with an ET120 head stage (eDAQ, Sidney, Australia). The C4D excitation frequency was set at 1200 kHz and amplitude at 100 Vpp (peak-to-peak). A bare fused silica capillary (Hebei Ruifeng Instrumental Co., Hebei, China) was used, with a size of 50 μm i.d. (365 μm o.d.) × 50 cm (for cations)/20 cm (for anions). The new capillary was conditioned by flushing it with 0.1 M NaOH for 20 min, water for 2 min, and running buffer for 10 min. Each day before starting CE, the installed capillary was rinsed with 0.1 M NaOH for 2 min and running buffer for 5 min. After every three runs the capillary was rinsed for 2 min with the running buffer to maintain the reproducibility of the analysis. The system was pre-run at 18 kV for about 5 min after every cycle of flushing. Injection of cations was carried out first by siphoning from the positive end at 10 cm drop for 15 s and then anions from the other end by electrokinetic injection at −1.2 kV for 5 s. The separation was immediately started after injection by application of +18 kV across the whole capillary. The data were collected at 10 Hz and analyzed by eDAQ PowerChrom v2.5 software. All experiments were performed at room temperature (ca. 25 °C).
Sample preparation
A total of 21 different canned beers that belong to four brands were tested. In each brand, beers of different marketing time between August 14, 2017 to August 25, 2018 were purchased from local markets in China, i.e., four Yanjing beers (Y1–Y4), four Tsingtao beer (T1–T4), five Harbin beer (H1–H5), four Snow beer A (SA1–SA4), and four Snow beer B (SB1–SB4), with detailed information in Table S1 in the Electronic Supplementary Material (ESM). Before CE, beers were left open for 2 h at room temperature to release the carbon dioxide. A sample (90 μL) was spiked with 10 μL aniline (internal standard, IS) at 2 mM and sonicated for 1 min before analysis of the cations. Specifically, the beers were diluted to 1:10 (v/v) with water for the quantification of ammonium, potassium, calcium, sodium, and magnesium, or to 1:5 (v/v) for quantification of anions and sonicated for 1 min before injection.
Method validation
Cations were quantified by calibration curves plotted from peak ratios of analytes to IS (always fixed at 200 μM) vs. concentration. Linear range was obtained by regression analysis and averaged over three independent CE of mixed amino acids, BAs, and inorganic cations at concentrations between 5 μM and 1000 μM (except for Pro, Cys, and Asp that were between 10 μM and 2000 μM). Anions were quantified by direct plots of peak area with concentration at 5–1000 μM, with linearity also fixed by regression analysis. Intra- or interday repeatability for all analytes was determined in three replicates in either 1 day or three non-consecutive days. The limit of detection (LOD) and limit of quantification (LOQ) were determined at the signal-to-noise ratios of 3 and 10, respectively. The recovery of the method was evaluated by spiking 40, 400, and 750 μM standards into beer samples.
Statistical analysis
The acquired multicomponent data was processed by principal component analysis (PCA) using SPSS 18.0 software, aiming at reducing the number of variables and producing new latent variables (principal components) to facilitate the identification or classification of beer brand and/or their authenticity. Additionally, this new variable space simplifies the way of explaining the variability contained in the discrete data.
Results and discussion
Critical capillary electrophoresis conditions
Bi-end injection design
To simultaneously separate cations and anions, bi-end injection was tried with different injection methods and their coupling modes. The most convenient operation is one-step bi-end electrokinetic injection which was performed by inserting the two capillary ends into two sample vials applied with either positive voltage at the normal end or negative voltage at the rare end. Unfortunately, the migration discrimination was too obvious to be used for quantitative analysis of slow cations (ESM Fig. S1). To reduce the degree of discrimination, the cations were first injected into the anodic end by siphoning for 15 s at a height drop of 10 cm (Fig. 1a) followed by electrokinetic injection of anions at the cathodic end at −1.2 kV for 5 s (Fig. 1b) and CE at +18 kV was applied immediately at the cationic end (Fig. 1c). This injection approach increased the peak height of the slow cations by at least 10 times, making the method applicable to quantitative analysis. Note that the injection sequence could not be reversed for better quantification performance; otherwise, the peaks of ions may have a problem in quantitative analysis.
Schematic steps for bi-end injection CE of cations and anions with C4D as a detector. a Siphoning injection of cations at the normal end for 15 s at a height drop of 10 cm. b Electrokinetic injection of anions at the rare end at −1.2 kV for 5 s. c CE separation at +18 kV with C4D detector at a position closer to the negative end
Low pH running buffer
The running buffer was selected on the basis of three considerations: (1) no loss of target analytes; (2) full separation; and (3) sensitive detection. For the first point of consideration, the EOF should be suppressed as much as possible. Accordingly, low pH buffers were tried and those around pH 2.0 were found to be better. The third consideration asks for a low conductive electrolyte while the second requires concentrated buffers. After compromised comparison, acetate buffers were selected and optimized at 2.5 M and pH 2.1, and full separation of 37 target constituents was achieved with acceptable detection sensitivity. At pH 2.1, all amino groups are protonated to become positively charged ions, which sped up the whole separation to about 25 min. Faster separation was possible, but at the cost of losing some resolution.
Capillary size and detector position
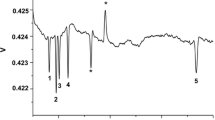
Similar to normal CE, capillary size impacts on performance. Narrowing the capillary bore increased separation and saved running time while shortening the capillary length reduced run time but lost resolution. To find a compromise between resolution and time, we selected a capillary 70 cm long and 50 μm i.d., which was shown to be capable of achieving full separation of all 37 ion targets. Different from normal CE, this method uses a conjugate capillary: increase of capillary length for cations will shorten the length for anions. Thus, for a given capillary, the cationic-to-anionic effective length ratio, which was fixed by the position of the C4D detector, should be a compromise between the resolutions of both cations and anions. Figure 2 illustrates a set of electropherograms measured at the length ratio of 45:25, 50:20, and 55:15, where the optimal ratio is around 50:20 (Fig. 2b). A low critical ratio is found at about 45:25 (Fig. 2a) to keep the closest anion and cation (sulfate and ammonium) baseline-separated; and a high critical ratio is also found at about 55:15 since the peaks of chloride and nitrate start to overlap (Fig. 2c), but an enhancing effect is not obvious for the separation of cations. At the compromised ratio of 50:20, nearly all the analytes were baseline-separated, with the two pairs of sodium/magnesium and Phe/Pro less separated, but remaining within acceptable limits.
Separations of 37 cations and anions in a–c standard solution and d a real beer at the positive-to-negative capillary length ratio of a 45 cm / 25 cm, b, d 50 cm / 20 cm, and c 55 cm / 15 cm. Sample: standard ions at 100 μM (except for Pro, Cys, and Asp at 200 μM); Run buffer: 2.5 M acetic acid at pH 2.1 (in water); Capillary: 50 μm i.d. and 365 μm o.d. × 70 cm; Separation voltage: +18 kV; Injection: Cations into the normal end at a gravity height of 10 cm for 15 s, followed by anions into the rare end at −1.2 kV for 5 s. Peak identifications: 1, Cl−; 2, NO3−; 3, SO42−; 4, NH4+; 5, K+; 6, Spm; 7, Spd; 8, Put; 9, HA; 10, Cad; 11, Ca2+; 12, Na+; 13, Mg2+; 14, Zn2+; 15, EA; 16, Li+; 17, Cu2+; 18, Lys; 19, Arg; 20, His; 21, Try; 22, DA; 23, NE; 24, Gly; 25, E; 26, Ala; 27, Val; 28, Ile; 29, Leu; 30, Thr; 31, Met; 32, Glu; 33, Phe; 34, Pro; 35, Tyr; 36, Cys; 37, Asp. IS, internal standard (200 μM)
Method validation
This method is suitable for high-throughput separation of positive and negative ions in a mixture of up to around 40 constituents (Fig. 2). Furthermore, according to the fine resolution and symmetric peaks of nearly all the ions, it should also be suitable for quantitative analysis of the separated and detected ions. Its performance has been evaluated firstly by using standard solutions to check linearity, precision, and sensitivity (Table 1). The linearity was found between peak area ratios of analyte/IS (cations) or peak area (anions) against concentration for about two orders of magnitude for all 37 ions between 10 and 2000 μM, with linear coefficients all at above 0.99, LOQ at 2.3–31.7 μM (Table 1) and LOD at 1.0–16.6 μM which is highly comparable with HPLC methods (LOD at mg/L BAs or amino acids [8]), and with other CE-C4D methods [22,23,24]. The repeatability was checked by the variation of peak time (t), at < 1.92% for intraday and < 1.37% for interday tests; while checked by the variation of peak area, it gave intraday and interday repeatability of 0.69–8.97% and 0.68–11.04%, respectively. The recovery measured by spiking technique was 73%–110% (Table 2). These results indicated that the method is fully suitable for determination of the target ions.
Analysis of beer samples
In order to assess the real applicability of the newly established CE method, various beers purchased from local markets were analyzed, and some interesting results were obtained in respect of the composition, peak profile, and their concentration of analytes, which vary widely among the three classes of inorganic ions, amines, and amino acids (ESM Table S2). Over all the major components of inorganic ions in beers, wide variation of concentration was detected from different beer samples with respect to brands (Fig. 3). These differences strongly suggest that the CE method may be suitable for brand recognition. This possible use is henceforth further discussed.
Heat map representation of concentration distribution of ions in beer samples. The analysis is based on the normalized content of the identified beer ions across all analyzed samples. The color-coding scale indicates the relative abundance within each ion: blue, low abundance; white, medium abundance; red, high abundance
Inorganic ions
The inorganic ions in beers mainly come from brewing water, malt, and accessories. In the tested beers, there were four major classes of ions (ESM Table S2): The first class is simply K+ at a concentration up to around 300 mg/L; the second includes Cl−, SO42−, and Mg2+ at around 100 mg/L; the third is Ca2+ and Na+ at about 10–100 mg/L; and the fourth is NH4+ and NO3− at 20 mg/L or below. Copper, zinc, and lithium were not found in any of the tested beers. It should be noted that an unknown peak interfered with the determination of trace levels of copper, but its content is much lower than the LOD according to previous reports [25,26,27]. In the case of the Tsingtao brand, its beers contained notably higher inorganic salt than other beers. This is parallel to the concentration difference of original gravity, 11 °P higher than others, which is one of the possible main reasons. By contrast, the total inorganic content in Yanjing beers was significantly lower, especially the content of ammonium, potassium, and magnesium. This can be attributed to the origins of juice beers which were fertilized with different raw materials containing lower inorganic ions. The two types of Snow beers, A and B, had similar content of inorganic ions because they were produced with similar raw materials and processes.
Biogenic amines
BAs in various foods have been widely studied because of their potential toxicity. The types and levels of BAs in beers are closely related to their raw materials, brewing process, and contamination during brewing and storage [28, 29]. Put, EA, and Spd were commonly found in beers. As shown in the left middle of Fig. 3, their content order was EA (2.1–6.1 mg/L) > Put (1.8–5.1 mg/L) > Spd (1.0–2.2 mg/L (except for five beers not detected or quantified). These values were similar to previous reports [5, 30, 31]. Other BAs, namely Spm, HA, Cad, Try, D, NE, and E, were not detected or quantified in all the samples. The summed concentration of total BAs in beers varied not very widely, between 4.3 mg/L and 12.3 mg/L at a mean of 8.5 mg/L, with relative standard deviation equal to 21.6%. Similar to inorganic ions, Tsingtao beers contained the highest levels of BAs (mean 11.7 mg/L) while Yanjing beer contained the lowest levels of BAs (mean 5.8 mg/L). They were all far below the health hazard warning limits.
Amino acids
Besides proteins and peptides, free amino acids are highly expected to exist in beers, mainly derived from the malt during brewing [32, 33]. In fact, Pro (ESM Table S2) was the second most abundant constituent after K+ in beers, but with more uniform concentrations than K+, ranging from 123 to 358 mg/L (mean 251 mg/L). Pro is also the most abundant amino acid in other fermented beverages, such as red wine, wherein its concentration is much higher [7], possibly as a result of its poor assimilation by the yeasts present [5]. Besides Pro, the other abundant amino acids in beers were in a content order of Ala > Tyr > Asp >Arg > Phe > Val ≫ Met (mean concentration 64.2, 63.7, 44.1, 36.4, 33.0, 30.4, and 5.3 mg/L, respectively), followed by Thr and Cys which were not detected in all samples we tested. This amino acid concentration distribution was similar to previous reports [5, 7], which demonstrated that the new method has enough sensitivity and specificity for practical applications.
Differentiation of beers
The above discussion has given a rough comparison of the beers based on the contents of inorganic ions, BAs, and amino acids, but the recognizable level is low according to the global concentration distribution of all the tested beers as shown in Fig. 3. PCA was used to analyze 24 quantified ions (24 ions), which were first used as inputs and then auto-ordered by SPSS software. PC1 (principal component 1) explains 68% of the variance, the second variable PC2 12%, and the third PC3 7%, together accounting for 87% of the variation, which allowed us to distinguish beer brands. 3D plots based on these PCs were obtained as illustrated in Fig. 4, which clearly sorts the beer brands: PC1 separates Tsingtao and Yanjing beer brands, which agrees with the analysis by all the 24 ions; PC2 separates Snow beer A from B and from Harbin brands, which agrees with the analysis mainly based upon the higher content of Cl−, Ca2+, and Mg2+and lower content of Gly, Ala, and Ile; additionally, Harbin brand and Snow beer B were further separated by PC3, which agrees with the content discriminations throughout the ions of K+, Mg2+, EA, Lys, Arg, Val, Leu, Phe, Pro, and Asp (Fig. 3 and ESM Table S3). Thus, PCA is suitable for distinguishing brands. This differentiation trial reveals a promising application of the CE method not just for brand recognition but also for verifying the authenticity of beer brand. Thus, the quantitative data measured by CE method are valuable and have much potential for further mining.
Conclusions
By using a home-made device, we developed a CE method for high-throughput separation, label-free detection, and quantitative determination of 11 inorganic ions, 10 BAs, and 16 amino acids. The method enables easy manipulation, with only a running buffer of 2.5 M acetic acid and two sequential steps to inject cations from the positive end by hydrokinetics and anions from the negative end by electrokinetics. The separation optimization can thus be very simple and easy, just needing to adjust the location of the detector to regulate the cationic-to-anionic separation length ratio, and to dilute the samples if a sample is too concentrated. The applicability of the method was evaluated by separation standard-spiked beer samples and 21 beers. The results proved that the method is quantitative and suitable not only for the high-performance determination of the 37 ions in beers but for brand (and potential authenticity) differentiation. The method is applicable to the quality and process control in beer productions. It remains exploitable with respect to screening and differentiation of other beverage like wines and even solid foods if sample pretreatment is further considered, and is worthy of further investigations and development.
References
Nord LI, Vaag P, Duus JØ. Quantification of organic and amino acids in beer by 1H NMR spectroscopy. Anal Chem. 2004;76(16):4790–8.
Cortacero-Ramı́Rez S, Segura-Carretero A, Cruces-Blanco C, Fernández-Gutiérrez A. Analysis of beer components by capillary electrophoretic methods. Trends Analyt Chem. 2003;22(7):440–55.
Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006;7(8):628–9.
Sindelar JJ, Milkowski AL. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide. 2012;26(4):259–66.
Redruello B, Ladero V, Del Rio B, Fernández M, Martín MC, Alvarez MA. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 2017;217:117–24.
Redruello B, Ladero V, Cuesta I, Álvarez-Buylla JR, Martín MC, Fernández M, et al. A fast, reliable, ultra high performance liquid chromatography method for the simultaneous determination of amino acids, biogenic amines and ammonium ions in cheese, using diethyl ethoxymethylenemalonate as a derivatising agent. Food Chem. 2013;139(1–4):1029–35.
Gómez-Alonso S, Hermosín-Gutiérrez I, García-Romero E. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J Agric Food Chem. 2007;55(3):608–13.
Pereira V, Pontes M, Câmara JS, Marques JC. Simultaneous analysis of free amino acids and biogenic amines in honey and wine samples using in loop orthophthalaldeyde derivatization procedure. J Chromatogr A. 2008;1189(1–2):435–43.
Parente E, Martuscelli M, Gardini F, Grieco S, Crudele M, Suzzi G. Evolution of microbial populations and biogenic amine production in dry sausages produced in southern Italy. J Appl Microbiol. 2001;90(6):882–91.
Eerola S, Sagués A-XR, Lilleberg L, Aalto H. Biogenic amines in dry sausages during shelf-life storage. Z Lebensm Forsch A. 1997;205(5):351–5.
Hernández-Jover T, Izquierdo-Pulido M, Veciana-Nogués MT, Mariné-Font A, Vidal-Carou MC. Biogenic amine and polyamine contents in meat and meat products. J Agric Food Chem. 1997;45(6):2098–102.
Bisson A, Anslyn E. Optical sensing of inorganic anions employing a synthetic receptor and ionic colorimetric dyes. J Chem Soc Perkin Trans. 1999;2(6):1111–4.
Cui L, Wu J, Ju H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron. 2015;63:276–86.
Arienzo M, Capasso R. Analysis of metal cations and inorganic anions in olive oil mill waste waters by atomic absorption spectroscopy and ion chromatography. Detection of metals bound mainly to the organic polymeric fraction. J Agric Food Chem. 2000;48(4):1405–10.
Harwood JJ, Wen S. Analysis of organic and inorganic selenium anions by ion chromatography-inductively coupled plasma atomic emission spectroscopy. J Chromatogr A. 1997;788(1–2):105–11.
Haddad PR, Jackson PE, Shaw MJ. Developments in suppressor technology for inorganic ion analysis by ion chromatography using conductivity detection. J Chromatogr A. 2003;1000(1–2):725–42.
Meng H-B, Wang T-R, Guo B-Y, Hashi Y, Guo C-X, Lin J-M. Simultaneous determination of inorganic anions and cations in explosive residues by ion chromatography. Talanta. 2008;76(2):241–5.
Ding M-Y, Suzuki Y, Koizumi H. Simultaneous determination of organic acids, inorganic anions and cations in beverages by ion chromatography with a mixed-bed stationary phase of anion and cation exchangers. Analyst. 1995;120(6):1773–7.
Sarazin C, Delaunay N, Varenne A, Vial J, Costanza C, Eudes V, et al. Identification and determination of inorganic anions in real extracts from pre-and post-blast residues by capillary electrophoresis. J Chromatogr A. 2010;1217(44):6971–8.
Kutlán D, Molnár-Perl I. New aspects of the simultaneous analysis of amino acids and amines as their o-phthaldialdehyde derivatives by high-performance liquid chromatography: analysis of wine, beer and vinegar. J Chromatogr A. 2003;987(1–2):311–22.
Rovio S, Sirén K, Sirén H. Application of capillary electrophoresis to determine metal cations, anions, organic acids, and carbohydrates in some Pinot Noir red wines. Food Chem. 2011;124(3):1194–200.
Unterholzner V, Macka M, Haddad PR, Zemann A. Simultaneous separation of inorganic anions and cations using capillary electrophoresis with a movable contactless conductivity detector. Analyst. 2002;127(6):715–8.
Sáiz J, Duc MT, Koenka IJ, Martín-Alberca C, Hauser PC, García-Ruiz C. Concurrent determination of anions and cations in consumer fireworks with a portable dual-capillary electrophoresis system. J Chromatogr A. 2014;1372:245–52.
Mai TD, Hauser PC. Simultaneous separations of cations and anions by capillary electrophoresis with contactless conductivity detection employing a sequential injection analysis manifold for flexible manipulation of sample plugs. J Chromatogr A. 2012;1267:266–72.
Agra-Gutiérrez C, Hardcastle JL, Ball JC, Compton RG. Anodic stripping voltammetry of copper at insonated glassy carbon-based electrodes: application to the determination of copper in beer. Analyst. 1999;124(7):1053–7.
Mayer H, Marconi O, Floridi S, Montanari L, Fantozzi P. Determination of Cu(II) in beer by derivative potentiometric stripping analysis. J Inst Brew. 2003;109(4):332–6.
Llobat-Estelles M, Mauri-Aucejo A, Marin-Saez R. Detection of bias errors in ETAAS: determination of copper in beer and wine samples. Talanta. 2006;68(5):1640–7.
Huang K-J, Jin C-X, Song S-L, Wei C-Y, Liu Y-M, Li J. Development of an ionic liquid-based ultrasonic-assisted liquid–liquid microextraction method for sensitive determination of biogenic amines: application to the analysis of octopamine, tyramine and phenethylamine in beer samples. J Chromatogr B. 2011;879(9–10):579–84.
Torrea D, Ancín C. Content of biogenic amines in a Chardonnay wine obtained through spontaneous and inoculated fermentations. J Agric Food Chem. 2002;50(17):4895–9.
He L, Xu Z, Hirokawa T, Shen L. Simultaneous determination of aliphatic, aromatic and heterocyclic biogenic amines without derivatization by capillary electrophoresis and application in beer analysis. J Chromatogr A. 2017;1482:109–14.
Izquierdo-Pulido M, Hernández-Jover T, Mariné-Font A, Vidal-Carou MC. Biogenic amines in European beers. J Agric Food Chem. 1996;44(10):3159–63.
Charalambous G. Involatile constituents of beer. Brewing Sci. 1981;2:167–254.
Klampfl CW. Analysis of organic acids and inorganic anions in different types of beer using capillary zone electrophoresis. J Agric Food Chem. 1999;47(3):987–90.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21727809, 21475136, 21235007 & 21621062) and Chinese Academy of Sciences (QYZDJ-SSW-SLH034).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest in this article.
Additional information
Published in the topical collection New Insights into Analytical Science in China with guest editors Lihua Zhang, Hua Cui, and Qiankun Zhuang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 333 kb)
Rights and permissions
About this article
Cite this article
Guo, C., Guo, Z. & Chen, Y. A bi-end injection capillary electrophoresis method for simultaneous determination of 37 cations and anions in beers. Anal Bioanal Chem 411, 4113–4121 (2019). https://doi.org/10.1007/s00216-018-1507-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1507-7