Abstract
It is important to profile steroids in many physiological and pathological processes. Recently, hair has been used for the long-term measurement of endogenous steroid hormones. Analyzing hair has advantages of being noninvasive and time sequential compared with other bio-specimens. Liquid chromatography-mass spectrometry (LC-MS) techniques have been widely used over the past decades; however, it is challenging to profile estrogens in hair by LC-MS, and more comprehensive steroid profiling is required. In this paper, an ultra high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was developed to simultaneously profile 28 multifunctional steroids, including corticosteroids (n = 6), estrogens (n = 13), androgens (n = 5) and progestogens (n = 4), in human scalp hair in a single run. To optimize the sample preparation procedure, we evaluated extraction time, post-incubation purification and hair fragment length; 30 mg hair samples were washed with hexane, cut into 5 mm pieces and incubated in methanol for 18 h at 25 °C. Methanol extraction derivatized using Girard P and dansyl chloride reagent was analyzed within 25 min using an automated injection program combined with a diverter valve switch and step analysis (AIDSA). The method was well validated in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, matrix effect and recovery, and was successfully applied to a steroid profile from male and female hairs. Significant differences were observed between genders. In addition, steroids showed a declining trend from the proximal to more distal hair segments; thus, care should be taken when obtaining hair samples for analysis to account for this difference in steroid levels along the length of hair.

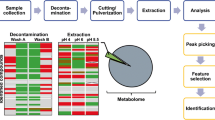
The workflow of the estabished UHPLC-MS/MS method
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroid hormones with strong biological activities, low concentrations and similar structures play important roles in a wide variety of developmental and physiological processes [1,2,3]. Hair analyses for endogenous steroids have drawn increasing attention over recent years, since increasing evidence shows relevant associations between hair cortisol concentrations and stress-related psychological, psychiatric process, and new method for hair steroid analysis has been established [4,5,6,7]. As steroids are incorporated into the growing hair, hair steroid concentration should provide a long-term retrospective view of integrated steroid levels over the previous several months [7]. As an important element of the hypothalamus–pituitary–adrenal (HPA) axis, hair cortisol is considered a crucial candidate biomarker for chronic stress diseases [8, 9], depressive disorders [10, 11], and anxiety disorders [12, 13]. Furthermore, long-term cortisol secretion in hair is used clinically in diagnosing Cushing’s disease [14] and in monitoring medical treatment [15]. Sex steroid metabolism has demonstrated physiological significance, including in reproduction, gene expression, and cardiovascular health [16], and has been implicated in the risk of diseases such as tumors of the reproductive system [17], neurodegenerative diseases [18], and cardiovascular disease [19]. Thus, a comprehensive analysis of long-term steroids in hair is significant for assessing developmental diseases.
Several analytical methods have been used for the determination of integrated steroids in hair, including immunoassays (IAs) [20], high-performance liquid chromatography with fluorescence detection (HPLC-FLU) [21], gas chromatography-mass spectrometry (GC-MS) [22, 23] and liquid chromatography-mass spectrometry (LC-MS) [4, 5, 8]. IAs are relatively sensitive, but it is important to note that they show high cross-reactivity with other endogenous steroids and lack specificity as the result of matrix interference [20], leading to false-positive results. HPLC-FLU requires a large amount of hair and has only been used for cortisol [21]. GC-MS has been successfully used for steroid profiling with high chromatographic resolution [22, 23]; however, GC-MS showed obvious shortcomings, such as the requirement for long throughput times and high costs. In addition, GC-MS methods published have tended to require larger amounts of hair (30–200 mg [22,23,24]) compared with most LC-MS methods (10 − 30 mg [4, 5, 8]). High-performance liquid chromatography-mass spectrometry (HPLC-MS), with its multi-analyte capabilities and its higher specificity and sensitivity, has become the preferred method for steroid analysis and has been attracting more attention in recent years. Several methods have been developed for the analysis of hair steroids by LC-MS. LC-electrospray ionization (ESI)-MS methods using the positive mode for simultaneous quantification of six steroids [5] and nine corticosteroids [25] in human hair have been reported. A negative ESI method for the determination of three steroids in hair [8] has also been reported. Furthermore, LC-APCI-MS methods have been used for the quantification of seven steroids in human hair [4]. Although several studies based on LC-MS have been conducted, a limited number of endogenous steroids were monitored. In addition to the reported steroids, other steroids also play critical roles in organism modulation [26,27,28]. It is important to develop a full-picture LC-MS endogenous steroid analysis. In addition, there is no LC-MS method available for detecting hair estrogens. Estrogen can change the cell morphology and contribute to the pituitary regulation function [29], an important part of the HPA axis involved in a variety of neural activities and endocrine system regulations. Furthermore, estrogen has been demonstrated to play an important role in breast cancer [30], ovarian cancer [31], and endometrial cancer [32]. Therefore, long-term monitoring of hair estrogen fluctuations should help us to better study metabolism and pathogenesis.
Recently, we reported a method to profile 20 sex steroids using a diverter valve switch and step analysis (AIDSA) [33]. The human serum was prepared through liquid-liquid extraction (LLE) and derivatized using Girard P offline. Samples were automatically injected twice under the automated injection program, and both ketolic and phenolic sex steroids were determined. Therefore, we attempt to establish a method to comprehensively profile hair steroids in the metabolic pathway (Fig. 1) based on AIDSA, because hair steroids have great clinical importance and physiological significance. The established AIDSA method was further investigated and validated to meet the hair analysis requirement, and 28 multifunctional steroids, including corticosteroids, estrogens, androgens, and progestogens (Fig. 2), in hair were profiled in a single run. Chemical derivatization has advantages of significant improvement in ionization efficiency, chromatographic separation of isomers and reduction of the matrix effect [34]. The methanol extraction was derivatized using Girard P and dansyl chloride reagent. The hair preparation method for steroid analysis was optimized for extraction time, post-incubation purification, and hair fragment length. To the best of our knowledge, this study is first to determine hair estrogens by LC-MS, and a profile of 28 steroids were determined by ultra high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method using only 30 mg of scalp hair and overcoming disadvantages such as low concentration, poor response to mass spectrometry, and separation difficulties of estrogen isomers. The method was applied to characterize steroid hormones in male and female hairs and to observe differences in the proximal to more distal hair segments.
Metabolic pathways of steroids. The rectangles shown in pink, yellow, blue, and white represent steroids with only carbonyl groups, only phenolic hydroxyl groups, both carbonyl and phenolic hydroxyl groups, and no functional groups, respectively. The gray rectangles represent compounds not involved in the established method
Materials and methods
Chemicals and reagents
Steroid standards, including estrone (E1), estradiol (E2), estriol (E3), 16-keto-Estradiol (16-ketoE2), 4-methoxy 17β-estradiol (4-MeOE2), 2-hydroxy-17β-estradiol (2-OHE2), 2-hydroxy estrone (2-OHE1), 4-hydroxyestrone (4-OHE1), 16-epi-estriol (16-epiE3), pregnenolone (PREG), progesterone (PROG), 17-hydroxypregnenolone (17-OHPREG),17-hydroxyprogesterone (17-OHPROG), dehydroepiandrosterone (DHEA), androstenedione (AN), testosterone (TES), 2-methoxyestrone (2-MeOE1), 2-methoxy-estradiol (2-MeOE2), 4-methoxyestrone (4-MeOE1), 20α-dihydroprogesterone (20α-DHP), deoxycorticosterone (DOC), cortisol (COL), tetrahydrocortisol (TH-COL), tetrahydrocortisone (TH-COR), epi-testosterone (epi-T), and corticosterone (CORT), were purchased from Steraloid Inc. (Newport, RI, USA). 16-OH-estrone (16-OHE1), corticosterone (A) and cortisone (COR) were purchased from J&K Chemical Industry (Beijing, China). The purities of the above ingredients were greater than 98%. Deuterated internal standards (E1-d4, E2-d4) were obtained from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, Canada). Dansyl chloride was obtained from Tokyo Chemical Industry (Tokyo, Japan). Girard P was purchased from the J&K Chemical Industry (Beijing, China). All solvents were higher than HPLC grade. Ultra-pure water was prepared using a Milli-Q purification system (Millipore, Bedford, MA, USA). The Oasis HLB cartridges (3 mL, 60 mg) were purchased from Waters (Milford, MA, USA). The UHPLC-MS/MS assay was performed using an Agilent 1290 Infinity LC and 6490 triple quadrupole mass spectrometer (Agilent Technologies, Inc. Santa Clara, CA).
Preparation of stock solutions, calibration standards and quality control samples
Each steroid standard was prepared in methanol containing 0.1% ascorbic acid at a final concentration of 1 mg/mL as respective stock solutions. These stock solutions were mixed and serially diluted to obtain working standard solutions at concentrations of 0.05, 0.10, 0.20, 0.50, 1, 5, 10, and 20 ng/mL, except for E2 and 4-MeOE2 with concentrations at 0.01, 0.02, 0.04, 0.10, 0.20, 1, 2, and 4 ng/mL. An internal standard mixture solution of E1-d4 and E2-d4 was prepared in methanol containing 0.1% ascorbic acid at a final concentration of 0.5 ng/mL.
For calibration standards, 2 mL of blank matrix (10 mg/mL) was spiked with 100 μL of working standard solutions and 200 μL of IS solution was added afterwards. The samples were evaporated to dryness and re-dissolved in 100 μL of Girard P solution. Finally, the calibration samples were at eight levels of 0.25, 0.50, 1, 2.5, 5, 25, 50, and 100 pg/mg for 26 steroids and 0.05, 0.10, 0.20, 0.50, 1, 5, 10, and 20 pg/mg for E2 and 4-MeOE2.
Limit of detection (LOD), limit of quantification (LOQ), quality control (QC) samples at low-level concentration (LQC), medium level concentration (MQC), and high-level concentration (HQC) were also prepared in the same manner as the calibration standards. For the 26 steroids, the final concentrations of LOQ, LQC, MQC, and HQC were 0.25, 1, 5 and 50 pg/mg, respectively. For E2 and 4-MeOE2, the final concentrations of LOQ, LQC, MQC, and HQC were 0.05, 0.20, 1, and 10 pg/mg, respectively. All solutions were stored at −20 °C when not in use.
Sample preparation
Sample collection and extraction
Hair strands were carefully cut with surgical scissors from a posterior vertex position. As hair grows regularly at a growth rate of 1 cm/month, the proximal 3 cm of hair was obtained. The fibers were washed with plant shampoo for 1 min and rinsed with water for 5 min, which was repeated twice. After drying at 30 °C for 12 h, the dried fibers were washed with hexane for 3 min. After drying under a constant stream of nitrogen for 12 h, the fibers were cut into 5 mm pieces. Then, 30 mg hair was accurately weighed and transferred into a glass tube. Subsequently, 2.7 mL of methanol and 0.3 mL of IS solution were added, and the hair sample was incubated for 18 h at 25 °C for extraction. After centrifugation at 4500 rpm for 5 min, 2 mL of the clear supernatant was transferred to a new glass tube. The methanol extraction was dried under a gentle nitrogen stream. Then, 100 μL of Girard P solution (2.5 mg/mL in 70% methanol containing 1% formic acid) was added, and the mixture was incubated for 3 h at 30 °C to generate steroid-Girard P derivatives. After derivatization, all samples were analyzed without further purification.
Blank matrix
For method validation, a blank hair matrix was prepared. Although some steroids, including COL, COR, TH-COL, DHEA, AN, E2, 4-MeOE2, 16-ketoE2, 4-OHE1, 2-OHE1 and PROG, were not detected in the hair segment distant from scalp (20–30 cm), others (TES, epi-TES, PREG, 4-MeOE1, 2-MeOE1, and 17-OHPREG) can still be detected. Thus, it is impossible for us to find a blank hair without any steroids. Similar dilemmas also occurred with steroids analysis in other complicated bio-matrix, such as serum [35,36,37] and urine [38]. For method validation, prior studies [35,36,37] purified a blank matrix by charcoal extraction. Following their works, we stripped endogenous steroids by charcoal purification and obtained a relative clean blank hair which was further applied to method validation. The proximal 3 cm of hair of six humans was collected from posterior vertex position with surgical scissors. Hair was black and had not been treated with dye or other chemical reagents. For this step method validation, methanol was added to pool hair, followed by incubation for 18 h at 25 °C to obtain hair extraction at 10 mg/mL. The dry residue was re-dissolved using 2% methanol (5 mg/mL) and vortexed for 1 min. To strip steroids from the above solution, 30 mg of activated charcoal was added per milliliter of solution, followed by vortexing for 30 min and centrifugation for 10 min. The supernatant was lyophilized to dryness and re-dissolved with methanol to obtain the blank hair matrix (10 mg/mL).
Optimization of sample preparation
To maximize the extraction efficiency of hair steroids, a series of experiments was carried out to optimize the preparation method. Natural human hair was collected from posterior vertex position with surgical scissors. The proximal 3 cm of hair was obtained. Hair was black or brown, had not been treated with dye or other chemical reagents. Washed hair was cut to 5 mm fragments. The results were calculated by comparing the peak area ratios of the analytes under each condition. Firstly, different extraction times and conditions were initially tested, including an 18-h incubation, a 6-h incubation, and a 1-h sonication in methanol. The hair was collected from one human; each condition was performed with three replicates.
Then, different post-incubation purifications were compared after 18 h methanol extraction. For solid-phase extraction (SPE), methanol extraction was cleaned by Oasis HLB cartridge. Detailed procedures can be found in the Electronic supplementary material (ESM; 1. Detailed procedures of the post-incubation purifications). For LLE, dryness of methanol extraction was dissolved using methanol/water 2/98 (v/v), followed by extraction of dichloromethane. Detailed procedures can be found in the ESM (Detailed procedures of the post-incubation purifications). In case of no SPE/LLE, 2 mL methanol extraction was evaporated to dryness without any post-incubation purification. The hair was collected from one human; each condition was performed with three replicates. The impact of the sample preparation on the matrix effect was evaluated using LQC and HQC samples. Detailed procedures can be found in the ESM (2. The impact of the sample preparation on the matrix effect).
Then, the effect of hair powder and different lengths of hair pieces on the extraction of steroids was evaluated. The hair collected from three humans was divided into four samples: one was milled into powder in a stainless-steel mortar, and the others were cut into small pieces of 1–2 mm, 5 mm, and 1 cm. The samples were then prepared as in “Sample collection and extraction,” with two replicates per hair state. A total of 17 steroids were detected in human scalp hair, and the peak area ratios were calculated for comparison with hair powder as the base value.
LC-MS/MS analysis
An Agilent 1290 Infinity LC and 6490 triple quadrupole mass spectrometer (Agilent Technologies, MA) equipped with an ESI ion source was employed for the analysis of the steroids. Chromatographic separation was performed using an Agilent Zorbax Eclipse Plus C18 Rapid Resolution HD (1.8 μm, 2.1 mm × 50 mm). The mobile phase consisted of solvent A (0.1% formic acid and 10 mM ammonium formate in deionized water) and solvent B (acetonitrile:methanol (1:1, v/v) with 0.1% formic acid and 1 mM ammonium fluoride). The elution was performed using the following gradient program at 0.5 mL/min: 0–2 min, 15% B; 2–6 min, 15–22% B; 6–8 min, 22–25% B; 8–10 min, 25–35% B; 10–11 min, 35% B; 11–12 min, 35–45% B; 12–18 min, 45–75% B; 18–19 min, 75% B; 19–21 min, 75–55% B; 21–22 min, 55–65% B; 22–22.1 min, 65–85% B; and 22.1–25 min, 85% B. The column temperature was maintained at 45 °C.
Prior to injection, a 1 M sodium carbonate and dansyl chloride solution dissolved in acetone at 10 mg/mL was separately placed in positions “P1-A-1” and “P1-A-2.” The temperature of the injection plate was controlled at 30 °C. The sample injection procedure was programmed with the following steps [33]: step 1, draw 3 μL from the sample; Step 2, inject; Step 3, draw 2 μL from the sample; Step 4, wash the needle; step 5, draw 1 μL from location “P1-A-1”; step 6, mix 3 μL of air; step 7, wait 0.3 min; step 8, wash the needle; step 9, draw 2.5 μL from “P1-A-2”; step 10, mix 6 μL of air; step 11, wait 15 min; and step 12, inject (at approximately 15.8 min).
The mass spectrometer conditions were as follows: source, ESI; polarity, positive; nebulizer, 20 psi; sheath gas temp, 250 °C; multiplier voltage (delta EMV), 400 V; and fragmentor, 380 V. The LC eluent flow during the periods from 0.0 to 3.0 min and 13.5–16.5 min was not introduced to the mass spectrometer for data acquisition. The optimum conditions for ionization and fragmentation selectivity are listed in Table 1.
Method validation
The newly established method was carefully validated in terms of the linear range, LOD, LOQ, precision, accuracy, matrix effect, and recovery.
Calibration curves, LOD, and LOQ
Calibration standards were prepared as described in “Materials and methods.” The calibration curve was constructed using the peak area ratios of compound to IS versus the concentration of compound at eight levels of matrix-matched calibration standards and applying a weighted (1/x) least squares linear regression analysis. The correlation coefficients (R) were calculated. LOD was tested at a signal to noise (S/N) of 3. The accuracy and precision of LOQ were both validated. The criteria for acceptability of the data included accuracy within 80–120% and precision with a relative standard deviation (RSD) of less than 20%.
Accuracy and precision
The intra-precision and accuracy of the method were assessed by performing six replicates of LOQ, LQC, MQC, and HQC samples. The inter-day precision was performed on three separate days. For QC samples, 2 mL of blank matrix (10 mg/mL) was spiked with 100 μL of working standard solutions and 200 μL of IS solution. Accuracy was calculated as the averaged percentage of the measured concentrations to the real concentrations. Precision was expressed as the RSDs of the measured concentrations. The criteria for acceptability of the data included accuracy within 80–120% and precision with RSD of less than 20%.
Matrix effect and recovery
To evaluate the matrix effect (ME) and recovery (R), LOQ, LQC, MQC, and HQC samples (n = 6) were assessed. The matrix effect value was calculated as ME (%) = B/A × 100, where A is the compound peak area of 100 μL pure standard sample (i.e., in the absence of hair matrix) and B is the compound peak area of 2 mL blank matrix spiked with 100 μL standard sample after extraction. The recovery value was calculated as R (%) = C/B × 100, where C is the compound peak area of 2 mL blank matrix spiked with 100 μL standard sample before extraction.
Profile of hair samples
Hair samples were collected from 22 healthy adults, including 12 males and 10 females (matched in age and education level) aged 22 to 30 years. Hair had not been treated with dye or other chemical reagents. Natural scalp hairs were prepared and analyzed to profile 28 steroids using the established method.
Segmental analysis of steroids in human hair
The growth rate of hair is approximately 1 cm/month [39], recording steroid fluctuation in chronological order. We collected six consecutive segments of 1 cm from proximal to distal hair from two females. Therefore, long-term steroids provided a retrospective period of 6 months to evaluate the changes in steroid concentration along successive hair segments.
Data processing and statistical analysis
Agilent MassHunter Quantitative Analysis B.05.02 (Agilent Technologies, Inc., Santa Clara, CA) was employed for peak integration and steroid concentration calculation. As for profile of hair samples, the concentration results were subjected to bilateral t test using IBM SPSS 21 (Armonk, New York, United States). P < 0.05 was considered significant. Principal component analysis (PCA) was used to visually discriminate groups and orthogonal partial least squares discriminant analysis (OPLS-DA) was used to discriminate groups in classification using SIMCA 13.0 software (Umetrics, Umeå, Sweden).
Results and discussion
Optimization of sample preparation
As shown in Fig. 3A, using 18 h incubation, a higher extraction efficiency for most of the analytes were obtained, when compared with ultrasonication (14/17 analytes) and 6 h incubation (17/17 analytes). Therefore, a methanol incubation of 18 h at 25 °C was chosen as the final incubation condition.
(A) Comparison of different methods of incubation. The extraction efficiencies were calculated with 18 h and 25 °C as the base values. (B) Comparison of post-incubation purifications. The extraction efficiencies were calculated using methanol extraction as the base value. (C) Comparison of hair powder and pieces. The extraction efficiencies were calculated using hair powder as the base value
Figure 3B showed the result of different post-incubation purifications and detailed ratio information can be found in Table S1 in the ESM. Most analytes of SPE (15/17) and LLE (13/17) procedure were under the normal instrument fluctuation, showing a ratio between 80 and 120% [40, 41]. However, the extraction efficiency of two analytes in SPE (74.4% for PROG and 76.0% for epi-T) and four analytes in LLE was below 80% (69.4% for 4-OHE1, 78.6% for 2-MeOE1, 79.6% for 16-ketoE2, and 79.4% for TES), indicating the loss of analytes. The matrix effect of LLE and SPE were calculated (see ESM, Table S2). Corticosterone (A) showed a positive matrix effect (130–135% for SPE, 129–130% for LLE), suggesting ionization enhancement effects for this analyte. 17-OHPROG showed a negative matrix effect (77.2–75.3% for SPE, 77.8–78.3% for LLE), suggesting that there was suppression of ionization for 17-OHPROG in hair. For the rest of the steroids, the matrix effect of LLE was between 82.1 and 114% and the matrix effect of SPE was between 86.5 and 117%, indicating that the impact of SPE and LLE on the matrix effect was not obvious. Considering SPE and LLE is time consuming and labor required, we choose methanol extraction.
As shown in Fig. 3C, hair powder showed relative higher (highest peak area ratios in 13 analytes out of 17) and 1 cm fragment showed relative lower (lowest peak area ratios in 10 analytes out of 17) extraction efficiency. For 4-MeOE2 and 2-MeOE1, extraction efficiency of hair pieces decreased but was constant and reproducible. Compared with hair cut to small pieces, hair milled to powder was more easily adsorbed to the container due to electrostatic interaction and resulted in more waste of hair in the preparation procedure. Considering all variables together, including manual handling time, sample waste in the preparation procedure and sample throughput, 5 mm was chosen as the most suitable length for hair extraction, especially when a larger number of samples need to be processed.
LC-MS/MS method development
In a previous study, we developed a method for human serum to determine 20 steroids in a single run [33]. In the present study, to monitor the dynamic changes of upstream and downstream metabolites in the steroid metabolic network, 28 target steroids needed to be simultaneously monitored in a single run. We optimized the chromatographic elution gradient to achieve the rapid separation of multiple isomers and a reduced analysis time of 28 to 25 min (Fig. 4). We analyzed blank hair samples as shown in Fig. 4A; there was some interference in blank hair matrix since compounds with the same functional groups as steroid hormones could be derivatized by Girard P and dansyl chloride. As shown in Fig. 4C, however, these derivatized interference had different retention times with target compounds. Thus, interfering compounds had no overlap with the target compounds.
The keto group of corticosteroids, estrogens, androgens, and progestogens was directly derivatized with Girard P. Derivatization on the keto groups improved ionization efficiency using electrospray ionization. Based on Girard P derivatization, the established method can monitor six corticosteroids, seven ketolic estrogens, five androgens, and four progestogens in a single run, and we determined three corticosteroids, five ketolic estrogens, four androgens, and three progestogens in the hair. For the analysis of phenolic estrogen in hair, many factors, including low concentration, low ionization efficiency, and the complex matrix of hair, make it difficult. Dansyl chloride rapidly and quantitatively reacts with the phenolic hydroxyl group of estrogens to form the sulfone ester. Derivatives are readily cleaved by the low CID voltage to give an intense and characteristic product ion at m/z 171, and the ESI ionization efficiency and response to the mass spectrometry are thus enhanced. Based on Dansyl chloride derivatization, the established method can monitor six phenolic estrogens; we determined two phenolic estrogens in the hair. The ability to cover hair corticosteroids, estrogens, androgens and progestogens in a single LC-MS run will likely provide a long-term retrospective view and be very useful in physiological and pathological investigations.
Method validation
Linearity, LOD, and LOQ
A detailed summary of the calibration curves, linear dynamic range, LOQ, and LOD is provided in Table 2. The correlation coefficients (R) were greater than 0.9924, indicating good linearity of the method. For E2 and 4-MeOE2, linear dynamic ranges were 0.05–20 pg/mg and LOQ was 0.05 pg/mg. For other steroids, linear dynamic ranges were 0.25–100 pg/mg and LOQ was 0.25 pg/mg. LODs for these analytes were 0.05–0.1 pg/mg.
Accuracy and precision
The results of accuracy and intra-day and inter-day precision are presented in Table 3. The accuracy is between 79.5 and 115%. The intra-day precision is 17.9% or less and the inter-day precision is 16.5% or less. The method was considered suitable in terms of accuracy and precision.
Matrix effect and recovery
The matrix effect and recovery of 28 analytes were calculated (Table 4). Corticosterone (A) showed a positive matrix effect (between 127 and 134%), suggesting ion enhancement effects for this analyte. 17-OHPROG showed a negative matrix effect (between 67.6 and 72.5%), suggesting that there was suppression of ionization for 17-OHPROG in hair. For the rest of the steroids, the matrix effect was between 81.5 and 119%. The recovery of each analyte at each concentration was between 77.1 and 106%.
Profile of hair samples
We determined the steroid concentration in the hair of 22 adults, and 17 steroids were detected. The multivariate statistical analysis showed that male and female hairs could be clearly distinguished by PCA and OPLS-DA (Fig. 5A), indicating that the established method was reliable for monitoring the differences between different groups. Our investigation was confined to individuals aged 22–30 years, and we found five analytes (16-ketoE2, DHEA, TES, AN, and epi-T) showing significant differences by t test (Fig. 5B) between male and female. The concentration and p value of five analytes can be found in the ESM (Table S3). In a study of the hair steroids of 30 adults aged 21–58 years [4], DHEA, TES, and AN were also detected and hair testosterone levels of males were significantly higher than females. Our results of hair testosterone are consistent with previous studies [4]. However, DHEA and AN did not show significant differences in the previous study. Androgen secretion declined from 20 to 60 years [33]. Androgen has relative strong secretion at 20–30 years; this may lead to gender difference of DHEA and AN in hair in our study. The reliability of the method is further verified by the experimental results.
Segmental analysis of steroids in human hair
The segmental analysis (1 cm) of hair samples from two females are shown in Fig. 6, and we found that the endogenous steroid concentrations decrease gradually from the root-side end toward the distal side hair segments. This trend was particularly obvious in glucocorticoids (cortisone and cortisol), which suggested that the clinical collection of hair samples should follow the standards of “scalp hair” and avoid interference with natural wash out and degradation. The results of successive hair segments are shown in Fig. 6, and the endogenous steroid concentrations decrease gradually from the root-side end toward the distal side hair segments, which set a natural limit to the period of retrospective steroids determination. The “wash-out” effect is consistent with other researches [10, 42, 43]. To ensure the results not confounded by a potential wash-out effect, we suggest to take the first scalp-near 3 cm hair segment to measure hair steroids. However, the concentration of five steroids (epi-T, 16-ketoE2, 2-MeOE1, 4-MeOE2, 4-OHE1) were relatively stable and did not decrease too much. F2 had facial acne at the time corresponding to 5 cm, resulting in an increase in glucocorticoids (COL, TH-COL), androgens (TES, AN, DHEA, epi-T), progesterones (PREG, PEOG, 17-OHPREG), and estrogens (E2, 16-ketoE2, 2-MeOE1, 4-MeOE1, 4-OHE1). The increase in androgen, a crucial metabolite in the central metabolic network of steroids, is an important cause of acne, leading to a general increase in the metabolism of other steroids and demonstrating that the established method has good applicability for monitoring human health and metabolism.
Conclusion
In this study, a reliable and stable method was established to comprehensively profile and determine 28 human hair steroids, including corticosteroids (n = 6), estrogens (n = 13), androgens (n = 5), and progestogens (n = 4). Compared with other reported LC-MS methods, the established method covered more types of steroids. To the best of our knowledge, this is the first time that a new method has been established to simultaneously profile corticosteroids, estrogens, androgens, and progestogens in a single run, and 17 steroids, including corticosteroids (n = 3), estrogens (n = 7), androgens (n = 4), and progestogens (n = 3) in the hair were detected by UHPLC-MS/MS. Considering that the level of metabolites in the hair is easily affected by diet, mood, and external environment, clinical research of hair samples often requires epidemiological studies of samples on a large scale; therefore, we optimized the sample preparation procedure for high-throughput hair analysis. The final procedure involved 5 mm hair fragments incubated for 18 h in methanol, followed by derivatization, which saves time. The concentration of steroids in human hair using the established method was consistent with that reported in a previous study [4]. This method has great potential as a useful tool for comprehensive profiling and quantification of hair steroids and investigating chronic disease.
References
Eser D, et al. Neuroactive steroids and affective disorders. Pharmacol Biochem Behav. 2006;84(4):656–66.
van Wingen GA, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13(3):325–33.
Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–9.
Gao W, et al. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:1–8.
Noppe G, et al. LC-MS/MS-based method for long-term steroid profiling in human scalp hair. Clin Endocrinol. 2015;83(2):162–6.
Iglesias S, et al. Hair cortisol: a new tool for evaluating stress in programs of stress management. Life Sci. 2015;141:188–92.
Staufenbiel SM, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–35.
Chen Z, et al. Simultaneous determination of hair cortisol, cortisone and DHEAS with liquid chromatography-electrospray ionization-tandem mass spectrometry in negative mode. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;929:187–94.
Dettenborn L, et al. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. 2012;15(6):578–88.
Dettenborn L, et al. Introducing a novel method to assess cumulative steroid concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 2012;15(3):348–53.
Streit F, et al. Perceived stress and hair cortisol: differences in bipolar disorder and schizophrenia. Psychoneuroendocrinology. 2016;69:26–34.
Steudte-Schmiedgen S, et al. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology. 2015;59:123–33.
Manenschijn L, et al. Long-term cortisol in bipolar disorder: associations with age of onset and psychiatric co-morbidity. Psychoneuroendocrinology. 2012;37(12):1960–8.
Manenschijn L, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing’s syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab. 2012;97(10):E1836–43.
Noppe G, et al. Elevated hair cortisol concentrations in children with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol. 2014;81(6):820–5.
Gruber CJ, et al. Production and actions of estrogens. N Engl J Med. 2002;346(5):340–52.
Arnett-Mansfield RL, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61(11):4576–82.
Chakraborti A, Gulati K, Ray A. Estrogen actions on brain and behavior: recent insights and future challenges. Rev Neurosci. 2007;18(5):395–416.
Bechlioulis A, et al. Cardiovascular effects of endogenous estrogen and hormone therapy. Curr Vasc Pharmacol. 2010;8(2):249–58.
Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–7.
Gao W, et al. HPLC-FLU detection of cortisol distribution in human hair. Clin Biochem. 2010;43(7–8):677–82.
Choi MH, Kim KR, Chung BC. Determination of estrone and 17 beta-estradiol in human hair by gas chromatography-mass spectrometry. Analyst. 2000;125(4):711–4.
Jung HJ, et al. Gas chromatography/mass spectrometry based hair steroid profiling may reveal pathogenesis in hair follicles of the scalp. Rapid Commun Mass Spectrom. 2011;25(9):1184–92.
Choi MH, Chung BC. GC-MS determination of steroids related to androgen biosynthesis in human hair with pentafluorophenyl-dimethylsilyl-trimethylsilyl derivatisation. Analyst. 1999;124(9):1297–300.
Bevalot F, et al. Analysis of corticosteroids in hair by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;740(2):227–36.
Pellegrini M, et al. Role of the sex hormone estrogen in the prevention of lipid disorder. Curr Med Chem. 2014;21(24):2734–42.
Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33(1):1–47.
Walf AA, et al. I. Levels of 5alpha-reduced progesterone metabolite in the midbrain account for variability in reproductive behavior of middle-aged female rats. Brain Res. 2011;1379:137–48.
Shivers K-Y, et al. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine. 2015;72(2):121–9.
Zhao Y, et al. Liquid chromatography tandem mass spectrometry determination of free and conjugated estrogens in breast cancer patients before and after exemestane treatment. Anal Chim Acta. 2014;806:172–9.
Jeon S-Y, Hwang K-A, Choi K-C. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol. 2016;158:1–8.
Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99:8–10.
Wang C, et al. Ultraperformance liquid chromatography-tandem mass spectrometry method for profiling ketolic and phenolic sex steroids using an automated injection program combined with diverter valve switch and step analysis. Anal Chem. 2016;88(16):7878–84.
Higashi T, Ogawa S. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review. J Steroid Biochem Mol Biol. 2016;162:57–69.
Weisser JJ, et al. Two simple cleanup methods combined with LC-MS/MS for quantification of steroid hormones in in vivo and in vitro assays. Anal Bioanal Chem. 2016;408(18):4883–95.
Xu X, et al. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–21.
Xu, W., et al.. A rapid and simple liquid chromatography-tandem mass spectrometry method for the measurement of testosterone, androstenedione, and dehydroepiandrosterone in human serum. J Clin Lab Anal. 2016.
Xu X, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77(20):6646–54.
Thomson S, et al. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diabetes. 2010;118(2):133–8.
Zelena E, et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal Chem. 2009;81(4):1357–64.
Dunn WB, et al. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis. 2012;4(18):2249–64.
Gao W, et al. LC-MS based analysis of endogenous steroid hormones in human hair. J Steroid Biochem Mol Biol. 2016;162:92–9.
Dettenborn L, et al. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–9.
Acknowledgments
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2016ZX09101017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants who provided hair samples. Ethics approval for this study has been obtained from the Ethics Committee, Peking Union Medical College Hospital, China.
Electronic supplementary material
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Dong, Z., Wang, C., Zhang, J. et al. A UHPLC-MS/MS method for profiling multifunctional steroids in human hair. Anal Bioanal Chem 409, 4751–4769 (2017). https://doi.org/10.1007/s00216-017-0419-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0419-2