Abstract
Circulating exosomal microRNAs (miRNAs) are valuable biomarker candidates; however, information on the characterization and mutual agreement of commercial kits for circulating exosomal miRNA profiling is scarce. Here, we analyzed the advantages and weaknesses of four commonly used commercial kits for exosomal miRNA profiling and their application to the sample of serum and/or plasma, respectively. NanoSight and Western blotting were conducted to evaluate the efficiency and purity of the isolated exosomes. In our conditions, the size distribution of the isolated particles was appropriate (40–150 nm), and ExoQuick™ Exosome Precipitation Solution (EXQ) generated a relatively high yield of exosomes. Nevertheless, albumin impurity was ubiquitous for all the four kits, and Total Exosome Isolation for serum or plasma (TEI) yielded a relatively pure isolation. We further performed Illumina sequencing combined with RT-qPCR to determine the ability of these kits for miRNA profiling. There was significant correlation of the exosomal miRNA profile and specific miRNAs between kits, but with differences depending on methods. exoRNeasy Serum/Plasma Midi Kit (EXR) and EXQ performed better in the specific exosomal miRNAs recovery. Intraassay CVs for specific miRNA measurement were 0.88–3.82, 1.19–3.77, 0–2.70, and 1.23–9.11% for EXR, TEI, EXQ, and RIBO™ Exosome Isolation Reagent (REI), respectively. In each kit, serum yielded a higher abundance of exosomes and exosomal miRNAs than plasma, yet with more albumin impurity. In conclusion, our data provide some valuable guidance for the methodology of disease biomarker identification of circulation exosomal miRNAs.

Circulating exosomal microRNAs (miRNAs) are valuable biomarker candidates; however, information on the characterization and mutual agreement of commercial kits for circulating exosomal miRNA profiling is scarce. In this study, we compared four commonly used commercially available kits for exosomal miRNAsextraction and analyzed the advantages and weaknesses of each kit and their application to the sample ofserum and/or plasma
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are mainly composed of exosomes and microvesicles (MVs). Exosomes are nano-sized (30–150 nm) membrane-bound vesicles actively released in the extracellular medium from a variety of cells and are originated from the endosomal compartment by the fusion of multivesicular bodies within the plasma membrane, whereas MVs (150 nm–1 μm) bud directly from the plasma membrane and are often hallmarked with cell apoptosis [1,2,3,4]. Exosomes and MVs carry a wide variety of functional proteins and nucleic acids (particularly mRNAs and miRNAs) that circulate in various biofluids (e.g., blood, urine) and are subsequently transferred to neighboring or distant cells and tissues [5]. Exosomes and MVs play key roles in cell-cell communication and impact multiple dimensions of recipient cellular life by shuttling bioactive molecules [2, 6, 7]. Moreover, exosomes in bodily fluids are a highly stable resource of disease biomarkers [8]. Recently, exosome-encapsulated miRNAs (exosomal miRNAs) in circulation have been proposed as excellent novel biomarker candidates for disease monitoring and prognosis in the surveillance/monitoring of a variety of diseases [9,10,11,12]. However, amid growing interest in this research area, there is a fundamental issue that is not satisfactorily addressed, i.e., the technical standardization of exosome isolation [13].
Exosome isolation is a vital step for the accurate detection of exosomal miRNAs [13]. Many methodologies have been used to isolate exosomes, including ultracentrifugation (UC), size exclusion chromatography (SEC), ultrafiltration, immunoaffinity isolation, microfluidics techniques, and polymeric precipitation methods [13,14,15,16]. Unfortunately, no standard methods are available for exosome extraction. A few groups have conducted comparative studies on different methodologies [13, 17,18,19,20]. UC is regarded as the most commonly used method to isolate exosomes from plasma/serum; however, UC is technically more demanding, labor-intensive and time-consuming isolation method for exosomes. In addition, some concerns on the efficiency and purity of UC-based methods have been raised [13, 15]. SEC exosome isolation from plasma can be performed without significant impurities; however, the efficiency of SEC exosome isolation is still debated [14, 21, 22]. Additionally, SEC generates a low vesicle yield compared with UC [13]. Compared to UC, ultrafiltration and polymeric precipitation techniques are much faster and easier, but these methods also have limitations [17, 23, 24]. With increasing interest in the pathological and physiological roles of exosomal miRNAs, commercial kits promising “easy and quick isolation procedures” have been rapidly developed and are available for use. The commercial kits are robust, are fast, use little sample, and hence serve as ideal choices for the identification of exosomal miRNA disease biomarkers. Higher miRNA yield in serum exosomes was detected by some commercial kits compared with UC [18, 25, 26]. However, the majority of these kits isolate or precipitate exosomes and inevitably suffer from the co-isolation of other EVs and protein complexes. To determine the most suitable exosome extraction protocols for circulating exosomal miRNA analysis, it is necessary to evaluate the commercial kits for exosome extraction used in clinical settings. However, so far, comparative analyses on the quantitative and qualitative performances of these kits have rarely been reported.
Here, we performed an unbiased side-by-side rigorous comparison of four widely used commercial exosome isolation kits. The exosomes derived from these approaches were first assessed for the quantity and quality of specific marker proteins and then examined for the recovery of serum/plasma exosomal miRNAs. In addition, we also compared the exosomal miRNAs between serum and plasma using RNA extracted from the isolated exosomes to increase the current understanding of plasma/serum differences and how these variations are affected by exosome isolation protocols.
Materials and methods
Sample collection
In the present study, we obtained serum and paired plasma samples from 65 random adult volunteers seeking routine checkups at Jinling Hospital, Nanjing, China, according to protocols approved by the ethics committee of Jinling Hospital. All blood donors participating in the present study provided written informed consent and simultaneously contributed one serum sample and one paired plasma sample. Clot Activator Tubes and sodium citrate-containing tubes (BD biosciences, NY, USA) were used for serum and plasma sample collection, respectively. All blood samples were centrifuged at 1500×g for 10 min at room temperature, and subsequently the supernatants were centrifuged at 2000×g for 10 min at 4 °C to remove debris. After centrifugation, the samples were divided into aliquots and stored at − 80 °C until further analysis.
Exosome isolation from serum and plasma
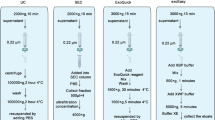
To compare different exosome enrichment methods, three widely used commercial exosome isolation kits, including Total Exosome Isolation for serum or plasma (Invitrogen, MA, USA) (TEI), ExoQuick™ Exosome Precipitation Solution (System Biosciences, CA, USA) (EXQ), and RIBO™ Exosome Isolation Reagent (RIBO, Guangzhou, China) (REI), were examined. Serum and plasma samples from 65 healthy donors were collected and analyzed independently. Pre-study, serum and plasma samples were centrifuged at 10, 000×g at 4 °C for 20 min to remove larger EVs. A 450-μL aliquot of the serum or corresponding plasma sample from each donor was collected and vortexed. Subsequently, each sample was divided into 3 aliquots of 150 μL each to perform three exosome enrichment protocols according to manufacturer’s instructions. Figure S1 illustrates the main steps of each method (see the Electronic Supplementary Material, ESM).
Nanoparticle tracking analysis (NanoSight™)
Concentration, size, and size distribution profile of the particles isolated from the serum or plasma by the three exosome isolation kits described above was evaluated using a NanoSight NS500 instrument (NanoSight Technology, Malvern, UK) and the NTA 2.3 software. Videos were recorded at camera level 15 with the minimal expected particle size, minimum track length, and blur setting, all set to automatic. Each sample was diluted in pre-filtered PBS, and the concentration was between 1 × 109 and 10 × 109 particles/mL. For each sample, six videos of 30–60 s in duration were recorded and analyzed in batch-processing mode.
Protein extraction and Western blot analysis
For protein extraction from the exosomes obtained using the TEI, EXQ, and REI methods, after the last centrifugation, each sample was suspended in 20 μL of PBS, mixed with 20 μL of RIPA Lysis Buffer and then incubated for 30 min on ice. The subsequent extraction and Western blot analyses were performed as previously reported [27]. The primary antibodies included anti-CD63 (Abcam, Cambridge, UK), anti-TSG101 (Abcam, Cambridge, UK), and anti-albumin (Santa Cruz Biotechnology, CA, USA). HRP-conjugated anti-mouse and anti-rabbit antibodies were purchased from Santa Cruz (Santa Cruz Biotechnology, CA, USA).
RNA extraction
For RNA extraction from the exosomes obtained using the TEI, EXQ, and REI methods, after the last centrifugation, each sample was suspended in 100 μL PBS and then mixed with 1 mL of Trizol reagent (Invitrogen, MA, USA) and 200 μL of chloroform. The following extraction was performed as previously reported [27], and the details are provided in the ESM (section Supplementary Methods). Finally, the pellet was dissolved in 20 μL of ribonuclease-free water and stored at − 80 °C until further analysis.
In addition, we also extracted serum/plasma vesicular RNA using the exoRNeasy Serum/Plasma Midi Kit (QIAGEN, Hilden, Germany) (EXR), which is designed for the direct purification of total vesicular RNA from the serum or plasma without the intermediate isolation of EVs. The extraction of RNA with EXR was performed according to the manufacturer’s instructions, and the final elution volume was adjusted to 20 μL, consistent with that of the other kits (see ESM Fig. S1).
miRNA profiling by Illumina sequencing via synthesis (SBS) technology
For SBS, equal volumes of serum and plasma (1.2 mL each) from 20 volunteers were pooled to form serum and plasma samples (24 mL each). Each pool was then divided into 4 identical aliquots of 6 mL each to perform four protocols. Total RNA extraction from the exosomes of serum or plasma was performed as described above. SBS (Annoroad Gene Technology Corporation, Beijing, China) was performed as previously described [27]. The details are provided in the ESM (section Supplementary Methods).
RT-qPCR analysis of miRNAs
A hydrolysis probe-based RT-qPCR assay was performed to measure exosomal miRNAs according to the instructions by the manufacturer (Roche Light Cycler® 480 II, Roche Diagnostics Ltd., Rotkreuz, Switzerland), with a minor modification as previously reported [28]. The details are provided in the ESM (section Supplementary Methods). All reactions, including the no-template controls, were performed in triplicate. The miRNA Cq values of exosomes from serum or plasma were normalized to the sample volume.
Statistical analysis
For statistical analyses, SPSS 21.0 (IBM, NY, USA) and GraphPad Prism 6 (GraphPad Software, CA, USA) were used. The miRNA Cq values were presented as the means ± SD. An unpaired t test was used to compare the differences in the Cq values of the miRNAs between the groups. For correlation analyses, Pearson’s correlation coefficient was calculated. A P value < 0.05 was considered statistically significant.
Results
Comparison of the yield and purity of exosomes isolated from human serum or plasma using different commercial kits
In the present study, exosomes were separately isolated from the serum or plasma of 5 volunteers by using three different commercial exosome extraction kits, including TEI, EXQ, and REI. The isolations were then analyzed by the NanoSight NS 300 System (NanoSight) to track the concentrations and sizes of the exosomes. As it is designed for the direct purification of total vesicular RNA from serum or plasma without intermediate EVs, EXR was not included in this comparison. As shown in Fig. 1a, there were statistically significant differences in the quantities of the yielded particles between methods, and REI generated the highest particle yields. The diameter of the majority of particles isolated by the three methods was approximately 30–150 nm, consistent with previously reported exosome size distributions [29] (Fig. 1b–g). In addition, we also observed that serum yielded more particles and contained higher proportions of particles with diameters larger than 200 nm than paired plasma samples isolated by each kit (Fig. 1a–g).
Exosome yield and purity of different commercial kits from human serum or plasma. a The concentrations of particles yielded by three methods (n = 5). b–g Size distribution of particles isolated with nanoparticle tracking analysis (averages of n = 5). h Protein analyses of EV samples. Lysates of EV enriched with the different protocols from serum or plasma were assessed by Western blot for the presence of the exosome markers CD63 and TSG101 and albumin impurity. *P < 0.05, **P < 0.01, ***P < 0.001
We next compared the three methods for their efficiency to enrich for typical exosome proteins and depleting high abundant albumin from the samples. We used Western blotting to analyze the presence of two classical exosome protein markers, CD63 and TSG101, which are associated with exosomes [30]. By loading equal volumes of lysate sample isolated by each protocol, we observed that EXQ was the most efficient method for maximizing CD63 and TSG101 contents, and the lowest TSG101 signal was found in pellets isolated by TEI (Fig. 1h). In addition, serum yielded higher amounts of the two protein markers than plasma (Fig. 1h). Nevertheless, purity test revealed that albumin contamination was present at different degrees in all samples, and TEI yielded the least contamination, while REI showed the most (Fig. 1h). Similar to CD63 and TSG101 contents, more albumin contamination was found in serum than in plasma.
Exosomal miRNA analysis by Illumina SBS technology
Next, we focused on the miRNA recovery by different exosome extraction kits. We extracted the exosomal RNA from the samples pooled from 20 independent serum or plasma samples using four kits, including TEI, EXQ, REI, and EXR, and compared the exosomal RNA characterization between the different methods. All extracted RNA was of good quality, with a primary RNA peak between 20 and 25 nucleotides (see ESM Fig. S2), except for RNA isolated from plasma by EXR, which was excluded for insufficient RNA quantity for SBS analysis. SBS was then used to analyze the miRNA profiling of exosomes isolated by the four kits. Among the 797 miRNAs scanned by SBS, 385, 411, 296, 448, 316, 457, and 268 miRNAs (> 10 copies) were detected in EXR-serum, EXQ-serum, EXQ-plasma, TEI-serum, TEI-plasma, REI-serum, and REI-plasma, respectively (see ESM Table S1 and Fig. 2). The heatmap showed that different methods yielded slightly different signal intensity of exosomal miRNAs (Fig. 2). Furthermore, we performed a correlation analysis of signal intensity of each detected exosomal miRNA by each method, and a strong correlation was observed between the methods (R2 was above of 0.93 between all the kits) (P < 0.001) (see ESM Fig. S3). In addition, comparisons of exosomal miRNA profile between plasma and serum showed that serum exosomal miRNA profiling had higher signal intensity than the corresponding plasma exosomal miRNA for each kit (Fig. 2).
Exosomal miRNAs analysis by RT-qPCR assay
We then explored potential differences between the four methods for specific miRNAs. We selected four miRNAs, including miR-127-3p, miR-25, miR-16, and let-7d, ranging from low to high signal intensity in SBS, and measured these molecules using RT-qPCR in 20 independent serum/plasma samples. As shown in Fig. 3, the four commercial kits demonstrated different performances in terms of the Cq values of the examined miRNAs: EXR and EXQ both showed good performances in detecting let-7d, miR-16, and miR-127-3p, and the only significant difference was found in miR-25 concentrations, which were more enriched using EXQ than using EXR (P < 0.001). REI also recovered higher quantities of miR-127-3p, miR-25, and serum let-7d, comparable to EXQ or EXR, but recovered relatively low quantities of miR-16 and plasma let-7d (P < 0.001 compared with EXR). Furthermore, correlation analyses of the miRNA Cq values between the four kits showed that the R2 coefficient between EXQ and EXR in both serum and plasma samples were the highest among the comparisons (see ESM Fig. S4). In addition, REI showed a better correlation with TEI than with the other two methods (see ESM Fig. S4).
Exosomal miRNAs analysis by RT-qPCR analysis. a–h Cq values of the four exosomal miRNAs enriched by examined four kits were measured in 20 individuals with a hydrolysis probe-based RT-qPCR assay. Each point represents the mean of the results for triplicate samples. *P < 0.05, **P < 0.01, ***P < 0.001
When comparing the Cq values of the detected miRNAs in isolations between serum and paired plasma samples, the exosomal miRNAs recovered from serum were significantly higher than those from plasma in each kit, consistent with the results of SBS (Fig. 4). The R2 values of the correlations between serum and plasma in both the EXQ and EXR methods were more meaningful than those of the other two methods (R2 > 0.6) (see ESM Fig. S4).
Cq values of exosomal miRNAs with each kit from serum and paired plasma samples. a–p The Cq values of the four exosomal miRNAs enriched by the four kits were compared between serum sample and paired plasma sample. Each point represents the mean of the results for triplicate samples. *P < 0.05, **P < 0.01, ***P < 0.001
These results suggested that although a strong correlation of the exosomal miRNA profiles was obtained between the four isolation methods, the detected levels of specific miRNAs, including let-7d, miR-16, miR-25, and miR-127-3p, were influenced by the exosome isolation kit used.
Reproducibility
To evaluate the reproducibility of the four commercial kits in exosomal miRNAs, we used each of the kits to analyze samples obtained from a pooled serum or paired plasma from 20 volunteers, which were separately divided into 20 aliquots of 150 μL each. As shown in Fig. 5, all methods had an acceptable intraassay CV < 10% for the detection of specific miRNAs: 0.88–3.82, 1.19–3.77, 0–2.70, and 1.23–9.11% for EXR, TEI, EXQ, and REI, respectively (Fig. 5).
Reproducibility of each kits with serum or plasma. a–h To evaluate the reproducibility of each kit in detecting exosomal miRNAs, samples obtained from pooled serum or paired plasma from 20 volunteers were separately divided into 20 aliquots of 150 μL each, and the intraassay CV of each method was calculated from 20 duplicate measurements
Discussion
Since part of EVs, such as exosomes, is actively released from specific tissues or cells, these particles may reflect the pathological or physiological status of the producing cells or tissues. Exosomal miRNAs, which are also present in human biofluids, show greater potential as prognostic and diagnostic disease biomarkers. Therefore, research should focus on the miRNAs found in extracellular vesicles [31]. However, the technical standardization of exosome isolation is a necessary prerequisite for the translation of exosomal miRNAs into clinical practice, as EVs will be useful for narrowing the search for diagnostic and prognostic miRNAs only if they can be reproducibly isolated to high purity while retaining sufficient material for downstream analyses [31]. To determine the most suitable exosome extraction protocols for exosomal miRNA analysis, it is necessary to evaluate the currently available commercial kits for exosome extraction. Previous studies have characterized the efficiency of several commercial exosome isolation kits for serum; however, the understanding of the efficiency of commercial kits in terms of circulating miRNAs is still in its infancy [18, 25, 26]. Rekker et al. studied only one commercial kit, EXQ, by conducting a comparative study of serum exosomal miRNA profiling with UC [18]. Andreu et al. assessed four commercial kits, including EXQ and TEI, by comparing the levels of a set of serum exosomal miRNA with their levels in total serum [25]. Helwa et al. evaluated three commercial exosome kits, including EXQ and TEI, for their ability to measure two specific exosomal miRNAs in serum [26]. However, a comprehensive comparative study of circulating exosomal miRNA profiling of different commercial exosome extraction kits has not been reported. In the present study, we compared the efficiencies of four commonly used commercial exosome isolation kits for the measurement of the serum/plasma exosomal miRNA profile as well as specific miRNAs as first step for biomarker discovery.
Because exosome extraction represents an important source of variability in the analysis of exosomal miRNAs, we first characterized the efficiency of studied commercial exosome isolation kits and the purity of isolates for serum/plasma by NanoSight and Western blot assays. In the Western blot assay, two representative proteins associated with exosomes were analyzed. CD63 is tetraspanin protein in exosomes, and TSG101, another exosomal marker, is a commonly accepted cytosolic ESCRT protein marker of exosomes involved in the biogenesis of multivesicular bodies [30]. We showed that REI yielded the highest amounts of particles, whereas TEI yielded the least. Although EXQ generated markedly fewer particles compared with REI, this method captured higher amounts of the exosome protein markers CD63 and TSG101. In addition, we also examined the presence of albumin, a major soluble plasma/serum protein, in particles isolated by the three kits, since it is recommended to assess not only the presence of exosome markers but also the absence of contaminants [13]. The most albumin contamination was observed in particles isolated by REI, which may be the reason why the Western blot results on exosome markers CD63 and TSG101 did not correlate with the NanoSight findings showing that REI yielded the most particles as observed in the present study. Recent studies on protein impurity analyses of EVs isolated from cell culture media and urine by commercial kits, as well as in EVs isolated from plasma by differential UC and SEC have been reported [32, 33]. These studies demonstrated that most isolation protocols do not isolate a pure population. In the present study, albumin was a predominant component of the isolates present in almost all the samples isolated from plasma/serum by commercial kits. Nevertheless, besides albumin, we suspect that some other contamination particles, including high-density lipoproteins and RNA binding protein (Argonaute 1 and 2) aggregates, may also be present in the isolations, which needs further identification [33, 34].
After the identification of exosomes, we focused on the miRNA recovery by different kits, including the three kits analyzed above and EXR. First, we analyzed the miRNA profiling of 797 miRNAs in pooled serum/plasma exosome samples isolated with these kits as determined by SBS. We observed a significant correlation of the exosomal miRNA profile between kits, but with differences depending on the isolation method. Notably, the EXR-plasma sample was not included in the comparison, as an insufficient RNA quantity for SBS was obtained from plasma treated by EXR, which needs further study in the future. Furthermore, we validated these results by RT-qPCR analysis of four specific miRNAs, including miR-127-3p, miR-25, miR-16, and let-7d. Numerous studies had indicated that these four exosomal miRNAs may be useful as potential biomarkers of disease, especially for cancer [34,35,36,37,38]. The validation results in the present study showed that although a correlation of Cq values of the four exosomal miRNA was obtained between the four isolation methods, the detected levels of the four miRNAs were influenced by the kits used. Among these kits, EXQ showed the best performance in all the four miRNAs. In addition, EXQ has been previously compared to the UC method, and the results demonstrated that two studied miRNAs, miR-486-5p and miR-92a, showed higher relative expression by EXQ [18]. The good performance of EXQ in enriching miRNAs may be attributed to its effectiveness in the isolation of exosomes as observed in the present study. For TEI, its low recovery of miRNAs is also consistent with its relatively low ability in achieving exosomes as demonstrated by NanoSight and Western blot assays. For exosomal miRNAs extraction, reproducibility remains a challenge, especially for circulating exosomal miRNAs. Under the conditions of the present study, the extraction with all the four kits achieved good performance in terms of reproducibility (intraassay CV < 10%). However, compared with the other methods, REI showed a relative lower reproducibility for all studied miRNAs. We suspect that the reason for this difference could be a relatively heterogeneous population of particles with abundant albumin contamination achieved by the REI method. The variation of specific miRNA recovery between the four commercial kits could be explained by the technical differences between the exosome isolation methods.
The choice of plasma or serum for circulating exosomal miRNA biomarker studies remains an open question. Some studies have shown that platelets can release many EVs after blood collection during clot formation, and platelet-derived EVs may account for more than 50% of the EVs in serum, which was more than tenfold greater than that observed for plasma [39]. This finding might suggest that plasma is the physiological medium of EVs in the blood. In the present study, by comparison, we also observed that serum yielded a larger number of exosomes than paired plasma in each studied commercial kit. In addition, particles isolated from serum showed more albumin impurities. Furthermore, higher miRNA levels were also found in serum compared to the corresponding plasma. Nevertheless, there were significant correlations in the Cq values of exosomal miRNAs between serum and plasma, either in SBS or RT-qPCR assay. Specifically, the correlation of the results obtained from serum and plasma samples with EXR and EXQ was much better than that obtained with REI, suggesting that some interfering artifacts, for example, albumin contamination as observed in the present study, may impact this last kit. The comparison between serum and plasma may provide a reference for selecting serum or plasma to analyze exosomal miRNAs as potent non-invasive biomarkers. Additional studies are recommended to increase the current understanding of plasma/serum differences and determine how these differences are affected by sample processing protocols.
In conclusion, here, we highlight the characteristics of each kit, providing the most appropriate method according to different study requirements. Furthermore, we demonstrated that a serum sample could possess a higher abundance of exosomal miRNAs than plasma, but with more albumin contamination. Hence, it is highly advisable to carefully select the appropriate exosomal miRNA extraction method as well as sample type for the detection of exosomal miRNAs.
References
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81.
Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93.
Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23:91–7.
Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–8.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–33.
Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358–67.
Pfeffer SR, Grossmann KF, Cassidy PB, Yang CH, Fan M, Kopelovich L, et al. Detection of exosomal miRNAs in the plasma of melanoma patients. J Clin Med. 2015;4:2012–27.
Hosseini M, Khatamianfar S, Hassanian SM, Nedaeinia R, Shafiee M, Maftouh M, et al. Exosome-encapsulated microRNAs as potential circulating biomarkers in colon cancer. Curr Pharm Des. 2017;23:1705–9.
Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R, et al. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget. 2017;8:6513–25.
Dolz S, Gorriz D, Tembl JI, Sanchez D, Fortea G, Parkhutik V, et al. Circulating microRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. 2017;48:10–6.
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2
Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3
Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013;394:1253–62.
Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6:33935.
Royo F, Diwan I, Tackett MR, Zuniga P, Sanchez-Mosquera P, Loizaga-Iriarte A, Ugalde-Olano A, Lacasa I, Perez A, Unda M, Carracedo A, Falcon-Perez JM. Comparative miRNA analysis of urine extracellular vesicles isolated through five different methods. Cancers (Basel) 2016;8.
Rekker K, Saare M, Roost AM, Kubo AL, Zarovni N, Chiesi A, et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem. 2014;47:135–8.
Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209.
Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R, et al. Human saliva-derived exosomes: comparing methods of isolation. J Histochem Cytochem : Off J Histochem Soc. 2015;63:181–9.
Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65.
Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031.
Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–32.
Rood IM, Deegens JK, Merchant ML, Tamboer WP, Wilkey DW, Wetzels JF, et al. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010;78:810–6.
Andreu Z, Rivas E, Sanguino-Pascual A, Lamana A, Marazuela M, Gonzalez-Alvaro I, et al. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracell Vesicles. 2016;5:31655.
Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One. 2017;12:e0170628.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, et al. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–9.
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7:780–8.
Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1
Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem. 2015;61:56–63.
Royo F, Zuniga-Garcia P, Sanchez-Mosquera P, Egia A, Perez A, Loizaga A, et al. Different EV enrichment methods suitable for clinical settings yield different subpopulations of urinary extracellular vesicles from human samples. J Extracell Vesicles. 2016;5:29497.
Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10:e0145686.
Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888–93.
Chiam K, Wang T, Watson DI, Mayne GC, Irvine TS, Bright T, et al. Circulating serum exosomal miRNAs as potential biomarkers for esophageal adenocarcinoma. J Gastrointest Surg. 2015;19:1208–15.
Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH, et al. Exosome-derived miR-25-3p and miR-92a-3p stimulate liposarcoma progression. Cancer Res. 2017;77:3846–56.
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7:14293.
Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sultmann H, et al. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11:e1004712.
George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–40.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81472021 and no. 81672102) and Fund of State Key Laboratory of Analytical Chemistry for Life Science (no. 5431ZZXM1601) to C. Zhang, the National Basic Research Program of China (no. 2014CB542300) to C.-Y. Zhang, the National Natural Science Foundation of China (no. 81772282 and no. 81401257) and Foundation of Jiangsu Provincial Medical Youth Talent (QNRC2016893) to C. Wang, and the Scientific Research Foundation of Graduate School of Nanjing University (no. 2016CL09) to M. Ding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The Ethics Committee of Jinling Hospital (Nanjing, China) approved the present study. Written informed consent was obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 2460 kb)
Rights and permissions
About this article
Cite this article
Ding, M., Wang, C., Lu, X. et al. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal Bioanal Chem 410, 3805–3814 (2018). https://doi.org/10.1007/s00216-018-1052-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1052-4