Abstract
If the biomarker potential of intact heteromers and their free subunits is different, differentiation between these forms may reveal important clinical information. Such differentiation may however be analytically challenging. One possible way of circumventing this challenge is by performing a dual-immuno-MS approach. In the present paper, a two-step immunoaffinity sample preparation step is succeeded by digestion and subsequent LC-MS analysis to provide high-sensitivity quantification and differentiation between the heterodimer human chorionic gonadotropin (hCG) and its free β-subunit in serum. Intact and free variants are captured in two separate immunoextraction steps in order to increase the differentiation power of the method. Intact heterodimer variants were depleted prior to free subunit variants in order to incorporate a method quality control. The method was optimized for serum samples. A fully validated immuno-MS method was used as foundation, and partial validation according to the European Medicines Agency’s (EMA) guidelines on validation of bioanalytical methods was performed for the dual approach. An accelerated digestion step was incorporated making batch processing of samples within 1 day possible (approx. 3.5 h of sample preparation including digestion). Acceptable linearity (R 2 ≥ 0.990 for four variants and R 2 of 0.920 and 0.966 for the remaining two) and specificity were demonstrated, and the method was robust toward varying levels of intact heterodimer versus free subunit. The method was also successfully tested on realistic samples, demonstrating both the differences in total hCG and the distribution between intact hCG and its free β-subunit in real samples.

Schematic overview of the dual immuno-MS process
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heteromeric protein biomarkers used for clinical purposes have been suffering from difficulties when it comes to differentiation between the intact heteromer and the free subunits. This has somehow hampered the utilization of the inherent potential of some of these biomarkers, as free subunits may report different biological conditions than the intact heteromer. Examples of such heteromeric protein biomarkers with differential biomarker potential are neuron-specific enolase (NSE) (heterodimer; homodimeric and monomeric conformations also occur) [1–4], coagulation factor XIII (FXIII; heterotetramer) [5–12], and human chorionic gonadotropin (hCG; heterodimer) [13].
hCG is a glycoprotein hormone (GPH), which for several years has been utilized as a biomarker for the selection of biological and pathological conditions, first and foremost in pregnancy diagnostics, predicting the pregnancy outcome and revealing abnormalities [13–19]. hCG is also an important biomarker for several types of cancer [13, 18–29]. The prognostic value of hCG is of such strength that it alone can be an incentive for the onset of cancer treatment [13, 29–32]; reliable high-sensitivity quantification methods are therefore a premise for clinical use of hCG analysis.
hCG consists of an α-subunit (hCGα, 92 amino acids), common to all GPHs, and a β-subunit (hCGβ, 145 amino acids), which is specific for hCG [13]. The most common protein backbone variants of hCG are the intact heterodimer (intact hCG) and the free subunits, most importantly the specific β-subunit (hCGβ) and its degradation products, including hCGβ core fragment (hCGβcf) and nicked hCG variants (hCGn, hCGβn)—all gathered under the collective term hCG [29, 33, 34].
Different variants of hCG are associated with different biological/pathological conditions or stages of these [19, 35]. As a cancer biomarker, intact hCG is observed in elevated concentrations in most cases of gestational trophoblastic disease (GTD) and about half of the cases of testicular germ cell tumors [13]. Raised levels of hCGβ are found in 10–30 % of most cancers, and for some types of cancers, the percentage is even higher. In addition, nontrophoblastic tumors are often associated with increased production of free hCGβ alone [29, 35]. High serum levels of free hCGβ are strongly connected with poor disease outcome for the cancer patient, as it is usually a clear sign of aggressive disease and therewith poor prognosis [29]. Free hCGβ is a valuable prognostic marker for most cancers, which can help distinguish malignant tumors from benign [13, 29, 35–37]. Monitoring of hCGβ levels can also reveal development of therapy resistance [37–39] and thus evoke for personalized medicine.
The biomarker quality of free hCGβ emphasizes the importance of being able to analytically differentiate between free hCGβ and hCGβ as part of the intact dimer. Unfortunately, many immunoassays are designed to detect hCG and hCGβ together. These assays cannot differentiate between the two, and the “total hCG” concentration is based on the biological activity (international units, IU) of hCG, which is close to 15-fold higher than that of hCGβ (if compared in molar concentrations) [13, 29]. The contribution of hCGβ to the measured “total hCG” concentration will thus often be negligible, even if the levels of hCGβ are elevated. Specific immunoassays for hCGβ are available but these are often of insufficient sensitivity for detection of the serum hCGβ concentrations typically occurring in cancer patients [13]. Serum measurements are preferred for the quantification of hCG and hCGβ, rather than urine measurements, with the large day-to-day variation of protein concentrations in urine taken into account [13, 29]. Assays used for the quantification of serum hCG should ideally detect both intact hCG, hCGβ, nicked hCG forms, and hCGβcf, as degraded hCG variants may be found in the serum of cancer patients [29, 33, 34].
Previously, our group has developed a highly specific and sensitive immuno-MS method for quantification of intact hCG, hCGβ, hCGβn 45/46, hCGβn 47/48, and hCGβcf, all in one analysis [40]. One major drawback of this method is its incapability to differentiate between intact hCG and free hCGβ. Recently, this was partly addressed, and a method able to differentiate between the most common hCG variants in urine was described [41]. A two-step immunoextraction procedure was applied; first free hCGβ and hCGβcf were isolated and then intact hCG, and the complete sample preparation time was close to 24 h.
As serum is the most common matrix for the determination of cancer markers, the present work was set out to develop a setup capable to differentiate between all hCG variants (including the nicked ones) in serum through a dual-immuno-MS analysis. In order to incorporate a quality control feature, intact hCG variants were extracted in the first step. In addition, efforts were made to reduce the total sample preparation time (including digestion) to within one working day.
Materials and methods
Chemicals
Different hCG sources were used; Ovitrelle® (recombinant hCG) was obtained from Merck Serono Europe Limited (London, UK) and Pregnyl® (hCG extracted from human urine from pregnant women) from N.V. Organon (Oss, Netherlands). In addition, the WHO 1st international reference reagents (IRRs) for intact hCG (IRR 99/688), hCGβ (IRR 99/650), hCGn (IRR 99/642), and hCGβn (IRR 99/692) respectively, were obtained from the National Institute of Biological Standards and Controls (NIBSC) (Hertfordshire, UK). The monoclonal antibodies (mAbs) used (ISOBM-387, ISOBM-389, ISOBM-411, ISOBM-414, ISOBM-419, ISOBM-425, ISOBM-436, ISOBM-446, and ISOBM-447) were from the second ISOBM TD-7 workshop [42], and 1,4-dithiothreitol (DTT) (analytical grade), iodoacetic acid (IAA) (analytical grade), formic acid for mass spectrometry (∼98 %), TPCK-treated and lyophilized sequencing grade trypsin from bovine pancreas (T8802), and the stable isotope-labeled (SIL) internal standard peptides were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade. Human serum from healthy individuals was provided by Oslo University Hospital, Ullevaal (Oslo, Norway).
Preparation of hCG-spiked serum samples
Initial experiments were performed with either one of the pharmaceutical preparations Pregnyl® or Ovitrelle® as hCG source. For the method development experiments, urinary hCG isolated from pregnant women (Pregnyl®) was mainly used as hCG source in serum samples spiked prior to the immunoextraction procedure. Due to its origin, Pregnyl® may contain free subunits and degradation products of hCG. Recombinant intact hCG (Ovitrelle®) was used in some experiments. The hCG source used is specified for the respective experiments.
The hCG concentrations of Pregnyl® and Ovitrelle® were converted from IU/milliliter to molar by the conversion factor given by Stenman et al. [13].
Pregnyl® samples
A stock solution of hCG was made by resolving one ampulla of Pregnyl® (5000 IU hCG ∼15 nmol) in 1 mL of Milli-Q™ water. The stock solution was further diluted to 1.5 μM hCG with water of the same quality. Subsequently, human serum was spiked with this stock solution to the desired hCG concentration.
Ovitrelle® samples
One Ovitrelle® syringe with a concentration of ∼40 μM was diluted to a stock solution of 1.5 μM with Milli-Q™ water. The stock solution was used to spike human serum to the desired hCG concentration.
Reference reagents for hCG and associated molecules
Standard solutions of IRRs for intact hCG (IRR 99/688), hCGβ (IRR 99/650), intact hCGn (IRR 99/642), and hCGβn (IRR 99/692) were prepared separately by reconstitution of the lyophilized content of the respective ampoules with phosphate buffer saline pH 7.4 (PBS) containing 10 mg/mL bovine serum albumin added in volumes obtaining an IRR concentration of 1 μM. Aliquots of standard solutions were stored at −32 °C and used to spike human serum samples.
Dual immunocapture
The samples were run through a dual immunocapture procedure depicted in Fig. 1: the first step capturing intact hCG variants with mAb ISOBM-414 and the second step capturing hCGβ and other free variants with mAb ISOBM-419. A method previously developed by our group [40] was used as basis for both immunoextraction steps. Apart from the choice of mAb in step 1, the only differences were that tryptic digestion was adapted to fit into a high-speed format, the DTT reduction temperature was reduced from 95 to 60 °C to reduce the chances of protein aggregation [43] (no decrease in signal intensity was observed; data not shown), and the solid-phase extraction step was omitted. The derived immunoextraction method was found to have similar performance as the conventional method (see Electronic supplementary material (ESM) Fig. S1).
Dual-immuno-MS workflow. The numbering corresponds to the numbers in the procedure description in the “Dual immunocapture” section
The immunocapture method (based on [44]) was performed as follows: Step 1: (1) 500 μL PBS with 0.05 % Tween 20 was added to a LoBind Eppendorf® tube from Eppendorf AG (Hamburg, Germany), and (2) 40 μL magnetic beads (10 mg beads/mL) coated with 15 μg mAb ISOMB-414/mg beads was added to the tube. (3) The sample tube was mixed before it was placed in an Invitrogen DynaMag-2 magnetic rack (Carlsbad, CA, USA) withholding the magnetic beads, and the washing solution was removed. (4) A 1-mL serum sample was added before the sample was incubated for 1 h on a HulaMixerTM at room temperature (Invitrogen). (5) The sample was placed in the magnet rack and the sample solution transferred to a new tube (further preparation of the transferred sample solution is described in point (11)). (6) The magnetic beads were then washed in the given order: (I) 500 μL PBS with 0.05 % Tween 20, (II) 500 μL PBS, and (III) 500 μL of 10 mM Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) pH 7.4. Between all washing steps, the Eppendorf tube was mixed before the washing solution was removed. (7) One hundred microliters of freshly prepared 50 mM ammonium bicarbonate (ABC) with 0.001 % polyethylene glycol (PEG) was added. Step 2 (derived from [40]): (8) point (1) was repeated with a new Eppendorf® tube, and (9) 20 μL magnetic beads coated with 15 μg mAb ISOMB-419/mg beads (10 mg beads/mL) were added to the tube. (10) Point (3) was repeated before (11) 1 mL of the serum sample from point (5) was transferred to the new tube and extracted for 1 h on a HulaMixerTM at room temperature. (12) The serum was removed and the magnetic beads were washed as described in points (6) and (7).
Tryptic digestion and internal standards
In accordance with a previous work [45], an accelerated digestion method with a 1:1 (w/w) trypsin-to-protein ratio and only 45 min digestion time was performed subsequent to the immunocapture. The 100-μL samples obtained from steps 1 and 2 of the dual immunocapture were run separately through tryptic digestion in the following manner: 6 μL of 100 mM DTT (freshly prepared in Milli-Q™ water) was added to each sample and the samples were left at 60 °C for 15 min on a Thermomixer comfort (Eppendorf®) at 1100 rpm. After being cooled to room temperature, 9 μL of 400 mM IAA (freshly prepared in Milli-Q™ water) was added to the samples, which were then placed in a dark environment for 15 min. Seven microliters of 500 μg/mL trypsin (freshly prepared in 50 mM ABC with 0.001 % PEG) was added to the samples, which were subsequently kept at 37 °C for 45 min on a Thermomixer comfort at 1100 rpm. The sample tubes were mixed before they were placed in a magnetic rack withholding the magnetic beads, allowing the sample solution to be transferred to the new tubes. As the sample solutions were removed from the beads, they were immediately mixed with 4 μL concentrated formic acid to stop the digestion process. No further sample cleanup was performed prior to injection to the LC-MS system.
Heavy labeled synthetic analogs (SIL peptides) of the hCGα (hCGα-T2; [H]AYPTPLR[OH]) and hCGβ (hCGβ-T5; [H]VLQGVLPALPQVVCNYR[OH]) signature peptides were used as internal standards (IS): [H]AYPTPL[R13C15N][OH] and [H]VLQGVLPALPQVVCNY[R13C15N][OH], respectively. The SIL IS peptides were added to each sample to a final concentration of 10 nM, before injection to the LC-MS system. Signal intensities of hCGα-T2 and hCGβ-T5 were monitored and displayed relative to the signal intensity of their respective SIL IS peptide. The IS was introduced after the dual immunocapture digestion and corrected for MS-variability only. Both IS′ were prepared according to recommendations in the product description and as described by Lund et al. [40].
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The LC-MS/MS analysis was carried out by the use of a TSQ Quantum Access triple quadrupole (TSQ) from Thermo Scientific (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled to a Dionex UltiMate 3000 chromatographic system. This instrumentation, the LC-MS/MS method, mobile phases, and column were identical to those used by Lund et al. [40]: Chromatographic separation was carried out on a Biobasic-C8 column (50 mm × 1 mm i.d., pore size 300 Å, particle diameter 5 μm from Thermo Scientific) using a flow rate of 50 μL/min. The mobile phases consisted of the following—A: 20 mM formic acid and MeCN (95:5 v/v) and B: 20 mM formic acid and MeCN (5:95 v/v). A linear gradient was run from 0 to 40 % B in 8 min, followed by a linear gradient from 40 to 46 % in 4 min. Then, the elution strength was increased to 95 % B within 0.1 min and kept constant for 2 min prior to returning to starting conditions within 1 min. The column was regenerated to starting conditions for at least 10 column volumes. Sample aliquots of 40 μL were injected to the system. An overview of the signature peptides and their SRM transitions can be found in Table 1. Details about the mass spectrometric detection are provided in the Experimental part of the ESM.
Method validation
The cross-validation of the complete dual-immuno-MS method with the previously published method [40] was carried out in accordance with key elements from the European Medicines Agency’s (EMA) guidelines on validation of bioanalytical methods [46].
Data processing
The data was processed manually by the use of the Thermo Xcalibur™ Qual Browser (version 2.0.7 software, Thermo Scientific).
Results and discussion
The aim of the present work was to explore the possibility to differentiate between heterodimers and free subunits using hCG as a model analyte. A dual-immuno-MS method capable to differentiate between intact hCG, free hCGβ, and associated variants of these, through two separate immunoextraction steps, was developed. By capturing intact hCG variants in the first immunoextraction step and free hCGβ variants in the second, the present method also incorporates a quality control of the method efficiency, as any hCGα detected in step 2 will be a sign of insufficient capture of intact hCG in step 1. Tryptic peptides were obtained subsequent to immunoextraction and used as basis for the LC-MS analysis; the signature peptides (previously defined by Lund et al. [40, 47]) and the hCG variants they originate from are listed in Table 1.
Optimization of immunoextraction step 1
Choice of mAb for the capture of intact hCG
To enable the capture of intact hCG in a separate immunoextraction step prior to the extraction of remaining free hCGβ variants in the serum sample, a range of ISOBM-mAbs (ESM Fig. S2) specific for intact hCG variants only, with affinity for either the c1, c2, or c3 epitope on hCG, were selected and screened based on previous mAb mapping [42, 44]. Efficient step 1 capture of all intact hCG variants is crucial, as the ISOBM-mAb used in step 2, ISOBM-419, has affinity to the β4-epitope on hCGβ and thus has potential antigens in both intact hCG and free hCGβ variants.
The efficiency of all ISOBM-mAbs screened for in immunoextraction step 1 was evaluated by means of signal intensity of hCGα-T2 and hCGβ-T5 in step 1 and the remaining intact hCG in step 2 (signal of hCGα-T2 in step 2). The less hCGα-T2 detected in step 2, the more efficient the mAb in step 1. The relative standard deviation (RSD) was <10 % for both hCGα-T2 and hCGβ-T5 in step 1 for all but two of the mAbs (ISOBM-436 and ISOBM-446); the higher RSDs observed for these two were most likely due to their low signal intensities in step 1, as shown in Fig. 2. From Fig. 2, it can also be seen that ISOBM-414 was superior regarding both extraction efficiency in step 1 and remains in step 2: The use of ISOBM-414 in the first immunoextraction step yielded significantly higher signal intensities of both hCGα-T2 and hCGβ-T5 compared to the other ISOBM-mAbs screened (and with satisfactory repeatability: RSD 2.6 and 8.2 % for hCGα-T2 and hCGβ-T5, respectively (n = 5, both)), and the remains of intact hCG in step 2 were lower than the method’s lower limit of quantification (LLOQ). mAb ISOBM-414 was consequently chosen for extraction of intact hCG in step 1 and was followed by ISOBM-419 in step 2.
Step 1 mAb screening. The diagram presents signal intensities of hCGα-T2 (blue) and hCGβ-T5 (red) (relative to corresponding IS) in both steps of dual-immuno-MS runs of 1.5 nM hCG (Pregnyl®) in serum (n = 5). The mAb in step 1 was interchanged, while ISOBM-419 was used in step 2 for all experiments. The data is normalized and the signal intensity ratio of the most efficient antibody (ISOBM-414) is set to 100 %
The order in which the intact versus free protein variants are captured combined with the choice of antibody in step 2 supplies an important quality control feature to the method: Any remaining intact hCG from step 1 will be captured by the ISOBM-419 mAb and revealed by a hCGα signal in the MS analysis of step 2.
Capacity of step 1 and optimization of mAb-bead concentration
The capacity of the first immunoextraction step was determined by analysis of spiked serum samples of increasing hCG level (Pregnyl®) after preparation by the dual-immuno-MS method. At levels of 6 nM and above, the capacity of the mAbs in step 1 was surpassed (see ESM Fig. S3). These results indicated an upper LOQ of <6 nM for intact hCG (step 1). As this capacity was considered to be too low, the amount of ISOBM-414-coated magnetic beads (initially 20 μL) added in step 1 was optimized. Increasing volumes (20–100 μL) of mAb ISOBM-414-coated magnetic beads (10 mg beads/mL, 15 μg mAb ISOBM-414/mg beads) were added to serum samples spiked with 6 nM hCG (Pregnyl®). Significantly increased hCGβ-T5 signal intensity was seen in step 1 of the immunoextraction when the ISOBM-414 amount was raised from 20 to 40 μL (ESM Fig. S4), and the corresponding hCGβ-T5 signal in step 2 was close to be eliminated. No significant increase in hCGβ-T5 signal intensity in step 1 or decrease in step 2 was detected when the ISOBM-414 amount was further increased (>40 μL); hence, the ISOBM-414 amount in the final dual-immuno-MS method was set to 40 μL.
The capacity of the method (≥6 nM) is by such sufficiently high for diagnostic purposes, and due to the quality control feature of the dual-immuno-MS method, intact hCG concentrations beyond the extraction capacity of step 1 will be reported by the remains of intact hCG (presented as hCGα-T2) in step 2. If this is observed, the samples should be diluted and reanalyzed in order to fit within the concentration limits. Lund et al. have previously appointed a capacity ≥15 nM for the ISOBM-419 extraction constituting the second immunoextraction step of the present method [40].
Cutoff values and reference levels of serum hCG
hCG and hCGβ may occur in healthy men and nonpregnant women at lows levels (hCG: ∼3 pM (men), ∼9 pM (women); hCGβ: ∼2 pM (men/women) [13, 33]). In addition, due to structural similarities, cross-reactivity with other GPHs may occur; the mAb used in the first step of immunoextraction, ISOBM-414, is known to have cross-reactivity with LH, and ISOBM-419 in step 2 may also have modest affinity to LH [42]. In healthy individuals, GPHα is produced in quite high levels in the pituitary, and LH normally occurs in concentrations more than 10-fold that of hCG [13]. Due to the natural occurring levels of GPHα, a cutoff value for baseline hCGα (or GPHα), monitored through the signature peptide hCGα-T2, was therefore assessed for the method. The baseline was determined by running blank serum samples from four different healthy individuals through the dual-immuno-MS method (n = 2). In immunoextraction step 1, the hCGα-T2 cutoff value was set to 4 % (area of peak relative to area of internal standard hCGα-T2 in sample); in step 2, the cutoff value was 1 %. The cutoff value for hCGβ-T5 in both immunoextraction steps was less than the limit of detection (LOD).
Method validation
As the basis for the dual-immuno-MS method was a fully validated method [40], partial validation in accordance with the EMA guidelines on bioanalytical method validation [46] was carried out to evaluate the dual-immuno-MS method. For the validation, the 1st IRR preparations of intact hCG (IRR 99/688), hCGβ (IRR 99/650), hCGn (IRR 99/642), and hCGβn (IRR 99/692) were used to spike serum samples. hCGβcf was considered specific and compatible with the dual-immuno-MS method through analysis of Pregnyl®-spiked serum samples and based on previous experiments performed by Lund et al. [40, 47].
Linearity
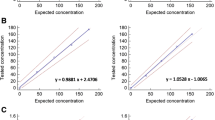
The linearity of the present dual-immuno-MS method was investigated by separate analyses of IRR serum preparations of hCG (IRR 99/688), hCGn (IRR 99/642), hCGβ (IRR 99/650), and hCGβn (IRR 99/692). As can be seen from Fig. 3, the method is linear for all hCG variants, R 2 = 0.920–0.999, within a concentration range of 10–3000 pM for intact hCG (Fig. 3a) and hCGβ (Fig. 3c) and 100–2000 pM for intact hCGn (Fig. 3b(i, ii)) and hCGβn (Fig. 3d(i, ii)). The intact hCGn and free hCGβn were included to give an indication of the performance of the nicked variants. As the IRRs containing hCGn (IRR 99/642 and IRR 99/692) are combinations of two nicked variants and, in addition as discussed in the next section, also contain a known contamination of intact hCG, only three concentration levels were applied for these IRRs.
Calibration curves, including R 2 values, for the different hCG variants. Spiked serum samples of a intact hCG (IRR 99/688), b intact hCGn (IRR 99/642), c free hCGβ (IRR 99/650), and d free hCGβn (IRR 99/692) were run separately through the dual-immuno-MS method (n ≥ 2). hCG and hCGβ are monitored through hCGβ-T5 (a, c). hCGn and hCGβn are monitored through hCGβn-T5 (b i, d i) and hCGβn-T5′ (b ii, d ii). Signal intensities are given relative to IS of respective signature peptides
Specificity: free versus intact variants
The specificity of the separate dual-immuno-MS extraction steps was characterized through analysis of the different IRR serum preparations within a concentration range of 10–3000 pM for hCG and hCGβ and 100–2000 pM for hCGn and hCGβn.
Intact hCG (IRR 99/688) was efficiently depleted in step 1 of the dual-immuno-MS method, as shown in Fig. 4a. At intact hCG concentrations ≤500 pM, hCGβ-T5 in step 2 remains below the LOQ, and at intact hCG concentrations >500 pM, the hCGβ-T5 signals in step 2 were below 4 % of the signal in step 1. Free hCGβ (IRR 99/650), on the other hand, was efficiently captured in step 2. At hCGβ concentrations <500 pM, hCGβ-T5 was observed in step 1 in amounts below LOQ, and at hCGβ concentrations ≥500 pM, hCGβ-T5 signals in step 1 were below 4 % of the amount in step 2. This can be seen in Fig. 4b. The nicked free variant, hCGβn (IRR 99/692), was detected in step 2 only, and no traces of hCGβn-T5 (Fig. 4d(i)) or hCGβn-T5′ (Fig. 4d(ii)) were observed in step 1. For the nicked intact variant, hCGn (IRR 99/642) on the contrary (Fig. 4c(i, ii)), intact hCGn seems to be insufficiently captured in step 1. This is concurrent with the known fact that ISOBM-414 (step 1) has lower affinity to hCGn than ISOBM-419 (step 2) [44], although it also might be due to a hCGβn contamination of the intact nicked standard. In addition, hCGn (IRR 99/642) has previously been shown to contain a cross-contamination of ∼20 % intact hCG [42, 44], and Lund et al. suggested the possibility of a free hCGβn contamination in the intact hCGn standard (IRR 99/642) [44, 47]. From ESM Fig. S5a, it can be seen that intact hCG (detected in step 1) is a main contaminant of IRR 99/642. In addition, hCGα-T2 is also detected in both steps, indicating that at part of the nicked variants detected in step 2 is due to insufficient capture of the intact nicked variants in step 1. Minute amounts of free hCGβ can also be seen at the highest concentration level (detected in step 2). The free nicked standard (IRR 99/692) is much cleaner (ESM Fig. S5b), and only small amounts of free hCGβ are seen as the highest level, similar to those in the intact nicked standard (IRR 99/642). The cross-contamination of hCGn (IRR 99/642) and the fact that the nicked standards are combinations of two nicked forms complicate the use of these IRR preparations for standardized method validation for hCGn (intact and nicked). The linearity described above for the intact nicked standard (IRR 99/642) was based on the step 1 analysis of hCGn as only these signals were considered relevant for the analysis of intact hCGn.
The specificity of the separate dual-immuno-MS extraction steps for a intact hCG (IRR 99/688), b free hCGβ (IRR 99/650), c intact hCGn (IRR 99/642), and d free hCGβn (IRR 99/692). hCG and hCGβ are monitored through hCGβ-T5 (a, b). hCGn and hCGβn are monitored through hCGβn-T5 (c i, d i) and hCGβn-T5′ (c ii, d ii). Signal intensity is given relative to IS for their respective signature peptides (n = 2)
Robustness toward varying variant levels
To investigate if the specificity of the dual-immuno-MS method was influenced by varying analyte levels, serum samples were spiked with intact hCG (IRR 99/688) and/or free hCGβ (IRR 99/650) at different levels (high = 1000 pM, low = 100 pM, zero = not added). Eight concentration combinations of hCG (IRR 99/688) and/or free hCGβ (IRR 99/650) were investigated: high-high, low-high, high-low, low-low, high-zero, low-zero, zero-high, or zero-low. From Fig. 5a, it can be seen that the captured amount of intact hCG (determined in step 1) was independent of the level of free hCGβ in the sample: The circle representing the signal ratio is of similar size for all three conditions at low-level intact hCG and at high-level intact hCG. A similar result is seen for free hCGβ (determined in step 2) in Fig. 5b. These results demonstrate that varying levels of hCG variants do not influence the specificity of the dual-immuno-MS method. Small amounts of hCGβ-T5 were detected in step 1 at the “zero-high” concentration of free hCGβ, as well as in step 2 at the “high-zero” concentration of intact hCG (Fig. 5). These amounts constituted of less than 2 % of the measured total hCGβ-T5 and by such did not significantly reduce the method specificity although it indicates some cross-reactivity with hCGβ in step 1 (Fig. 5a; condition zero-high) and some overload of the mAb in step 1 at high levels of intact hCG (Fig. 5b; condition high-zero). These results are concurrent with the observations in the specificity experiments.
Robustness toward varying levels of intact hCG and free hCGβ. Signal intensities of hCGβ-T5 in step 1 (a) and step 2 (b) of dual-immuno-MS runs of spiked serum samples are shown as circles sized corresponding to their signal (relative to IS). The samples were spiked with either intact hCG (IRR 99/688) and free hCGβ (IRR 99/650) at different concentration levels (high-high, low-high, high-low, or low-low) (n = 6), or intact hCG (IRR 99/688) or free hCGβ (IRR 99/650) (high-zero, low-zero, zero-high, or zero-low) (n = 3)
Sensitivity, precision, and recovery
The sensitivity of the dual-immuno-MS method was determined on the basis of hCGβ-T5 signal intensities from serum samples spiked with intact hCG (IRR 99/688) in step 1, or free hCGβ (IRR 99/650) in step 2. LOD was defined as 3-fold the signal in a blank sample (noise) and LOQ 10 times the “noise.” For both intact hCG and free hCGβ the LOD was 3 pM and the LOQ 10 pM. These limits are considered sufficiently low for cancer diagnostics, thus providing gained diagnostic power, as intact and free variants are differentiated in serum.
The repeatability (precision) of the method was evaluated using coefficients of variation (CV), and the results for the dual-immuno-MS method are given in Table 2. At the low concentration level, CV was ≤28 %, and at the high concentration level, the CV was ≤11 %. Although the CV of the low concentration samples is slightly above the requirements set by EMA [46], the repeatability of the dual-immuno-MS method was considered sufficient.
The recovery was determined on the basis of hCGβ-T5 signals from hCG (Pregnyl®)-spiked serum samples (1500 pM) run through the dual-immuno-MS method, which was compared to aqueous hCG (Pregnyl®) samples (150 nM) digested in solution without prior immunoextraction. The ratio between extracted and nonextracted hCGβ-T5 was multiplied by a correction factor, taking into account the differences in injection volumes and preconcentration throughout the sample preparation. The dual-immuno-MS method was found to have a mean recovery of 34 % (CV = 8 %) in step 1, which is in accordance with that determined by Lund et al. [40] (∼40 %; that method included a solid-phase extraction preconcentration subsequent to the immunoextraction). It was not investigated which parts of the procedure had the biggest impact on recovery. Most likely, it is a combination of the capture step and the tryptic digest step. The ISOBM-414 was shown to be considerably more efficient in capturing intact hCG than the other antibodies evaluated, so it might be reasonable to assume that the major contribution to the low recovery is the combined step of releasing hCG from the antibody and digestion. It has previously been shown [47] that hCG is partly released from ISOBM-419 (used in step 2) during the reduction and alkylation step and it is likely to assume that the same is true for ISOBM-414. In addition, the efficiency of in-solution digestion is dependent on various factors such as trypsin-to-protein ratio and absolute protein concentration [45]. As standards and unknown samples will be treated in the same manner, the recovery was considered satisfactory as sufficient sensitivity was achieved with satisfactory repeatability (CV = 8 % in the recovery experiment).
In contrast to a previously published work [41], the present dual-immuno-MS method provides a serum-tailored method capable to differentiate between all intact hCG and free hCG protein backbone variants. By permutation of the immunoextraction steps, and thus incorporation of the quality control feature, efficient method performance for every sample is ensured, which is of high importance for the utilization of such methods for clinical purposes. In addition, the incorporation of the accelerated digestion step reduces the procedure to sample preparation time of 3.5 h providing the possibility of batch processing of samples within one working day.
Application to realistic samples
Urinary hCG-spiked serum samples
To demonstrate the differentiation power of the present dual-immuno-MS method, serum samples spiked with urinary hCG (Pregnyl®) were analyzed. One concentration within the method capacity range (1.5 nM) and one concentration exceeding the method capacity (12 nM) was chosen to give both an example of efficient capture of intact hCG in step 1 and illustrate the method quality control concept when swamped mAb capacity in step 1 results in intact hCG remains in step 2. The highest concentration also ensures signals above the LOD for all hCG variants. As can be seen from both panels a and c of Fig. 6, intact hCG is captured in step 1 and reported by hCGβ-T5 and associated hCGα-T2. In step 2, Fig. 6b, d, free hCGβ variants are captured; these include free hCGβ, free hCGn47/48, free hCGn44/45, and hCGβcf. Only in step 2 analysis of the high concentration (Fig. 6d) can all these variants be seen, as the concentration of the degradation products, as well as free hCGβ, is <LOD at the low Pregnyl® concentration (Fig. 6b), and consequently, only the internal standards are detected. In addition, hCGα-T2 is observed in Fig. 6d as a result of swamped mAb capacity in step 1. Due to high initial hCG concentration exceeding the capacity of the method, only hCGβcf can be reliably tracked back to a free hCGβ variant in Fig. 6d. The signature peptides hCGβ-T5, hCGβn-T5, and hCGβn-T5′ can also originate from intact hCG variants as hCGα-T2 is detected, reporting the presence of intact hCG in step 2, a cause of insufficient intact hCG capture in step 1. However, detection of nicked variants only in step 2 indicates that these variants most likely are free subunits. Figure 6 illustrates two important features of the dual-immuno-MS method: the ability to detect intact and free hCG variants from the same signature peptides in two different immunoextraction steps, and the incorporated quality control offered by hCGα-T2, as remains of this signature peptide in step 2 is a sign of insufficient step 1 extraction.
SRM chromatograms of the fractions from step 1 and step 2 of dual immunoextractions of 1.5 nM (a, b) and 12 nM (c, d) hCG (Pregnyl®)-spiked serum samples after separate LC-MS analyses. In step 1 (a, c), intact hCG variants are captured. Step 2 (b, d) captures free hCG variants (and potentially excess of intact hCG variants from step 1). Retention times are given above each peak
Cancer patient samples
In addition to the Pregnyl®-spiked samples, a set of cancer patient serum samples were analyzed to investigate if the dual-immuno-MS method allowed for the determination of ratios between intact hCG and free hCGβ variants in real samples. The cancer patients were previously diagnosed with testicular cancer. As can be seen from Fig. 7, there are large variations in both total hCG content and ratio between intact hCG and free hCGβ. Sample numbers 1 and 4 contain considerably lower amounts of total hCG compared to sample numbers 2, 3, and 5 (see Fig. 7a). Sample numbers 1 and 5 show a high ratio of intact hCG to free hCGβ (5.6 and 8.2, respectively), while samples 2, 3, and 4 have a low ratio of intact hCG to free hCGβ (0.54, 0.52, and 0.42, respectively). A low ratio is the result of high amounts of free hCGβ compared to intact hCG and indicates that the patients behind these samples are most likely to suffer from aggressive cancer [29]. These results present the indisputable strength of the present method; by combining the specificity and differentiation power of dual immunoextraction and the sensitivity of the MS, the dual-immuno-MS method provides extended knowledge regarding the proportions of intact hCG versus free hCGβ variants compared to existing immuno-MS methods developed for the clinics [40]. As large proportions of free hCGβ may be a poor prognosis indicator for the patient, this additional information may be crucial in order to tailor the cancer treatment for the individual and by such hopefully increase the survival rate.
Concluding remarks
For heteromeric proteins, when the intact protein and its free subunits have different biomarker potential, differentiation between these forms potentially will lead to a more precise diagnosis. The current paper demonstrates how this can be enabled in LC-MS/MS-based targeted biomarker analysis of hCG by including a dual immunocapture step prior to LC-MS/MS analysis. It is shown that efficient depletion of the desired variants can be achieved by careful selection of the antibodies, and how this in turn ensures robust quantification. We believe that also other existing immuno-MS methods can be appended by the concept of dual-immuno-MS, introducing strengthened differentiation power into the targeted biomarker analysis.
References
Torsetnes SB, Løvbak SG, Claus C, Lund H, Nordlund MS, Paus E, et al. Immunocapture and LC–MS/MS for selective quantification and differentiation of the isozymes of the biomarker neuron-specific enolase in serum. J Chromatogr B. 2013;929:125–32.
Paus E, Myklebust AT. Expression and interconversion of neuron-specific enolase in patient sera and extracts from small-cell lung cancer cells. Tumor Biol. 1996;17:271–80.
Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–20.
Kaiser E, Kuzmits R, Pregant P, Burghuber O, Worofka W. Clinical biochemistry of neuron specific enolase. Clin Chim Acta. 1989;183:13–31.
Schroeder V, Kohler HP. New developments in the area of factor XIII. J Thromb Haemostasis. 2013;11:234–44.
Ashcroft AE, Grant PJ, Ariëns RAS. A study of human coagulation factor XIII A-subunit by electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:1607–11.
Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931–72.
Osaki T, Sugiyama D, Magari Y, Souri M, Ichinose A. Rapid immunochromatographic test for detection of anti-factor XIII A subunit antibodies can diagnose 90 % of cases with autoimmune haemorrhaphilia XIII/13. Thromb Haemostasis. 2015;113:1347–56.
Souri M, Osaki T, Ichinose A. The non-catalytic B subunit of coagulation factor XIII accelerates fibrin cross-linking. J Biol Chem. 2015;290:12027–39.
Mezei Z, Bereczky Z, Katona É, Gindele R, Balogh E, Fiatal S, et al. Factor XIII B subunit polymorphisms and the risk of coronary artery disease. Int J Mol Sci. 2015;16:1143–59.
Katona É, Pénzes K, Csapó A, Fazakas F, Udvardy ML, Bagoly Z, et al. Interaction of factor XIII subunits. Blood. 2014;123:1757–63.
Kiss C, Gyurina K, Csáthy L, Bresolin S, Kronnie G, Hevessy Z, et al. Subunit A of coagulation factor XIII as a new biomarker in childhood acute lymphoblastic leukemia? Blood. 2014;124:5346.
Stenman U-H, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of hCG. Hum Reprod Update. 2006;12:769–84.
Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostics. Clin Chim Acta. 2011;412:1515–20.
Larsen J, Buchanan P, Johnson S, Godbert S, Zinaman M. Human chorionic gonadotropin as a measure of pregnancy duration. Int J Gynecol Obstet. 2013;123:189–95.
Ong CYT, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free β human chorionic gonadotrophin and pregnancy associated plasma protein a as predictors of pregnancy complications. BJOG-Int J Obstet Gy. 2000;107:1265–70.
Sutton-Riley JM, Khanlian SA, Byrn FW, Cole LA. A single serum test for measuring early pregnancy outcome with high predictive value. Clin Biochem. 2006;39:682–7.
Berger P, Sturgeon C. Human chorionic gonadotropin isoforms and their epitopes: diagnostic utility in pregnancy and cancer. Expert Opin Med Diagn. 2008;2:1347–64.
Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8.
Cole LA, Butler S. Hyperglycosylated hCG, hCGβ and hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol. 2012;349:232–8.
Cole LA, Wang Y, Elliott M, Latif M, Chambers JT, Chambers SK, et al. Urinary human chorionic gonadotropin free β-subunit and β-core fragment: a new marker of gynecological cancers. Cancer Res. 1988;48:1356–60.
Iles RK. Ectopic hCGβ expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007;260–262:264–70.
Iles RK, Delves PJ, Butler SA. Does hCG or hCGβ play a role in cancer cell biology? Mol Cell Endocrinol. 2010;329:62–70.
Lempiäinen A, Stenman U-H, Blomquist C, Hotakainen K. Free β-subunit of human chorionic gonadotropin in serum is a diagnostically sensitive marker of seminomatous testicular cancer. Clin Chem. 2008;54:1840–3.
Marcillac I, Cottu P, Théodore C, Terrier-Lacombe M-J, Bellet D, Droz J-P. Free hCG-β subunit as tumour marker in urothelial cancer. Lancet. 1993;341:1354–5.
Michel RM, Aguilar JL, Arrieta O. Human chorionic gonadotropin as an angiogenic factor in breast cancer during pregnancy. Med Hypotheses. 2007;68:1035–40.
Marcillac I, Troalen F, Bidart J-M, Ghillani P, Ribrag V, Escudier B, et al. Free human chorionic gonadotropin β subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901–7.
Cole LA. 32—hCG, free β-subunit, and β-core fragment as markers of malignancies. In: Cole LA, Butler SA, editors. Human chorionic gonadotropin. London: Elsevier; 2010. p. 339–44.
Stenman U-H, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem. 2004;37:549–61.
Butler SA, Cole LA. Falsely elevated human chorionic gonadotropin leading to unnecessary therapy. Obstet Gynecol. 2002;99:516–7.
Olsen TG, Hubert PR, Nycum LR. Falsely elevated human chorionic gonadotropin leading to unnecessary therapy. Obstet Gynecol. 2001;98:843–5.
Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet. 2000;355:712–5.
Alfthan H, Haglund C, Dabek J, Stenman U-H. Concentrations of human choriogonadotropin, its β-subunit, and the core fragment of the β-subunit in serum and urine of men and nonpregnant women. Clin Chem. 1992;38:1981–7.
Cole LA, Kardana A, Ying FC, Birken S. The biological and clinical significance of nicks in human chorionic gonadotropin and its free beta-subunit. Yale J Biol Med. 1991;64:627–37.
Cole LA. Human chorionic gonadotropin and associated molecules. Expert Rev Mol Diagn. 2009;9:51–73.
Weinberg BD, Newell KL, Wang F. A case of a beta-human chorionic gonadotropin secreting sinonasal teratocarcinosarcoma. J Neurol Surg Rep. 2014;75:e103–e7.
Butler SA, Iles RK. Ectopic human chorionic gonadotropin β secretion by epithelial tumors and human chorionic gonadotropin β-induced apoptosis in Kaposi’s sarcoma: is there a connection? Clin Cancer Res. 2003;9:4666–73.
Vartiainen J, Alfthan H, Lehtovirta P, Stenman U-H. Elevated hCG and a high proportion of hCGβ in serum preceding the diagnosis of trophoblastic disease by seven months. BJOG-Int J Obstet Gy. 2002;109:589–90.
Vaitukaitis JL, Ebersole ER. Evidence for altered synthesis of human chorionic gonadotropin in gestational trophoblastic tumors. J Clin Endocrinol Metab. 1976;42:1048–55.
Lund H, Løvsletten K, Paus E, Halvorsen TG, Reubsaet L. Immuno–MS based targeted proteomics: highly specific, sensitive, and reproducible human chorionic gonadotropin determination for clinical diagnostics and doping analysis. Anal Chem. 2012;84:7926–32.
Woldemariam GA, Butch AW. Immunoextraction–tandem mass spectrometry method for measuring intact human chorionic gonadotropin, free β-subunit, and β-subunit core fragment in urine. Clin Chem. 2014;60:1089–97.
Berger P, Paus E, Hemken PM, Sturgeon C, Stewart WW, Skinner JP, et al. Candidate epitopes for measurement of hCG and related molecules: the second ISOBM TD-7 workshop. Tumor Biol. 2013;34:4033–57.
Hildonen S, Halvorsen TG, Reubsaet L. Why less is more when generating tryptic peptides in bottom-up proteomics. PROTEOMICS. 2014;14:2031–41.
Lund H, Paus E, Berger P, Stenman U-H, Torcellini T, Halvorsen T, et al. Epitope analysis and detection of human chorionic gonadotropin (hCG) variants by monoclonal antibodies and mass spectrometry. Tumor Biol. 2014;35:1013–22.
Egeland SV, Reubsaet L, Halvorsen TG. The pros and cons of increased trypsin-to-protein ratio in targeted protein analysis. J Pharm Biomed Anal. 2016;123:155–61.
EMA. European Medicines Agency. Guideline on validation of bioanalytical methods. 2009. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed June 2014.
Lund H, Torsetnes SB, Paus E, Nustad K, Reubsaet L, Halvorsen TG. Exploring the complementary selectivity of immunocapture and MS detection for the differentiation between hCG isoforms in clinically relevant samples. J Proteome Res. 2009;8:5241–52.
Acknowledgments
Professor Ulf-Håkan Stenman, Department of Clinical Chemistry, Helsinki University Central Hospital (Helsinki, Finland); Professor Peter Berger, Institute for Biomedical Aging Research, University of Innsbruck (Innsbruck, Austria); Medix Biochemica (Kauniainen, Finland); and Roche (Basel, Switzerland) are acknowledged for providing antibodies for the screening of antibodies in the first immunoextraction step.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for research involving human participants.
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent has been obtained from the participants involved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1273 kb)
Rights and permissions
About this article
Cite this article
Egeland, S.V., Reubsaet, L., Paus, E. et al. Dual-immuno-MS technique for improved differentiation power in heterodimeric protein biomarker analysis: determination and differentiation of human chorionic gonadotropin variants in serum. Anal Bioanal Chem 408, 7379–7391 (2016). https://doi.org/10.1007/s00216-016-9818-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9818-z