Abstract
We developed and evaluated a candidate serum reference material to help improve clinical routine measurement, and to provide traceability of the measurement results. D8-Homocystine, dithiothreitol, and acetonitrile were used as an internal standard, the reducing agent, and the protein precipitating agent, respectively. A triple quadrupole mass spectrometer with an electrospray ionization source was used for monitoring the transitions (m/z 140.0 → 94.0, 136.0 → 90.0) in multiple-reaction-monitoring mode. We used a calibration model relying on bracketing and gravimetric measurements to give SI-traceability and higher accuracy to serum value assignments. The method was evaluated for accuracy using NIST Standard Reference Material SRM1955. The results of the three concentrations (1, 2, and 3) of total homocysteine in human serum samples were determined by an isotope-dilution liquid chromatography-tandem mass spectrometry method; tHcy 1 is 28.8 ± 1.1 μmol/L, tHcy 2 is 17.93 ± 0.57 μmol/L, and tHcy 3 is 14.38 ± 0.46 μmol/L.

The workflow diagram.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homocysteine (Hcy) is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine, an essential amino acid. Hcy is regarded as an independent risk factor for cardiovascular disease. Increased levels of Hcy in plasma are strongly correlated with coronary artery disease [1–4], cerebrovascular disease [5, 6], and venous thrombosis [7, 8].
Various methods have been developed to determine the concentration of total homocysteine (tHcy) in serum or plasma. Radioimmunoassay (RDA), fluorescence polarization immunoassay (FPIA), enzyme-linked immunosorbent assay (ELISA), enzymatic cycling assay (ECA), and liquid chromatography with either fluorescence detection (LC-FD) or electrochemical detection (LC-ED) are more traditional approaches and are widely used in routine clinical measurements [9–11]. However, the results obtained with different methods are often not comparable because of inter-method and inter-laboratory variability. Thus, establishing reference methods for value-assigning certified reference materials (CRMs) and external quality assessment is the best way to help verifying standardization effectiveness.
Most recently, isotope-dilution gas chromatography coupled with mass spectrometry (ID/GC/MS) and isotope-dilution liquid chromatography–tandem mass spectrometry (ID/LC/MS/MS) have been effectively used to measure plasma tHcy because of their specificity and accuracy [12–17]. There are currently three Reference Measurement Procedures for homocysteine in serum listed in the JCTLM database (GC/MS [14], LC/MS and LC/MS/MS [13] from NIST). The increased accuracy, provided by the use of stable-isotope-labeled internal standards, allows ID/MS to be an effective quantitative approach for the accurate determination of tHcy via minimizing the matrix effects in serum or plasma.
Experimental
Reagents and materials
The candidate reference material (RM) was prepared by Beijing Aerospace General Hospital (Beijing, China). Each donor unit of serum used in the preparation of this product was fresh and tested by the colloidal gold method and was found to be nonreactive for hepatitis B surface antigen (HbsAG), hepatitis C virus (HCV), and human immunodeficiency virus 1 (HIV-1) antibodies. However, since no known test method can offer complete assurance that infectious agents are absent from this material, this human blood-based product should be handled at Biosafety Level 2 or higher, as recommended for any potentially infectious human serum or blood specimen [18].
dl-Homocystine was obtained from Sigma Chemical Company (St. Louis, MO, USA), and the purity, determined by NMR, was calculated as 98.8%, the internal standard was acesulfame potassium (NIM CRM GBW(E)100065, the purity is 99.7%, and the uncertainty is 0.6%). dl-[2H8]-Homocystine was obtained from C.D.N. Isotopes (Pointe-Claire, Quebec, Canada). dl-[2H8]-Homocystine contains eight stable 2H isotopes (3, 3, 3′, 3′, 4, 4, 4′, 4′-d8) incorporated into its methylene groups, and the supplier’s listed purity was >99.5 atom-% deuterium. Dithiothreitol (DTT, purity ≥ 99%) was obtained from INALCO SPA Company, Milano, Italy.
NIST serum Standard Reference Material (SRM 1955: Homocysteine and Folate in Frozen Human Serum) was used to evaluate our method. Purified water (18.2 MΩ), prepared using a Millipore Milli-Q purification system, was used to prepare all samples and standards. All other solvents were HPLC grade, and chemicals were reagent grade unless stated otherwise.
Preparation of candidate material
The candidate serum material was prepared from 1000 patients in Beijing Aerospace General Hospital. According to the concentration of Hcy determined by a routine method, the 1000 serum samples of approximately 1 L were divided into three concentration levels. The volume of each pool was approximately 300 mL after abandoning the infected samples. Each pool was mixed magnetically stirring at room temperature (20–25 °C) for 30 min and then sub-packed in amber vials and stored at −80 °C.
Homogeneity study
The homogeneity study was delegated to the Beijing Aerospace General Hospital Reference Laboratory. Seventeen samples at each concentration level were selected in a stratified random manner and measured by enzymatic cycling assay (S-adenosine homocysteine hydrolase based on small molecule capture technology) in a Hitachi 7170A automatic analyzer. The injection volume was 18 μL, the linear range was 0–50 μmol/L, and each sample was analyzed in triplicate. Statistical tests for homogeneity of the standard materials were performed by means of variance analysis according to ISO Guide 35: 2006.
Stability study
The stability of reference materials is defined as the property of the standard substance under a specified time interval and set of environmental conditions. Serum tHcy standard material was stored at −80 °C under the protection of dry ice during transport. The long-term stability test was performed using the isotope dilution mass spectrometry method. The sample preservation temperature was −80 °C and all experiments were completed at room temperature in 2 h. The short-term stability test was completed by ECA in the Beijing Aerospace General Hospital Reference Laboratory. We tested the stability of the candidate RM stored under the following different conditions: three freezing and thawing cycles at −20 °C, storage at −20 °C for 30 days, storage at 4 °C for 7 days, and storage at room temperature for 3 days. For each concentration level, the three vials were selected in a stratified random manner at regular intervals and the average value was taken. Statistical tests for stability of the standard materials were performed according to ISO Guide 35: 2006.
Commutability study
Commutability with native clinical samples is a property that exists for a reference or trueness control material among different measurement procedures [19]. In this study, we chose the GCell kit (Beijing Strong Biotechnologies, Inc) coupled with a Hitachi 7170A automatic analyzer system used in Beijing Aerospace General Hospital Reference Laboratory. The principle of GCell kit is enzymatic cycling assay (ECA). Twenty fresh human serum samples covering the three concentrations of candidate reference material were selected as the object of investigation, and the commutability of this candidate RM was preliminarily evaluated in this conventional analysis system according to CLSI EP30-A [19].
Calibration preparation
Homocysteine (Hcy) and deuterated homocysteine ([2H4]-Hcy) stock solutions (∼10 μg/g) were prepared by chemically reducing the gravimetrically prepared homocystine and deuterated homocystine ([2H8]-homocystine) stock solutions (∼1000 μg/g), respectively. Briefly, dried Hcy or deuterated homocystine powder (∼3 mg) was weighed into a glass vial containing a known amount (∼3 g) of 0.1 mol/L HCl. The homocystine stock solution was then neutralized (pH ≈ 7) by the addition of a small portion (30 μL) of 10 mol/L NaOH. The aforementioned stock solutions were then reduced and diluted with 1.5% DTT. The final concentration of the Hcy stock solution was calculated on the basis of the relationship that 1 mole of homocystine is chemically reduced to 2 moles of Hcy by the action of DTT.
We prepared four calibration solutions by adding a series of different volumes of Hcy stock solutions to a constant volume of [2H4]-Hcy stock solution to achieve unlabeled-to-labeled Hcy mass ratios of 0.5, 0.8, 1.2, and 1.5.
Sample pre-treatment [13]
As with the preparation of the calibration solutions, all steps were performed gravimetrically. Serum samples were prepared by measuring 150 μL of serum and spiking it with sufficient [2H4]-Hcy stock solution to produce a final ratio of approximately 1:1 unlabeled-to-labeled Hcy. We calculated the amount of internal standard solution needed for this step from the approximate concentration of Hcy in the sample, as determined by a routine method. After 30 μL of 5% DTT was added, the solution was thoroughly mixed on a vortex mixer and allowed to stand at room temperature for at least 15 min. To precipitate the protein, 600 μL of 0.1% formic acid plus 0.05% trifluoroacetic acid in acetonitrile was added to the sample, and the solution was vortex-mixed for 2 min. After standing for another 15 min and being vortex-mixed again, the sample was centrifuged at 13,000g for 2 min, and the supernatant was filtrated through a 0.22-μm organic diameter filter for analysis.
At the same time, we treated SRM 1955 using the same procedures to complete the method validation.
LC-MS/MS conditions
We performed the analyses on an Agilent 6410 Triple Quad mass spectrometer equipped with an Agilent 1200 LC system. Samples were analyzed using a Supelcosil LC-CN analytical column (4.6 mm × 250 mm, 5 μm particle size) at a temperature of 30 °C and were eluted at 0.5 mL/min using a mobile phase of 10:90 acetonitrile/water with 0.1% formic acid (isocratic elution). The autosampler temperature was 4 °C and the injection volume was 10 μL. MS parameters were optimized by infusing the Hcy and [2H4]-Hcy stock solution. Full scan, selected-ion-monitoring (SIM), and multiple-reaction-monitoring (MRM) mode mass spectra of Hcy and [2H4]-Hcy were obtained and optimized via positive-ion ESI (Table 1).
Data analysis
We used Agilent MassHunter Workstation Software Qualitative Analysis (B.03.01) to integrate selected peaks. Quantification was performed with the peak area count ratio of the Hcy quantification ion over the [2H4]-Hcy quantification ion. We used the mean area count ratios from the three injections for data analysis. We plotted the area count ratios over the mass ratios of the calibration solutions and applied a linear regression model. The mass ratio of the sample was determined by applying the calibration curve to the area count ratio of the sample. We calculated the mass of Hcy in the sample with the mass ratio and the mass of [2H4]-Hcy added to the sample. The mass concentration of Hcy in the serum sample was determined from the mass of the serum analyzed.
Results and discussion
Homogeneity study
The single-factor variance analysis results are shown in Tables 2, 3, and 4.
Stability study
Because of the recent certification of this material, the three concentrations of total Hcy show good stability at −80 °C after 12 months. NIM will continue to monitor the long-term stability of the analyte in this material.
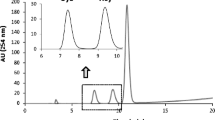
The short-term stability testing results are shown in Fig. 1.
In conclusion, a freezer temperature of −20 °C is acceptable for storage for up to 1 month or even longer, but continued monitoring is needed to determine the exact time limit. Storage of thawed material at room temperature for 3 days, 4 °C for 7 days, and repeated freeze-thaw for three cycles did not induce any significant changes in the analyte concentrations.
This candidate RM exhibits very good stability under different storage conditions; however, we still recommend using it under specified working conditions (room temperature or 4 °C placed in an airtight container within 1 day) to avoid the occurrence of unknown problems. If a longer storage time is anticipated, the material should be stored at −80 °C.
Commutability study
The result of the previously discussed commutability study indicates that the candidate RMs are commutable in the pairwise comparison involving IDMS and the GCell method using the prediction interval approach (Fig. 2).
Value assignment and the uncertainty evaluation
At the same time, NIST serum Standard Reference Material (SRM 1955: Homocysteine and Folate in Frozen Human Serum) was used to evaluate our method (Tables 5 and 6).
The combined standard uncertainty (u c ) of the tHcy concentration for the candidate RM was estimated by combining the contributions of the value assignment (u char ), the inhomogeneity of the material (u bb ), the uncertainty due to long-term instability (u lts ), and the uncertainty due to short-term instability (u sts ). The u c was calculation of using the following equation:
Because the transportation conditions (dry ice transport) are consistent with the long-term preservation conditions, we did not consider the uncertainty caused by transport instability. Under the guidance of ISO Guide 35: 2006, we performed T test on each slope of the four storage conditions in the short-term stability test, all t values are less than t α, ν, indicating there is no significant change; thus, the u sts can be ignored (Table 7).
From these values, the certified value of the candidate RM was determined as shown in Table 8.
Conclusion
The determination of total Hcy in human serum cannot only be used as diagnostic criterion for patients with cardio cerebral vascular disease but also is important for prognosis judgment. In the present study, we reported the development of a matrix-based candidate reference material of tHcy in human serum, the value of which was assigned by higher-order reference methods based on isotope dilution mass spectrometry, and the uncertainty was fully evaluated. The homogeneity and stability under different storage conditions were tested to provide users with more information. NIST has developed SRM 1955 (homocysteine and folate in human serum) to improve inter-laboratory and inter-method agreement [16], it ranges from 3.98 to 17.7 μmol/L, and this candidate RM can be complementary at the high concentration level. We also assessed the commutability on one routine clinical measurement method and intended to investigate with other more assays.
References
Palazhy S, Kamath P, Vasudevan DM. Elevated oxidative stress among coronary artery disease patients on statin therapy: a cross sectional study. Indian Heart J. 2015;67(3):227–32.
Verdoia M, Schaffer A, Pergolini P, et al. Homocysteine levels influence platelet reactivity in coronary artery disease patients treated with acetylsalicylic acid. J Cardiovasc Pharmacol. 2015;66(1):35–40.
Kumakura H, Fujita K, Kanai H, et al. High-sensitivity C-reactive protein, lipoprotein(a) and homocysteine are risk factors for coronary artery disease in Japanese patients with peripheral arterial disease. J Atheroscler Thromb. 2015;22(4):344–54.
Shenoy V, Mehendale V, Prabhu K, et al. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem. 2014;29(3):339–44.
Alvarez B, Yugueros X, Fernández E, et al. Relationship between plasma homocysteine and the morphological and immunohistochemical study of carotid plaques in patients with carotid stenosis over 70%. Ann Vasc Surg. 2012;26(4):500–5.
Choi JW, Lee MH, Fujii T, et al. Association of the urine homocysteine/creatinine ratio to proinflammatory cytokine, natural anticoagulant, and nitric oxide levels in cerebrovascular disease. Ann Clin Lab Sci. 2014;44(4):461–5.
Mouravas H, Verettas D, Kazakos K, et al. Homocysteine and its relationship to deep venous thrombosis in patients undergoing total knee or hip arthroplasty. Hippokratia. 2010;14(3):185–8.
Hoţoleanu C, Porojan-Iuga M, Rusu ML, et al. Hyperhomocysteinemia: clinical and therapeutical involvement in venous thrombosis. Rom J Intern Med. 2007;45(2):159–64.
Amores-Sánchez MI, Medina MA. Methods for the determination of plasma total homocysteine: a review. Clin Chem Lab Med. 2000;38(3):199–204.
Rasmussen K, Møller J. Total homocysteine measurement in clinical practice. Ann Clin Biochem. 2000;37(Pt 5):627–48.
Ubbink JB. Assay methods for the measurement of total homocyst(e)ine in plasma. Semin Thromb Hemost. 2000;26(3):233–41.
Magera MJ, Lacey JM, Casetta B, et al. Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 1999;45(9):1517–22.
Satterfield MB, Sniegoski LT, Welch MJ, et al. Comparison of isotope dilution mass spectrometry methods for the determination of total homocysteine in plasma and serum. Anal Chem. 2003;75:4631–8.
Nelson BC, Pfeiffer CM, Sniegoski LT, et al. Development and evaluation of an isotope dilution LC/MS method for the determination of total homocysteine in human plasma. Anal Chem. 2003;75:775–84.
Nelson BC, Satterfield MB, Sniegoski LT, et al. Simultaneous quantification of homocysteine and folate in human serum or plasma using liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:3586–93.
Satterfield MB, Sniegoski LT, Sharpless KE, et al. Development of a new standard reference material: SRM 1955 (homocysteine and folate in human serum). Anal Bioanal Chem. 2006;385:612–22.
Ducros V, Belva-Besnet H, Casetta B, et al. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin Chem Lab Med. 2006;44(8):987–90.
World Health Organization. Laboratory biosafety manual—third edition. WHO, 2004.
Clinical and Laboratory Standards Institute. EP30-A characterization and qualification of commutable reference materials for laboratory medicine; approved guideline. CLSI, 2015.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 21275134) and National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAK10B01, 2011FY130100, 2011BAI02B05, 2011AA02A111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Beijing Aerospace General Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Y., Song, D., Xu, B. et al. Development of a matrix-based candidate reference material of total homocysteine in human serum. Anal Bioanal Chem 409, 3329–3335 (2017). https://doi.org/10.1007/s00216-017-0272-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0272-3