Abstract
A label-free fluorescence assay has been developed for sensitive and selective detection of adenosine triphosphate (ATP) by using poly(thymine) (poly T)-templated copper nanoparticles (CuNPs) as fluorescent indicator. In our design, ATP aptamer was split into two fragments, both of which were elongated with poly T strands that can be utilized as efficient template for the formation of copper nanoparticles through the reduction of copper ions by sodium ascorbate. In the presence of ATP, the two split aptamers could be dragged to form aptamer-ATP aptamer complex, which drew the poly T strands close to each other and induced a remarkable fluorescence enhancement of poly T-templated CuNPs. Thus, an elevated fluorescence enhancement of poly T-templated CuNPs was obtained with the increase in ATP concentration. Under optimized conditions, a good linear range for ATP detection was realized from 100 nM to 100 μM with a detection limit of 10.29 nM. In addition, the application of this biosensing system in complex biological matrix was demonstrated with satisfactory results. This assay provided a simple, label-free, cost-effective, and sensitive platform for the detection of ATP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine triphosphate (ATP), acknowledged as an important energy resource, plays a critical role in the regulation of intracellular metabolism and takes on the role of energy supplier for all kinds of biochemical reaction in most animated beings [1]. As a consequence, the concentration and dissipative rate of ATP can reflect the cell activity and is implicated with cell viability and cell injuries. Aberrant ATP level is closely associated with many diseases such as Parkinson’s disease [2], hypoxia [3], hypoglycemia [4], ischemia [5], and some malignant tumors [6]. Therefore, due to its clinical and biological importance, development of simple and sensitive methods for the detection of ATP is of great importance in the field of biochemical and molecular biology research.
Over the past few decades, various approaches have been proposed and exploited for the detection of ATP, such as ultra performance liquid chromatography [7], fluorescence [8–12], chemiluminescence [13], and colorimetry [14, 15]. Among them, aptamer-based fluorescence aptasensors are gaining increasing attention on account of their sensitivity, specificity, convenience, and easy access instruments [16–18]. Aptamers are synthetic single-stranded DNA or RNA that are artificially selected by systematic evolution of ligands using exponential enrichment (SELEX), which can specifically recognize diverse targets including protein, metal ions, small molecules, and cells. The nucleic acid nature and intrinsic advantages of aptamers have promoted ATP aptasensor technologies growing rapidly in recent years, which were proved to be excellent tools for ATP detection. Recently, He and coworkers devised a sandwich-type fluorescence resonance energy transfer (FRET) assay for the detection of ATP based on split aptamer and upconverting nanoparticles [19]. By coupling FAM-modified ATP aptamer with carboxylated carbon nanoparticles (cCNPs), Liu et al. designed a platform for the detection of ATP in view of the quenching effect of cCNPs as well as the high affinity of the aptamer to ATP [20]. Liu et al. developed a sensitive and selective ATP assay based on split aptamer-labeled quantum dots. In the presence of ATP molecules, the aptamer-functionalized quantum dots were assembled together and thus resulting in fluorescence quenching due to the exciton energy transfer effect between interparticles [21]. Even though those strategies performed good sensitivity and selectivity for ATP, most of them suffer from the problem of high cost for fluorescent modification, tedious synthesis steps, toxicity of reagents, and complicated design of molecular probes. Furthermore, the modification process may weaken their affinities to the target molecules. Thus, it is still highly desirable to develop more convenient and sensitive methods for ATP analysis.
Recently, metal nanoclusters are drawing more and more attention due to their unique properties and have been applied in a wide range of fields including biosensing [22–24], bioimaging [25], and photovoltaic cells [26]. Qing et al. firstly reported that single-stranded poly T DNA could act as an efficient template for the formation of fluorescent CuNPs [27]. The synthesis of these fluorescent CuNPs that specifically based on poly T is highly efficient and can be accomplished within minutes, which is much faster and more facilitative compared with DNA-assisted synthesis of other fluorescent metal nanoparticles, such as silver nanoclusters [28], gold nanoclusters [29], platinum nanoclusters [30], etc. Meanwhile, poly T-templated CuNPs exhibit a maximum λ em at about 620 nm with large MegaStokes shifting (up to 275 nm), which is well-suited as a fluorescent probe for the detection of targets from complex biological matrix, as the MegaStokes shifting of fluorophore can enable the removal of strong background signal from complex biological systems. Recently, Jia et al. found that CuNPs exhibit an aggregation-induced emission (AIE) enhancement effect, which means that CuNPs can generate an obvious fluorescence intensity enhancement when CuNPs are close to each other [31]. Based on this fact, herein we proposed a novel and facile method for ATP detection by using fluorescence enhancement of poly T-templated copper nanoparticles as well as ATP aptamer. In our design, ATP aptamer was split into two fragments, both of which were elongated with poly T that could be used as efficient template for the formation of CuNPs. In the presence of ATP, the two split ATP aptamers resembled into an aptamer-ATP aptamer complex, which made the elongated poly T strands close to each other and thus the fluorescence of poly T-templated CuNPs was remarkably increased. With the increasing of ATP concentration, an elevated enhancement of the fluorescence intensity was obtained successfully. This proposed approach displayed excellent performance both in buffer solution and cell lysis with good selectivity and sensitivity.
Materials and methods
Reagents and chemicals
Aptamer 1 and aptamer 2 were synthesized and purified by Shanghai Sangon Biological Engineering Technology & Services Co. Ltd. (Shanghai, China). The sequence of the aptamers in italics is part of split ATP aptamers, which can specifically bind with ATP:
-
Aptamer 1 (Apt 1): TTTTTTTTTTTTTTTTTTACCTGGGGGAGTAT
-
Aptamer 2 (Apt 2): TGCGGAGGAAGGTTTTTTTTTTTTTTTTTTT
Other materials such as sodium ascorbate, CuSO4, tris-base, and 3-(N-morpholino)propanesulfonic acid (MOPS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). ATP, cytidine triphosphate (CTP), guanosine triphosphate (GTP), uridine triphosphate (UTP), and bovine serum albumin (BSA) were also obtained from Sigma-Aldrich. The reaction buffer solutions employed in this work were Tris–HCl buffer (10 mM Tris–HCl, 10 mM MgCl2, and 50 mM NaCl (pH 7.9). The MOPS buffer (10 mM MOPS, 150 mM NaCl, pH 7.6) acted as environment for the formation of fluorescent CuNPs. All the reagents were of analytical grade and used as received without any further purification. All the stock and buffer solutions were prepared using ultrapure water generated from a Millipore Milli-Q water purification system (Billerica, MA, USA) with an electric resistance of 18.2 MΩ.
Apparatus
All the fluorescence measurements were conducted on an F-4600 fluorescence spectrophotometer (Hitachi, Japan). The fluorescence emission spectra were collected from 500 to 680 nm with the excitation wavelength of 350 nm. All experiments were carried out at room temperature unless otherwise stated. Transmission electron microscope (TEM) analysis was conducted with a transmission electron microscope (FEI-Tecnai G2, USA) at an accelerating voltage of 200 kV.
Assay procedure in buffer solution
Typically, the procedure of detecting ATP was performed as follows: 2.5 μL of aptamer 1 (10 μM) and 2.5 μL of aptamer 2 (10 μM) were mixed together with 5 μL of Tris–HCl buffer containing 10 μL ATP of different concentrations. The mixture was incubated for 30 min at ambient temperature. Then, 20 μL of MOPs buffer was added into the solution for the formation of CuNPs, following which copper sulfate solution as well as sodium ascorbate were mingled with the solution above giving a final volume of 100 μL. The incubation time for the formation of CuNPs was 15 min, followed by the measurement with F-4600 at the excitation wavelength of 350 nm.
ATP detection in cell lysis
The cell extracts were obtained from the A549 human lung adenocarcinoma cell lines, which were cultured in RPMI 1640 medium supplemented with 12 % fetal calf serum, 100 μg mL−1 streptomycin, and 100 units mL−1 penicillin. The cell extracts were prepared according to the previous reports [32]. The collected cells were resuspended in 20 μL of 10 mM Tris–HCl (pH 7.8) with 150 mM NaCl. After adding 20 μL of 10 mM cell lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1 % Triton X-100, 0.4 mM phenylmethylsulfonyl fluoride, pH 7.5), the mixture was incubated at 4 °C for 1.5 h and shaken occasionally. Finally, the cell debris was removed by centrifugation at 10,000 rpm for 10 min, and the supernatant was recovered and used as the cell extracts. After dilution (10 %), 10 μL of the diluted cell extracts was added to the assay solution. Afterwards, the detection procedure was conducted as described in the aforementioned experiments.
Result and discussion
Sensor design
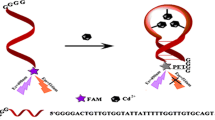
By taking advantage of the fact that poly T ssDNA can act as efficient template for the formation of copper nanoparticles, we developed a facile label-free aptasensor for the detection of ATP utilizing the fluorescence enhancement of the poly T-templated CuNPs. The principle of our proposed fluorescent strategy for the detection of ATP is illustrated in Scheme 1. DNA probe aptamer 1 (Apt 1) and aptamer 2 (Apt 2) are the two ssDNA oligonucleotides designed, both of which contains two parts. The purple part (b1) and dark blue part (b2) are the two split fragments of ATP aptamer. The dark green part (a) of the two aptamers is poly T exploited for the formation of fluorescent CuNPs. In the presence of ATP, Apt 1 and Apt 2 are induced to assemble into an Apt 1–ATP–Apt 2 complex, resulting from the specific binding between the aptamers and ATP. Due to the formation of Apt 1–ATP–Apt 2 complex, T-rich ssDNAs are staying close to each other, which results in a striking fluorescence intensity enhancement of CuNPs. While in the absence of ATP, Apt 1 and Apt 2 cannot form stable configuration and are spatially well-separated; thus, CuNPs exhibit relatively lower fluorescence. Therefore, by using this proposed aptasensor, ATP determination could be realized easily by monitoring the fluorescence enhancement of CuNPs.
Feasibility study
Before evaluating the feasibility of this assay, the generation of fluorescent CuNPs was confirmed by TEM as shown in Fig. 1, which indicates that Apt 1 and Apt 2 can be used as the template to produce fluorescent CuNPs. Afterwards, the feasibility of this strategy for constructing ATP aptasensor was investigated. The same gross concentration of Apt 1, Apt 2, and Apt 1/Apt 2 mixture was exploited as template for the formation of CuNPs in the absence and presence of ATP. As can be seen from Fig. 2, in the absence of ATP, Apt 1 (curve a), Apt 2 (curve c), and Apt 1/Apt 2 mixture (curve e) can all template the formation of fluorescent CuNPs whereas with relative faint fluorescence intensity. In the case of ATP incubated with Apt 1/Apt 2 mixture (curve f), the fluorescence intensity of CuNPs is increased intensively, which indicates the successful recognition of ATP by its aptamers. Additionally, when only one kind of aptamer (Apt 1 or Apt 2) exists in the detection system, no obvious signal enhancement can be observed even with the addition of ATP (curve b and curve d). The results demonstrate that this proposed aptasensor based on fluorescence enhancement of poly T-templated CuNPs could be successfully used to determine the concentration of ATP molecules.
Fluorescence emission spectra of the aptasensor under different conditions. (a) Apt 1 + ascorbate + Cu2+; (b) Apt 1 + ATP + ascorbate + Cu2+; (c) Apt 2 + ascorbate + Cu2+; (d) Apt 2 + ATP + ascorbate + Cu2+; (e) Apt 1 + Apt 2 + ascorbate + Cu2+; (f) Apt 1 + Apt 2 + ATP + ascorbate + Cu2+. (Concentration of Apt 1 and Apt 2 in (a), (b), (c), and (d) is 500 nM, while in (e) and (f), Apt 1 as well as Apt 2 are 250 nM, respectively. Ascorbate, 2 mM; Cu2+, 200 μM; ATP, 500 μM)
Optimization of assay condition
In order to achieve a better sensing performance, a series of experimental conditions were optimized. It was found that the concentrations of Cu2+ and sodium ascorbate were important factors influencing the fluorescent intensity of CuNPs. We firstly explored the effect of Cu2+ concentration on the fluorescence intensity of the poly T-templated CuNPs. As shown in Fig. 3a, the fluorescence intensity increases with the increasing concentration of Cu2+ and tends to level off after the concentration reaches 200 μM, so 200 μM of Cu2+ was used in further experiment. We also optimized the concentration of sodium ascorbate, and the result is shown in Fig. 3b. The fluorescence intensity increases abruptly and reaches a plateau over the concentration of 2 mM; thus, 2 mM of ascorbate was chosen for the formation of DNA-templated CuNPs. Under the optimum conditions above, the influence of incubation time on the binding extent of the aptamers with ATP was also investigated. As Fig. 3c indicates, the fluorescence intensity of the solution increases abruptly within 5 min and then reaches approximately the highest value after 30 min. Yet to get more accurate results, 30 min was used in further experiments. The kinetic behaviors of this developed aptasensor were also studied by monitoring the fluorescence intensity of the poly T-templated CuNPs as a function of time. Also it was found that the fluorescence induced by poly T-templated CuNPs could be generated very fast and stayed on a stable stage after 15 min (Fig. 3d). Thus, 15 min was adopted in the following experiment. Moreover, the temperature during the incubation of ATP with the aptamers was also investigated. Comparing the fluorescence intensity of CuNPs in the presence of and absence of ATP under different temperature, ambient temperature (around 20 °C) was found to be the optimal condition for this assay, not only for its relatively better performance but also for its convenience (Fig. 3e).
a The impact of Cu2+ concentration on the fluorescence of poly T-templated copper nanoparticles. b The impact of sodium ascorbate concentration on the fluorescence of poly T-templated copper nanoparticles. c Fluorescence intensity of copper nanoparticles as a function of the incubation time of ATP with Apt 1 and Apt 2. d Fluorescence intensity of copper nanoparticles as a function of time for the formation of copper nanoparticles. e Optimization of the temperature for the incubation of ATP with the aptamers. (Apt 1, 250 nM; Apt 2, 250 nM; Cu2+, 200 μM; ascorbate, 2 mM; ATP, 500 μM)
Fluorescence measurements of ATP in buffer solution
After the feasibility investigation and condition optimization, the detection capability of the proposed strategy for ATP sensing was evaluated. As shown in Fig. 4a, the relative fluorescence intensity increases gradually with the increase of ATP concentration, which indicates that the aptamer binding-induced fluorescence intensity enhancement of CuNPs is highly dependent on the concentration of ATP. Figure 4b depicts the relationship between the relative changes of the peak fluorescence intensity (F − F 0) / F 0 and the concentration of ATP. A good linear relationship (R 2 = 0.9969) as a function of the concentration of ATP is obtained in the range from 100 nM to 100 μM with a robust detection limit of 10.29 nM, which is based on the rule of three times standard deviation over the blank response (3σ/k, where σ is the standard deviation of the blank, k is the slope of the calibration curve). Besides, the limit of detection was also comparable to the results obtained from the previously reported methods (see Electronic Supplementary Material (ESM) Table S1). The results demonstrated that the proposed method could be used as a highly sensitive, label-free, and low-cost fluorescent biosensor for ATP detection. In addition, the specificity of this sensing assay towards ATP was also evaluated by using BSA, UTP, CTP, and GTP as the potential interferents for control experiments. As shown in Fig. 5, in the presence of ATP, a dramatic fluorescence increase is observed, whereas other analogs shows negligible effect on the fluorescence intensity, which demonstrates that this label-free aptasensor has superior specificity for ATP over other interferents. The high selectivity of this strategy might be attributed to the high binding affinity of ATP to its aptamers. Hence, this sensing strategy based on poly T-templated CuNPs and split ATP aptamers can be applied as a novel aptasensor for ATP determination with high sensitivity and selectivity.
a Fluorescence emission spectra of the sensing platform with the addition of different concentration of ATP ranging from 0 to 500 μM (from bottom to top: 0, 0.1, 1, 10, 25, 50, 75, 100, 250, and 500 μM). b Calibration curve for ATP detection. The error bars denote the standard deviation of three parallel measurements. F and F 0 represent the fluorescence intensity in the presence and absence of ATP, respectively. (Apt 1, 250 nM; Apt 2 250 nM; Cu2+, 200 μM; ascorbate, 2 mM)
The fluorescence enhancement factors (F − F 0) / F 0 of CuNPs in the presence of ATP, GTP, CTP, UTP, and BSA, respectively. The concentrations of ATP, GTP, CTP, and UTP are all 1 mM, and the concentration of BSA is 20 μg/mL. Error bars were estimated from three parallel experiments. (Apt 1, 250 nM; Apt 2, 250 nM; Cu2+, 200 μM; ascorbate, 2 mM)
Investigation of ATP in cell lysis
In order to assess the practicability, complex biological sample was used to examine the performance of the aptasensor. The A549 human lung adenocarcinoma cell extract aged for 2 days was chosen to simulate the intracellular environment of the label-free aptasensor during the process. As shown in Fig. 6a, the relative fluorescence intensity increases with the increasing of the concentration of ATP in the range from 1 to 500 μM in 1 % cell lysis. Figure 6b depicts that the relative changes of the fluorescence intensity (F − F 0) / F 0 exhibit a linear relationship as a function of the ATP concentration. To further confirm that our method can be applied to cellular ATP detection, 1 % fresh cell lysis was added into the assay solution, and the average concentration of cellular ATP was estimated to be 0.99 ± 0.03 mM (n = 3) (see ESM Table S2), which is in accordance with the previously reported work [33]. These results indicate the feasibility and reliability of the developed method for ATP detection in biological fluids, which holds great potential to be used in practical assay for real clinical samples.
a Fluorescence intensity of this aptasensor with different concentration of ATP in reaction buffer containing 1 % (v/v) cell extracts (from bottom to top: 0, 1, 10, 50, 75, 100, 250, and 500 μM). b The dependence of fluorescence intensity as a function of the ATP concentration in reaction buffer containing 1 % (v/v) cell extracts. The error bars denote the standard deviation of three parallel measurements. (Apt 1, 250 nM; Apt 2, 250 nM; Cu2+, 200 μM; ascorbate, 2 mM)
Conclusion
In summary, by using poly T-templated CuNPs as the signal reporter and split ATP aptamers as the target recognition sites, we designed a facile label-free method for the detection of ATP based on the fluorescence enhancement of CuNPs. Under optimal conditions, this strategy shows high sensitivity and selectivity for quantitative detection of ATP in a wide range from 100 nM to 100 μM with a low detection limit of 10.29 nM. Furthermore, this aptasensor shows satisfactory results for ATP detection in cell lysis, which is simple, cost-effective, needs no any sophisticated modification of aptamers, and provides a promising platform for the detection of ATP in biological samples.
References
Tatsumi T, Shiraishi J, Keira N, Akashi K, Mano A, Yamanaka S, et al. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res. 2003;59(2):428–40.
Janssens D, Michiels C, Delaive E, Eliaers F, Drieu K, Remacle J. Protection of hypoxia-induced ATP decrease in endothelial cells by Ginkgo biloba extract and bilobalide. Biochem Pharmacol. 1995;50(7):991–9.
Weille JD, Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci. 1988;85(4):1312–6.
Chaudry IH, Clemens MG, Ohkawa M, Schleck S, Baue AE. Restoration of hepatocellular function and blood flow following hepatic ischemia with atp-mgcl2. Adv Shock Res. 1982;8:177–86.
Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263(33):17422–8.
Li F, Du Z, Yang L, Tang B. Selective and sensitive turn-on detection of adenosine triphosphate and thrombin based on bifunctional fluorescent oligonucleotide probe. Biosens Bioelectron. 2013;41:907–10.
Schweinsberg PD, Loo TL. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr A. 1980;181(1):103–7.
Wang Y, Li Z, Hu D, Lin CT, Li J, Lin Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J Am Chem Soc. 2010;132(27):9274–6.
Wang Y, Li Z, Weber TJ, Hu D, Lin CT, Li J, et al. In situ live cell sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal Chem. 2013;85(14):6775–82.
Ma C, Chen H, Han R, He H, Zeng W. Fluorescence detection of adenosine triphosphate using smart probe. Anal Biochem. 2012;429(1):8–10.
Ma C, Tang Z, Wang K, Yang X, Tan W. A novel sensitive and selective ligation-based ATP assay using a molecular beacon. Analyst. 2013;138(10):3013–7.
Zhou Z, Du Y, Dong S. Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label-free aptamer sensor. Anal Chem. 2011;83(13):5122–7.
Mao Y, Liu J, He D, He X, Wang K, Shi H, et al. Aptamer/target binding-induced triple helix forming for signal-on electrochemical biosensing. Talanta. 2015;143:381–7.
Li C, Numata M, Takeuchi M, Shinkai S. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP. Angew Chem Int Ed. 2005;117(39):6529–32.
Zhang S, Wang K, Li J, Li Z, Sun T. Highly efficient colorimetric detection of ATP utilizing a split aptamer target binding strategy and superior catalytic activity of graphene oxide-platinum/gold nanoparticles. RSC Adv. 2015;5(92):75746–52.
Zhang J, Wang L, Zhang H, Boey F, Song S, Fan C. Aptamer-based multicolor fluorescent gold nanoprobes for multiplex detection in homogeneous solution. Small. 2010;6(2):201–4.
Zhu Y, Hu XC, Shi S, Gao RR, Huang HL, Zhu YY, et al. Ultrasensitive and universal fluorescent aptasensor for the detection of biomolecules (ATP, adenosine and thrombin) based on DNA/Ag nanoclusters fluorescence light-up system. Biosens Bioelectron. 2015;79:205–12.
Zhu H-W, Dai W-X, Yu X-D, Xu J-J, Chen H-Y. Poly thymine stabilized copper nanoclusters as a fluorescence probe for melamine sensing. Talanta. 2015;144:642–7.
He X, Li Z, Jia X, Wang K, Yin J. A highly selective sandwich-type FRET assay for ATP detection based on silica coated photon upconverting nanoparticles and split aptamer. Talanta. 2013;111:105–10.
Liu J, Yu J, Chen J, Shih K. Noncovalent assembly of carbon nanoparticles and aptamer for sensitive detection of ATP. RSC Adv. 2014;4(72):38199–205.
Liu J, Liu Y, Yang X, Wang K, Wang Q, Shi H, et al. Exciton energy transfer-based fluorescent sensing through aptamer-programmed self-assembly of quantum dots. Anal Chem. 2013;85(22):11121–8.
Zhang M, Guo SM, Li YR, Zuo P, Ye BC. A label-free fluorescent molecular beacon based on DNA-templated silver nanoclusters for detection of adenosine and adenosine deaminase. Chem Commun. 2012;48(44):5488–90.
Zhang K, Wang K, Zhu X, Xie M. A label-free kissing complexes-induced fluorescence aptasensor using DNA-templated silver nanoclusters as a signal transducer. Biosens Bioelectron. 2015;78:154–9.
Zhang J, Li C, Zhi X, Ramón GA, Liu Y, Zhang C, et al. Hairpin DNA-templated silver nanoclusters as novel beacons in strand displacement amplification for MicroRNA detection. Anal Chem. 2016;88(2):1294–302.
Wang C, Wang Y, Xu L, Zhang D, Liu M, Li X, et al. Facile aqueous-phase synthesis of biocompatible and fluorescent Ag2S nanoclusters for bioimaging: tunable photoluminescence from red to near infrared. Small. 2012;8(20):3137–42.
Yakimov A, Forrest SR. High photovoltage multiple-heterojunction organic solar cells incorporating interfacial metallic nanoclusters. Appl Phys Lett. 2002;80(9):1667–9.
Qing Z, He X, He D, Wang K, Xu F, Qing T, et al. Poly(thymine)-templated selective formation of fluorescent copper nanoparticles. Angew Chem Int Ed Engl. 2013;52(37):9719–22.
Gwinn EG, O’Neill P, Guerrero AJ, Bouwmeester D, Fygenson DK. Sequence-dependent fluorescence of DNA-hosted silver nanoclusters. Adv Mater. 2008;20(2):279–83.
Liu G, Shao Y, Ma K, Cui Q, Wu F, Xu S. Synthesis of DNA-templated fluorescent gold nanoclusters. Gold Bull. 2012;45(2):69–74.
Seidel R, Colombi Ciacchi L, Weigel M, Pompe W, Mertig M. Synthesis of platinum cluster chains on DNA templates: conditions for a template-controlled cluster growth. J Phys Chem B. 2004;108(30):10801–11.
Jia X, Li J, Wang E. Cu nanoclusters with aggregation induced emission enhancement. Small. 2013;9(22):3873–9.
Vodenicharov MD, Sallmann FR, Satoh MS, et al. Base excision repair is efficient in cells lacking poly (ADP-ribose) polymerase 1. Nucleic Acids Res. 2000;28(20):3887–96.
He Y, Wang ZG, Tang HW, Pang DW. Low background signal platform for the detection of ATP: when a molecular aptamer beacon meets graphene oxide. Biosens Bioelectron. 2011;29(1):76–81.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21205108, 21505122). The authors are very grateful to Professor Yongjun Wu (Zhengzhou University) for providing A549 human lung adenocarcinoma cells.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures were performed according to the Guideline for Experimentation of Zhengzhou University, and the protocol was reviewed and approved by the ethics committee of the institution.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 276 kb)
Rights and permissions
About this article
Cite this article
Zhou, SS., Zhang, L., Cai, QY. et al. A facile label-free aptasensor for detecting ATP based on fluorescence enhancement of poly(thymine)-templated copper nanoparticles. Anal Bioanal Chem 408, 6711–6717 (2016). https://doi.org/10.1007/s00216-016-9788-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9788-1