Abstract
Septic shock is the most severe form of sepsis, which is still one of the leading causes of death in the intensive care unit (ICU). Even though early prognosis and diagnosis are known to be indispensable for reaching an optimistic outcome, pathogenic complexities and the lack of specific treatment make it difficult to predict the outcome individually. In the present study, serum samples from surviving and non-surviving septic shock patients were drawn before clinical intervention at admission. Metabolic profiles of all the samples were analyzed by liquid chromatography-mass spectrometry (LC-MS)-based metabolomics. One thousand four hundred nineteen peaks in positive mode and 1878 peaks in negative mode were retained with their relative standard deviation (RSD) below 30 %, in which 187 metabolites were initially identified by retention time and database in the light of the exact molecular mass. Differences between samples from the survivors and the non-survivors were investigated using multivariate and univariate analysis. Finally, 43 significantly varied metabolites were found in the comparison between survivors and non-survivors. Concretely, metabolites in the tricarboxylic acid (TCA) cycle, amino acids, and several energy metabolism-related metabolites were up-regulated in the non-survivors, whereas those in the urea cycle and fatty acids were generally down-regulated. Metabolites such as lysine, alanine, and methionine did not present significant changes in the comparison. Six metabolites were further defined as primary discriminators differentiating the survivors from the non-survivors at the early stage of septic shock. Our findings reveal that LC-MS-based metabolomics is a useful tool for studying septic shock.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septic shock is the most severe form of sepsis leading to a high mortality rate [1, 2]. In recent years, although progress has been made with prompt initiation of therapy to treat underlying infection and support failing organs [3–5], mortality of septic shock remains high up to 30–60 % [6, 7], due to the pathogenic complexity and the lack of standard of specific treatment [8, 9]. It is known that early goal-directed therapy is highly significant to improve the outcome [10, 11]. However, as various kinds of bacteria can cause septic shock [12], it appears difficult to propose specific treatment with only a clinical examination [13] since it usually induces false-positive and false-negative results. Thus, an available prognostic method guiding specific treatment is still needed.
Past clinical studies have shown some biomarkers such as C-reactive protein (CRP); interleukin (IL) especially IL-6, IL-10, IL-8; and procalcitonin (PCT) distinguish septic patients from health controls [14, 15]. In the light of the proteomic approach, other proteins have also been proposed [5, 16]. Being able to show variations in the whole metabolic profile, metabolomics has been proved potent to be used in the clinical field in recent years [17]. Concerning sepsis research, studies in rodent animals and in human patients have revealed that some metabolites might be specific for the prediction and prognosis of sepsis or septic shock [18]. Using non-targeted method, Garcia et al. showed that a predictive model with up-regulated alanine, creatine, phosphoethanolamine, and acetoacetate distinguished septic rats from those in the control group [19]. Xu el al. revealed that several fatty acids might be correlative to the prediction of sepsis [20]. Fanos et al. separated early onset from late onset in neonatal sepsis [21]. Targeted profiling approach was also applied by Mickiewicz et al. who was able to predict the death in septic shock by their model within 31 metabolites identified [13]. Yet, to our knowledge, the prediction of the mortality of septic shock was rarely reported, and there has been no published study on mortality of septic shock employing the LC-MS approach. In our present work, in order to analyze the metabolic profiles of septic shock patients at admission, the LC-MS-based non-targeted metabolomics was applied to discover a possible relationship between the metabolic changes and the outcome.
Experimental section
Patients and sample collection
All human serum samples were collected before clinical intervention at the Jean Verdier Hospital, Bondy (F93140). The written informed consent was obtained from all subjects or their surrogate decision maker. Patients according with specific published criteria for septic shock at admission to the intensive care unit (ICU) were enrolled in this study [22]. The samplings were fulfilled from January 2009 to December 2011. In order to homogenize the samples and limit the impact from other complications, the screening standard included the following factors: (1) younger than 30 years old, (2) hematonosis, (3) cancer and other metabolic-related diseases, and (4) cirrhosis or chronic kidney disease. Included non-survivors died within 7 days after admission to the ICU, and other patients were still alive. The samples were stored by the CRB (liver disease biobank) Groupe Hospitalier Paris Seine-Saint-Denis BB-0033-00027.
Chemicals
HPLC-grade methanol was purchased from Merck (Darmstadt, Germany). Formic acid was obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was prepared by the Milli-Q system (Merck, Darmstadt, Germany).
Sample preparation
All the serum samples were stored at −80 °C until the beginning of sample preparation. After thawing, the samples on the ice, 100 μL from each sample, were primarily integrated to form the pooled serum. The pooled serum was then subpacked into equal aliquots being quality control (QC) samples. Another 100 μL serum was drawn from each real sample and QC sample; it was then extracted by 400 μL methanol solution (Methanol: H2O 4:1) on the ice. After vortexing for 1 min, the mixture was centrifuged at 16,000×g at 4 °C during 15 min. An equal volume of supernatant in each was then preserved. The supernatant of sera from non-survivors or survivors was randomized and transferred to an auto-sampler vial. Several QC samples were localized before the real samples to balance the equipment while other QC samples were inserted after each ten real samples in the sequence of analysis to affirm the stability of instrument and to obtain complementary product ion information.
UPLC-MS
An ultra performance liquid chromatography (UPLC) system (Waters, Milford, USA) was used to separate the metabolites in the serum. Five microliter aliquots were injected into a reversed-phase UPLC ACQUITY T3 column (Waters, 2.1 mm × 50 mm × 1.8 μm) maintained at 60 °C. Phase A corresponds to water with 0.1 % formic acid (FA); phase B corresponds to acetonitrile with 0.1 % FA. The gradient elution was as follows at a flow rate of 0.35 mL/min: 2 % B maintained for 1 min then linearly increased to 100 % B from 1 to 15 min, maintained 100 % B for 4 min, followed by equilibration at 2 % B for 2.9 min.
A LTQ-Orbitrap-XL (Thermo Fisher Scientific, Waltham, USA) is tandem to the UPLC system using an electrospray ionization (ESI) ion source. Samples were respectively tested in the positive and negative modes by a full scan from 50 to 1000 m/z at the mass resolving power of 30,000 at 400 m/z. The collision energy was at 35 eV. The I-spray voltage and capillary voltage were set to 4.5 and 49 kV for the positive mode and 4 and 37 kV for the negative mode.
Regarding the sequence of analysis, all the real samples were randomized. Blank samples containing barely the solvent were added after every 20 samples, aiming to elute residuals or other impurities which were retained by the chromatography column in the previous spectra records.
Data processing
After the recording of spectra by Xcalibur™ software (Thermo Fisher Scientific), the alignment and the peak detection were obtained by the SIEVE software version 2.2 (Thermo Fisher Scientific). Concretely, for peak framing, the retention time width of the alignment was set to 0.2 min with the mass width of 50 ppm. Ion peaks were filtered with an intensity threshold of 30,000 for both positive and negative modes. Normalization and auto-scaling of the ion peaks were then performed. The data set containing all the peak area, which corresponds to the concentration of certain metabolite, was exported to the SIMCA-P software (version 11, Umetrics, Umeå, Sweden) for the principal component analysis (PCA) and partial least square discrimination analysis (PLS-DA). In order to define significant variables between the survivors and the non-survivors, a student’s t test of all the screened peaks was carried out using SPSS statistics (version 19, IBM, Chicago, USA). The assignment of the significant ion peaks was proceeded by searching the METLIN database (https://metlin.scripps.edu) and the HMDB database (http://www.hmdb.ca). Metabolites, which were statistically significant for the separation between the two groups of patients, were selected. A hierarchical cluster analysis (HCA) was generated with these metabolites by the MultiExperiment Viewer (Mev, version 4.9.0, Dana-Farber Cancer Institute, MA, USA). Prime discriminating metabolites were selected by plotting the data set of all the discriminators in a volcano plot, and the boxcharts of these metabolites were drawn using the Origin software (version 9.2, OriginLab, Northampton, USA). Primary discriminators predicting early mortality of septic shock were afterward submitted to the SPSS software to calculate the area under the receiver operating characteristic (ROC) curve, which reflects the discriminatory power of the defined metabolites.
Results and discussion
Subjects
The study included a total of 50 patients who were screened from 92 patients with septic shock. Of these patients, 29 were dead within 7 days after admission to the ICU; other 21 patients were cured after therapies. Further clinical information of the patients at admission to the ICU was presented in Table 1. According to the data, there was only one significant difference (p < 0.05) in the concentration of lactate among the metabolites in measured sera between the two groups of patients. In addition, the two score systems, that is, the simplified acute physiological score II (SAPS II) and the sequential organ failure assessment score (SOFA) [23, 24], were found higher in the dead patients than those in the alive patients; these differences were statistically significant (P < 0.05). Other assessed components such as AST, ALT, and GGT, which are aminotransferases indicating the liver function, were shown increased in the non-survivors, even the disparity was not significant [25].
Assessment of QC samples and pre-attribution of peaks
Nine QC samples prepared as real ones were analyzed before real samples to test the repeatability and stability of the experiments. From the alignment of spectra, 3000 ion peaks within their area were recognized by the alignment software. On the basis of those normalized ion peaks, relative standard divergence (RSD) of each ion peak in all QC samples was calculated. We noted that ion peaks with RSD <30 % occupied 82.1 % of total peak area in positive mode, and this was 76 % in negative mode, indicating that acquiring of spectra was relatively reliable and the obtained data were eligible for statistical analysis. As regard to real samples data, those ion peaks with RSD higher than 30 % in QC samples were excluded before following analysis. In addition, peaks within excess zero area (zero area peaks occupied more than 20 % of integrated peak area) were also eliminated. Then, 1419 ion peaks and 1878 peaks were retained in positive and negative modes, respectively. All the 3297 peaks were integrated for all the QC samples and real samples, and a PCA model for all the samples was performed. Relative to other real samples, the QC samples were observed to be together, showing a stability of the experiment (see Electronic Supplementary Material (ESM) Fig. S1). The identification of the remained ion peaks was performed by searching the acquired exact molecular mass data (m/z) in the database (METLIN and HMDB) and in our previous work [26]. One hundred four metabolites in positive mode and 83 metabolites in negative mode were therefore identified (ESM Table S1).
Identification of varied metabolites
Two data sets were respectively established by the 1419 peaks in positive mode and the 1878 peaks in negative mode. The normalized ion peak areas were centered, and then submitted to the SIMCA-P software. In the PCA model, an apparent separation between the non-survivors and the alive patients has been displayed in positive mode (Fig. 1a). Although, in negative mode the discrimination between the two groups was shown less distinct than that in positive mode, a visible dispersal was still observed (Fig. 1b). In the PLS-DA model, the separation between the survivors and non-survivors was clearly presented with a Q2 = 0.43 (Fig. 1c). We validated this model with a permutation of 200 times; it showed that this model was reliable without overfitting (ESM Fig. S2).
Score plot of the multivariate analysis presenting the separation between non-survivors (red dots) and survivors (blue dots). a PCA score plot for the positive mode. b PCA score plot for the negative mode. c PLS-DA model showing the discrimination between the survivors (blue dots) and the non-survivors (red dots) in negative mode
Interestingly, we also noted a dispersion of sample from the non-survivors while the opposite was found in the alive group. This result implied that the differences existed not only between the survivals and the non-survivals but also longitudinally among the non-survivors. As it is known that the septic shock is possibly caused by various kinds of pathogenic bacteria and even leads to different organ dysfunctions [12], this finding might provide evidence that the complications aggravate the case and metabolic profiles of non-survivors may differ by leading to different complications.
In order to find out the metabolites whose concentrations were altered in non-survivors, compared to those in survivors, we defined all the ion peaks, with their variance importance (VIP) in the PLS-DA model, which is superior to 1. A student t test was also performed between the two groups of patients. Peak areas owing P value that were superior to 0.05 were excluded.
Further confirmation of our peak assignments was proceeded by the retention time (tR) of peak and by comparing the identified peak to MS/MS database. Three metabolites (tyrosine, phenylalanine, and glutamine) were defined as important discriminators by both positive and negative modes. We kept the result which presented a higher fold change. Finally, 22 significantly varied metabolites in positive mode and 21 in negative mode were obtained. The P value and the fold changes were calculated (ESM Table S2). A heat map was subsequently applied revealing the change direction of the discriminators (shown in Fig. 2). Significant variations between survivors and non-survivors have been found in metabolites included in the TCA cycle and in the urea cycle. Concretely, levels of TCA cycle metabolites such as alpha-KG, succinate, citrate, and malate have been determined elevated and various kinds of amino acids increased in non-survivor group; the declines of concentrations of long-chain (e.g., C18 and C16) and short-chain fatty acids in non-survivors were also found, accompanied by the changes in carnitines; other discriminating metabolites between the two groups of patients including bile acids, hypoxanthine, indoxysulfate, lysophosphatidylcholine (LPC), and lysophosphatidylethanolamine (LPE) were observed. Besides, certain amino acids such as alanine, methionine, and lysine were found not significant in the comparison between the two groups of patients. The same effect was also true for xanthine, some organic acids (such as benzoic acid, threonic acid, and quinic acid), and some dipeptides (such as glu-leu, ile-pro, phe-phe, etc.).
Heat map displaying the discriminating metabolites between the septic survivors and non-survivors with their trend of variation. Columns at the left side of the dotted line represent the samples from the non-survivors, while those on the right side of the dotted line represent the samples from the survivors. Level of the discriminating metabolites in all the samples is shown in lines. Up-regulated metabolites are presented with the yellow color while the down-regulated metabolites are shown with the blue color
Interpretation of the discriminators
A typical criterion for the diagnosis of septic shock is the symptom of ischemia and reperfusion, triggered by the endotoxin-induced shock, which leads to hypoxia [27, 28]. In this context, energy supply in septic patients is impaired by a decrease of ATP production [28]. Serum lactate is a direct indicator of hypoxia and anaerobic glycolysis; it was also reported to be specific in the contrast between septic patients and healthy controls. As to our results, an increment of lactate in non-surviving septic shock patients might implicate an increase of hypoxia in tissue and organs.
On the other hand, the deteriorated condition of energy supply in septic non-survivors was proved by the enhanced catabolism. Various amino acid concentrations increased in the non-survivors, which is in accord with Whelan et al. who summarized that the degradation of protein and the anaplerotic reaction contributed to the TCA cycle [29]. This effect might be also attributed to a sharper breakdown of proteins in organs and in muscle tissue in non-survivors [30]. Another point concerning the scarce of energy supply was that a decline of various fatty acids and a remarkable decrease of carnitine C 2:0 but increase in other carnitines were found in non-survivors. This result was corresponding to the depletion of lipids in the group of non-survivors since carnitine C 2:0 reacts initially with fatty acids during the beta-oxidation producing other long carbon chain carnitines [31]. Furthermore, we calculated the ratio of carnitine C 2:0 and carnitine which reflects the extent of the beta-oxidation [32]. We also found a sharp decrease of this ratio in the non-survivors, compared with that in the survivors (ESM Fig. S3). Thus, we demonstrated a faster beta-oxidation in the non-survivors.
Interestingly, it was reviewed by Trivai et al. that mortality was found lower in obese patients of sepsis [33]. The shift of mortality influenced by the obesity may be explained by the tolerance of sharp consumption of lipids.
The level of citrate, 2-ketoglutarate, succinate, and malate, which are metabolites included in the TCA cycle, increased generally in septic non-survivors. One reason could be both the anaplerotic reaction of amino acids and the enhanced beta-oxidation of fatty acids. Despite an increased level of these TCA cycle involved metabolites in the non-survivors, they were worse effectively applied to produce energy in injured mitochondria in the severe hypoxic condition [18].
Notably, the greater the effects above were induced by hypoxia, the more severe the organs such as liver and kidney would be injured. This would be a possible origin of aggravated organ dysfunctions in septic non-survivors. First, the up-regulated concentration of lactate and acids included in the TCA cycle might lead to a deviant acidosis in the blood [34]. Second, metabolites concerning the beta-oxidation could aggravate the metabolic stress for kidney and liver, while the damage on both organs also might lead to further impairment of detoxification [35]. Finally, due to an impaired metabolic capacity of ammonia clearance, the increment stress of transamination and deamination from the anaplerotic reactions of amino acids might aggravate the metabolic burden for the liver and kidney. Notable decline of argininosuccinate, citrulline, and orthinine in septic non-survivors, which are from the urea cycle in our findings, demonstrated our hypothesis because the urea cycle was bound up with the ammonia metabolism. This fact was in accordance with Steelman et al. who suggested that a down-regulated urea cycle-related metabolism might be useful to predict the outcome of sepsis [36].
Other statistically significant differences were identified in the present study such as bile acids, creatine, creatinine, indoxylactate, and indoxysulfate are also found to be significant to discriminate non-survival patients from survival ones. All these metabolites were up-regulated in dead patients. These findings were reinforced in previous studies [37–39]. We previously demonstrated that the concentration of bile acids in serum was widely found elevated in patients who suffered from liver injury [40–42]. Seymour et al. have also reported that increased bile acids from dead septic patients were due to the modified membranous receptor of hepatocyte and hepatocyte tight junctions [43].
Increased creatine and creatinine may be the proof of the damage in the muscle tissue producing added amino acids in the serum [44]. The finding of the up-regulated level of indoxylactate and indoxysulfate may issue from the disruption of intestinal environment [45]. Moreover, Liu et al. figured out that this effect indicated the damage of tissue in kidney [37]. A decreased level of LPE and an increased level of LPC in the non-survivors indicated a disorder of the metabolism of phosphatidylethanolamine N-methyltransferase (PEMT), which regulates the conversion from phosphatidylethanolamine (PE) to phosphatidylcholine (PC). This disorder might be associated with a more severe injury in hepatocytes [46]. To sum up all, a concluded evolution from septic shock to death in the non-survivors is shown in Fig. 3. In the non-survivors, a more severe shock-induced hypoxia might lead to a greater scarce of energy supply, triggering an up-regulated catabolism. Despite of that, energy supply is still insufficient, proved by the increased lactate in the non-survivors. However, organs like the liver and kidney are prone to be injured with an augmented metabolism stress issued from the up-regulated catabolism. All the resulting metabolic changes aggravating organ failure responds adversely to the case and should account for the death.
Determination of primary discriminators
To seek out primary discriminators differentiating the survivors from the non-survivors, further screening based on larger variation range among the discriminate metabolites was proceeded, namely the metabolites showing both considerable fold change and P value in the comparison between the survivals and the non-survivals were eligible for candidates. To this end, we plotted all the determined metabolites into the volcano plot (shown Fig. 4a). Citrulline, carnitine 2:0, valine, leucine, isoleucine, and betanine were the most correlated to the discrimination between the non-survivors and survivors. Boxcharts for the six metabolites were shown in Fig. 4b.
Determination of the six defined metabolites which are the most responsible for the distinction between the septic surviving patients and the non-surviving patients. a A volcano plot achieved by log2 (fold change of the metabolite) (abscissa) and the - log10 (P value of the metabolite) (ordinate). Marked metabolites are those which show an important variation among all the changing metabolites. b A set of boxcharts of the most varied metabolites between septic survivors and non-survivors. Samples are presented by star symbols. Asterisks stands for a P value inferior to 0.01 by the comparison between the two groups of samples. c ROC curve for the six potential precursory metabolites predicting the death in the septic patients. Left panel: ROC curve for the four up-regulated metabolites in septic non-surviving patients. AUROCleu = 1.00, AUROCiso = 1.00, AUROCval = 0.96; AUROCbetaine = 0.90; right panel: ROC curve for two down-regulated metabolites in septic non-surviving patients. AUROCCitrulline = 0.96, AUROCCarnitine C 2:0 = 0.93
To investigate the ability of predicting death in septic shock, area under receiver operating characteristic curve (AUROC) for the six pivotal metabolites was respectively calculated (Fig. 4c). For the up-regulated metabolites in non-survivors, the AUROC was equal to 1 for leucine and isoleucine; meanwhile, it was 0.96 and 0.90 for valine and betaine, respectively (Fig. 4c left panel). With regard to both the down-regulated metabolites in non-survivors, citrulline, and carnitine C 2:0, their AUROC were 0.97 and 0.93, respectively (Fig. 4c right panel). It showed that all the six defined important discriminators were fairly specific and sensitive to predict the septic death at the onset of septic shock.
Among the defined six metabolites, interestingly, leucine, isoleucine, and valine, are all branched chain amino acids (BCCAs), were universally up-regulated in the non-survivors. BCCAs were recently found to be relevant amino acids that indicated the breakdown of muscle proteins [47].
Beyond the metabolites discussed above, the higher level of betaine in non-surviving patients is also associated with export of hepatic lipids and enhanced the fatty acid oxidation [48]. Without doubt, all these findings were in support of our presumption about the evolution of septic shock, shown in Fig. 3.
Comparing with the results of traditional estimation (Table 1), we suggest that the LC-MS metabolomic approach is more sensitive to detect metabolic alterations in the early stage of septic shock. Accordingly, it may be possible to classify the patients by the particular metabolic alterations and thereby to execute personalized therapies. Meanwhile, as being limited by the amount of samples, we should notice that all the findings should be verified in another larger population of patients in the future.
Conclusions
In the present study, we collected the serum samples from septic shock patients at admission. We showed that differences in the serum of septic shock patients can be detected by analyzing their metabolic profiles with LC-MS-based metabolomics approach. One the basis of the results, when it is compared to those in septic shock survivors, various increases of amino acids and comprehensive depletion of fatty acids indicate an enhanced catabolism in septic shock non-survivors. The effects may be associated with a more severe hypoxia-induced scarce of energy supply, which accounts for aggravated injury in organs such as the liver and kidney, and therefore indicates a poor prognosis. Typically, six metabolites crucially contribute to the separation of the septic surviving patients and the non-surviving patients. Their potential in the prediction of death is demonstrated by their excellent performance in the ROC curve. Detection of the level of these metabolites and comparison to that in other survived patients at the onset of septic shock may be beneficial to predict the death for septic shock patients.
We have reason to believe that the LC-MS-based metabolomics approach help to better classify the patients in the early stage of septic shock, which may be an important evidence to personalize the patients and thereby better formulate personalized therapeutic plan. Following study will be a verification of the results in a larger population of septic patients.
References
Cao Z, Yende S, Kellum JA, Angus DC, Robinson RA. Proteomics reveals age-related differences in the host immune response to sepsis. J Proteome Res. 2014;13(2):422–32.
Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a prospal. J Surg Res. 1980;29(2):189–201.
Messing B, Peitracohen S, Debure A, Beliah M, Bernier JJ. Antibiotic-lock technique—a new approach to optimal therapy for catheter-related sepsis in home - parenteral nutrition patients. Jpen-Parenter Enter. 1988;12(2):185–9.
Vincent F-L. New therapies in sepsis. Chest. 1997;112(6):330S–8.
Cao Z, Robinson RA. The role of proteomics in understanding biological mechanisms of sepsis. Proteomics Clin Appl. 2014;8(1–2):35–52.
Zhao X, Chen YX, Li CS. Predictive value of the complement system for sepsis-induced disseminated intravascular coagulation in septic patients in emergency department. J Crit Care. 2015;30(2):290–5.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet. 2014;86(1):56–61.
Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68 Suppl 1:S24–32.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 2001;345(19):1368–77.
Lam SW, Bauer SR, Guzman JA. Septic shock: the initial moments and beyond. Cleve Clin J Med. 2013;80(3):175–84.
Antti H, Fahlgren A, Nasstrom E, Kouremenos K, Sunden-Cullberg J, Guo Y, et al. Metabolic profiling for detection of Staphylococcus aureus infection and antibiotic resistance. PLoS One. 2013;8(2):e56971.
Mickiewicz B, Duggan GE, Winston BW, Doig C, Kubes P, Vogel HJ, et al. Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit Care Med. 2014;42(5):1140–9.
Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46(1):1–12.
Henriquez-Camacho C, Losa J. Biomarkers for sepsis. Biomed Res Int. 2014;2014:547818.
Hinkelbein J, Kalenka A, Schubert C, Peterka A, Feldmann Jr RE. Proteome and metabolome alterations in heart and liver indicate compromised energy production during sepsis. Protein Peptide Lett. 2010;17(1):18–31.
Lindon JC, Holmes E, Nicholson JK. Metabonomics techniques and applications to pharmaceutical research & development. Pharm Res. 2006;23(6):1075–88.
Lin ZY, Xu PB, Yan SK, Meng HB, Yang GJ, Dai WX, et al. A metabonomic approach to early prognostic evaluation of experimental sepsis by (1)H NMR and pattern recognition. NMR Biomed. 2009;22(6):601–8.
Izquierdo-Garcia JL, Nin N, Ruiz-Cabello J, Rojas Y, de Paula M, Lopez-Cuenca S, et al. A metabolomic approach for diagnosis of experimental sepsis. Intensive Care Med. 2011;37(12):2023–32.
Xu PB, Lin ZY, Meng HB, Yan SK, Yang Y, Liu XR, et al. A metabonomic approach to early prognostic evaluation of experimental sepsis. J Infect. 2008;56(6):474–81.
Fanos V, Caboni P, Corsello G, Stronati M, Gazzolo D, Noto A, et al. Urinary 1H-NMR and GC-MS metabolomics predicts early and late onset neonatal sepsis. Early Hum Dev. 2014;90:S78–83.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327.
Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care. 2008;12(6):R161.
Vincent JL, Moreno R, Takala J, Willatts S, DeMendonca A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intens Care Med. 1996;22(7):707–10.
van Beek JH, de Moor MH, de Geus EJ, Lubke GH, Vink JM, Willemsen G, et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet. 2013;43(4):329–39.
Chen J, Zhao X, Fritsche J, Yin P, Schmitt-Kopplin P, Wang W, et al. Practical approach for the identification and isomer elucidation of biomarkers detected in a metabonomic study for the discovery of individuals at risk for diabetes by integrating the chromatographic and mass spectrometric information. Anal Chem. 2008;80(4):1280–9.
Gasparetto A, Corbucci GG, Candiani A, Gohil K, Edwards RHT. Effect of tissue hypoxia and septic shock on human skeletal muscle mitochondria. Lancet. 1983;322(8365–8366):1386.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Whelan SP, Carchman EH, Kautza B, Nassour I, Mollen K, Escobar D, et al. Polymicrobial sepsis is associated with decreased hepatic oxidative phosphorylation and an altered metabolic profile. J Surg Res. 2014;186(1):297–303.
Hasselgren PO, Fischer JE. Sepsis: stimulation of energy-dependent protein breakdown resulting in protein loss in skeletal muscle. World J Surg. 1998;22:203–8.
Bremer J. Carnitine—metabolism and functions. Physiol Rev. 1983;63(4):1420–80.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010;7:30.
Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: a systematic review. J Crit Care. 2015;30(3):518–24.
Forni LG, McKinnon W, Lord GA, Treacher DF, Peron JM, Hilton PJ. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care. 2005;9(5):R591–5.
Kim SC, Pierro A, Zamparelli M, Spitz L, Eaton S. Fatty acid oxidation in neonatal hepatocytes: effects of sepsis and glutamine. Nutrition. 2002;18(4):298–300.
Steelman SM, Johnson P, Jackson A, Schulze J, Chowdhary BP. Serum metabolomics identifies citrulline as a predictor of adverse outcomes in an equine model of gut-derived sepsis. Physiol Genomics. 2014;46(10):339–47.
Liu XR, Zheng XF, Ji SZ, Lv YH, Zheng DY, Xia ZF, et al. Metabolomic analysis of thermally injured and/or septic rats. Burns. 2010;36(7):992–8.
Luo L, Schomaker S, Houle C, Aubrecht J, Colangelo JL. Evaluation of serum bile acid profiles as biomarkers of liver injury in rodents. Toxicol Sci. 2014;137(1):12–25.
Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med. 2013;187(9):967–76.
Zhang W, Zhou L, Yin P, Wang J, Lu X, Wang X, et al. A weighted relative difference accumulation algorithm for dynamic metabolomics data: long-term elevated bile acids are risk factors for hepatocellular carcinoma. Sci Rep. 2015;5:8984.
Zhou L, Ding L, Yin P, Lu X, Wang X, Niu J, et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J Proteome Res. 2012;11(11):5433–42.
Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10(7):M110.004945.
Seymour CW, Yende S, Scott MJ, Pribis J, Mohney RP, Bell LN, et al. Metabolomics in pneumonia and sepsis: an analysis of the GenIMS cohort study. Intensive Care Med. 2013;39(8):1423–34.
Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachex Sarcopenia Muscle. 2013;4(1):19–29.
Yoshifuji A, Wakino S, Irie J, Tajima T, Hasegawa K, Kanda T, et al. Gut lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc. 2016;31(3):401–12.
Ridgway ND, Yao Z, Vance DE. Phosphatidylethanolamine levels and regulation of phosphatidylethanolamine N-methyltransferase. J Biol Chem. 1989;264(2):1203–7.
Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–8.
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr. 2015;113(12):1835–43.
Acknowledgments
We sincerely thank to the support of the University of Paris-XIII and the help from the Jean Verdier Hospital. The study has been supported by the key foundation (No. 21435006) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board of Jean Verdier University Hospital approved the protocol and the French Research Delegation Office accepted the creation of a dedicated bio-collection for the patients included in the protocol. The CNIL (National Informatics and Liberty Commission) also approved the creation of both bio-collection and database. Depending of the mental status of the patient, the written consent was obtained directly from the patient at inclusion or from the Person of Confidence designed by the patient (pre-emptively) or by the family relatives. In case of survival, a secondary written consent from the patient was systematically seek.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 428 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Yin, P., Amathieu, R. et al. Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal Bioanal Chem 408, 7641–7649 (2016). https://doi.org/10.1007/s00216-016-9845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9845-9


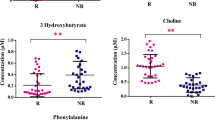




 : Measured increment before treatments and
: Measured increment before treatments and  : measured decrement before treatments in the non-surviving patients
: measured decrement before treatments in the non-surviving patients