Abstract
Synthetic pyrethroids are highly effective, widespread insecticides applied worldwide for different purposes. Among the possible sources of exposure for the general population, pyrethroid residues in food and their prominent use for the conservation of wool carpets or in indoor pest control might play a major role. On the basis of previous works, we have developed and validated a highly sensitive and specific GC/MS/MS-method to simultaneously quantify the metabolites of the most common synthetic pyrethroids in urine, namely cis- and trans-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid (DCCA), cis-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylic acid (DBCA), 4-fluoro-3-phenoxybenzoic acid (F-PBA), 3-phenoxybenzoic acid (3-PBA) as well as the metabolites cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl-cyclopropanecarboxylic acid (ClF3CA, λ-cyhalothrin/bifenthrin), 4-chloro-α-isopropylbenzene acetic acid (CPBA, esfenvalerate), and 2-methyl-3-phenylbenzoic acid (MPB, bifenthrin). After acidic hydrolysis to cleave conjugates in urine, the analytes are subjected to a pH-controlled extraction into n-hexane. After concentration, the analytes are derivatised using MTBSTFA and finally quantified by GC/MS/MS in EI-mode using d6-trans-DCCA and 13C6-3-PBA as internal standards. The limit of quantification for these metabolites was 0.01 μg/L urine. Precision within and between series was determined to range between 1.6 and 10.7 % using a native quality control sample as well as a urine sample spiked with 0.3 μg/L of the analytes. To investigate possible background excretions, we analysed spot urine samples of 38 persons of the general population in a pilot study. cis- and trans-DCCA as well as 3-PBA could be quantified in every urine sample investigated, while MPB and F-PBA could only be detected in two samples. The median levels for excretion of cis-DCCA, trans-DCCA, 3-PBA, ClF3CA, DBCA, CPBA, F-PBA and MPA were 0.08, 0.17, 0.22, 0.04, 0.04, <0.01, <0.01 and < 0.01 μg/L urine, respectively. The excretion of metabolites revealed excellent correlations between cyclopropane carboxylic acids and 3-PBA. Our method is highly suitable for human biomonitoring of exposures to synthetic pyrethroids in environmental medicine. Remarkable are the high detection rates for the metabolites ClF3CA (90 %) and CPBA (40 %), proving that their parent pyrethroids have entered the market in Germany.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic pyrethroids are highly effective insecticides that are used on a broad scale for pest control in agriculture and indoor environments. Other potential applications of pyrethroids include the impregnation of carpets or clothes (e.g. soldiers uniforms or protective clothes for forest workers [1, 2]) as contact repellent against vector controlled diseases as well as the use in shampoos and pet products for louse treatment. Pyrethroids are amongst the most frequently used insecticides and have replaced in many cases the insecticidal organophosphates, mainly because of their comparatively lower mammalian toxicity. According to US EPA, an estimated amount of two million pounds of permethrin (the most common synthetic pyrethroid) is annually applied for the above mentioned uses [3]. From all these applications, the general population is potentially exposed to pyrethroids via several routes, e.g. by oral dietary uptake of residues or by dermal as well as inhalative uptake from indoor applications [3].
Pyrethroids are generally neurotoxic, interacting with the sodium channel in the axons both in insects and mammals. After high exposure to pyrethroids (e.g. at the workplace using inadequate protection), humans showed mainly reversible and somehow unspecific symptoms like cough or respiratory irritation, headache, dizziness, nausea, vomiting, irritation or paresthesia (summarised in [4]). As these symptoms might be misinterpreted, human biomonitoring of pyrethroids might help to clarify the causative agent. Pyrethroids are quickly detoxified in mammals by carboxylesterases (resulting in reduced neurotoxicity). Nevertheless, their widespread use is under controversial discussion concerning possible public health effects. US EPA has classified permethrin as “likely to be carcinogenic” after oral uptake [3]. Furthermore, several epidemiological studies have found associations between pyrethroid exposures and effects on male human reproduction (altered semen quality, decreased sperm concentration, etc.) and changes in serum thyroid and male reproductive hormones (summarised in [4]). Recently, an increased risk for childhood acute lymphocytic leukaemia has been associated with elevated internal exposure to pyrethroids [5].
After uptake in humans, pyrethroids are readily cleaved at the central ester bondage. Further oxidation of the hydrolytic products leads to the formation of pyrethroid-specific carboxylic acids that are conjugated with glucuronide and/or sulphate and excreted via urine usually with half-lives ranging from 5 to 6 h [6, 7]. These urinary metabolites are important biomarkers of exposure to pyrethroids and have already been used for exposure assessment in large population studies like NHANES [8] or the German Environmental Survey (GerES) [9], where a widespread background exposure of the general population to pyrethroids was confirmed.
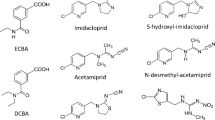
However, the low environmental exposure level of the general population requires highly sensitive methods for the determination of urinary pyrethroid metabolites. Most previous analytical methods for the determination of urinary pyrethroid metabolites are still not sensitive enough to quantify the levels of the general population on a broad basis. Furthermore, several pyrethroids (e.g. λ-cyhalothrin, bifenthrin, esfenvalerate) with specific metabolites not included in previous analytical methods have entered the market in the last years and evaded human biomonitoring in former population studies, still underestimating true exposure levels of the general population. Figure 1 depicts exemplarily the metabolism of the pyrethroid bifenthrin, which is mainly used in pest control, e.g. as termiticide [10].
Metabolism of bifenthrin in mammals [10]
Therefore, it was the aim of our study to develop a method for simultaneous quantification of eight metabolites of synthetic pyrethroids in human urine that allows a comprehensive exposure assessment of pyrethroid exposures in the general population. Based on our previous work [11], we have validated a gas chromatographic tandem mass spectrometric method using two labelled internal standards to allow accurate and specific determination of the analytes with a limit of quantification of 0.01 μg/L urine. This new method was applied in the analysis of spot urine samples of 38 persons of the general population. Table 1 shows the metabolites included and the parent pyrethroids for which internal exposure can be covered by this method.
Experimental
Reagents and standards
A mixture solution of cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis- and trans-DCCA, 10 mg/L in methanol, purity 99 %, cis/trans ratio of isomers 46/54) was obtained from Dr. Ehrenstorfer (Augsburg, Germany). Furthermore, solutions of cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (DBCA, 10 mg/L in methanol, purity 99 %), 4-fluoro-3-phenoxybenzoic acid (F-PBA, 100 mg/L in acetonitrile, purity 95.5 %), 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropancarboxyic acid (ClF3CA, bifenthrin free acid, 10 mg/L in cyclohexane, purity 99 %) and 4-chloro-α-isopropyl benzene acetic acid (CPBA, esfenvalerate free acid, 10 mg/L in cyclohexane, purity 98 %) were also obtained from Dr. Ehrenstorfer (Augsburg, Germany). 3-Phenoxybenzoic acid (3-PBA, 99 %) in neat form was purchased at Dr. Ehrenstorfer (Augsburg, Germany), and 2-methylbiphenyl-3-carboxylic acid (2-MPA, 98 %) in neat form was obtained from Novel Chemical Solutions (Crete, Nebraska, USA).
The labelled internal standard solution of trans-3-(2,2-dichlorovinyl)-2,2-dimethyl-D3-cyclopropane-1-carboxylic acid (d6-trans-DCCA, 100 mg/L in acetone, chemical purity 97 %, isotopic purity 99 %) was purchased from Dr. Ehrenstorfer (Augsburg, Germany), while the internal standard solution of 13C6-3-phenoxybenzoic acid (13C6-3-PBA, 100 mg/L in nonane, purity 99 %, isotopic purity 99 %) was obtained from Cambridge Isotope Laboratories (Andover, MA, USA).
N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamid (MTBSTFA, 98 %) was purchased from Merck-Schuchardt (Hohenbrunn, Germany). Toluene, ethanol, n-hexane and hydrochloric acid (37 %) were all of the highest analytical grade available and supplied by Merck (Darmstadt, Germany). To make 0.1 N NaOH, 400 mg of sodium hydroxide was dissolved in 100 ml of bidistilled water.
Preparation of standard solutions
Two separate stock solutions were prepared by dissolving 10 mg of the metabolites 3-PBA and 2-MPA with methanol in two separate 10-ml glass volumetric flasks (1 g/L). From these stock solutions, a combined intermediate solution is prepared by placing 200 μL of each of these stock solutions in a 20-ml glass volumetric flask and dilute to the mark with methanol (10 mg/L each).
Due to solubility problems with the different solvents of the commercially available stock solutions (cyclohexane, methanol), we had to prepare another intermediate solution in order to obtain a combined spiking solution of all analytes. Therefore, 200 μl of each of the stock solutions of 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropancarboxyic acid (ClF3CA) and 4-chloro-α-isopropyl benzene acetic acid (CPBA, 10 mg/L in cyclohexane) and 20 μl of the stock solution of 4-fluoro-3-phenoxybenzoic acid (F-PBA, 100 mg/L acetonitrile) were diluted with 1580 μl of ethanol to prepare a combined intermediate solution of those three analytes (1 mg/L in ethanol). One thousand microliters of this intermediate solution as well as 100 μL of the stock solutions of the mixture of cis- and trans-DCCA and DBCA (10 mg/L in methanol) and 100 μL of the intermediate solution of 3-PBA and 2-MPA (10 mg/L in methanol) are placed in a 10-ml volumetric flask and diluted to the mark with methanol. This combined multi-component spiking solution has a concentration of 100 μg/L for each analyte (cis-DCCA: 46 μg/L; trans-DCCA: 54 μg/L) and is used for the preparation of urinary standards and quality controls.
To prepare the working solution of the internal standards, 200 μL of the stock solution of 13C6-3-phenoxybenzoic acid (13C6-3-PBA, 100 mg/L in nonane) is diluted with 1800 μL 1,4-dioxane (c = 10 mg/L). One milliliter of this solution and 1 ml of the stock solution of d6-trans-DCCA (100 mg/L in acetone) are placed in a 10-ml glass volumetric flask and diluted to the mark with methanol (c = 1 mg/L). This working solution of the internal standards is ready for use in sample preparation.
Sample preparation
The sample preparation mainly followed the procedure of our previous method as described by Schettgen et al. [11]. Five milliliters of urine is pipetted into a 20-ml glass vial with teflon-lined screw top. Then, 25 μl of the working solution of the labelled internal standards (13C6-3-PBA and d6-trans-DCCA, 1 mg/L) is spiked. Hydrolysis of the conjugated carboxylic acids is performed by adding 1 ml of concentrated hydrochloric acid (37 %) and heating for 1 h at 90 °C in a waterbath. After cooling to room temperature, the samples are further processed.
The acidic urine samples were then extracted two times with 5 ml of n-hexane by short vortexing and subsequent mechanically shaking for 10 min. After centrifugation for 5 min at 1500g, the organic layers were taken up and combined in a 20-ml glass vial with teflon-lined screw top. For further cleanup, 2 ml of aqueous 0.1 N NaOH was added to the organic phase and the carboxylic metabolites were re-extracted into the aqueous phase by mechanically shaking for 10 min. After centrifugation for 5 min at 1500g, the organic phase was discarded.
The remaining aqueous phase was again acidified by adding 100 μl of concentrated hydrochloric acid (37 %) and once again extracted with 1.8 ml n-hexane. Following centrifugation at 1500g for 5 min, the upper layer was transferred to a 2-ml autosampler vial. Fifty microliters of toluene is added as a keeper, and the extract was evaporated under a gentle stream of nitrogen to a volume of approximately 50 μL (without heating). Then, 10 μl of n-tert-butyldimethylsilyl-n-methyltrifluoroacetamid (MTBSTFA) is pipetted into the glass vial, and the solution was transferred to microvials and sealed tightly with vial caps. For derivatisation, the vials are heated at 80 °C for 60 min in an oven. One microliter volume of this sample was then analysed by GC-MS/MS in EI-mode.
Urinary creatinine concentrations were determined photometrically according to Larsen using a 96-well-plate photometer [12].
Calibration procedure and quality control
Five calibration standards with concentrations ranging from 0.05 to 5 μg/L (cis-DCCA: 0.02–2.3 μg/L, trans-DCCA: 0.03–2.7 μg/L) were prepared from the multi-component spiking solution (see “Preparation of standard solutions”) by diluting with pooled urine (creatinine content: 0.45 g/L) or water. These calibration standards were stable for more than 12 months at −20 °C.
Linear calibration curves were obtained by plotting the quotients of the peak areas of the pyrethroid metabolites and the corresponding internal standard as a function of the spiked concentration. These graphs were used to ascertain the unknown concentrations of pyrethroid metabolites in urine samples. Due to chemical similarities, we evaluated d6-trans-DCCA as most suitable internal standard for cis-DCCA, trans-DCCA, ClF3CA and DBCA. The internal standard 13C6-3-PBA was used for the quantitation of CPBA, F-PBA, 3-PBA and 2-MPA.
For quality control purposes, we used an individual spot urine sample (creatinine: 0.52 g/L) that was spiked with metabolite concentrations of 0.3 μg/L (cis-DCCA: 0.14; trans-DCCA: 0.16 μg/L). Furthermore, we used another individual spot urine sample of a person not knowingly exposed to pyrethroids (creatinine: 0.61 g/L) that was not spiked with any metabolite. We used this urine sample as “native” quality control in order to give evidence for the precision of the method at environmental exposure levels.
The two quality control urines were divided into aliquots and stored at −20 °C. For quality assurance, both control samples were included in each analytical series. Accuracy was calculated by analysing the spiked and unspiked individual urine (spiked quality control, creatinine: 0.52 g/L) as described and comparing the results with the spiked amount of pyrethroid metabolites.
Within-day repeatability was determined by analysing both quality control samples six times in one analytical run. Between-day repeatability was determined by analysing the spiked and native quality control samples on 20 and 8 different days, respectively.
To estimate the influence of different urinary matrices on the results obtained, relative recoveries were also determined using five individual urine samples from different people without known exposure to pyrethroids, covering a wide range of creatinine contents (0.51–2.56 g/L). These urine samples were analysed unspiked and with a spiked concentration of 1 μg/L for each metabolite. Recoveries were calculated as described above.
Gas chromatography
Analysis was carried out on an Agilent 7890A gas chromatograph equipped with an Agilent G4513A autosampler and a split/splitless injector operating in splitless mode. The inlet purge off time was 1 min. The operating temperature of the injector was 280 °C. Chromatographic separation was performed using a HP-5-MS capillary column (crosslinked 5 %-Phenyl-95 %-dimethylpolysiloxane, 60 m × 0.25 mm I.D., 0.25 μm film thickness) purchased from Agilent (Waldbronn, Germany).
Helium 5.0 was used as the carrier gas at a constant flow of 1.2 ml/min. The initial column temperature of 90 °C was held for 1 min, then raised at a rate of 30 °C/min to 120 °C and held for 1 min. It was then raised at a rate of 5 °C/min to 255 °C and finally raised at 30 °C/min to 300 °C, remaining at this temperature for 8 min. The injection volume was 1 μl. The retention times for the MTBSTFA-derivatives of the metabolites and the internal standards under the described conditions are summarised in Table 2.
Tandem mass spectrometry
An Agilent 7000 tandem mass spectrometer was used in electron impact (EI) mode. The temperature of the ion source was kept at 230 °C, the temperature of the quadrupoles was kept at 150 °C and the temperature of the MSD transfer line was maintained at 300 °C.
EI mass spectra of the derivatised analytes were obtained at an energy level of 70 eV using a filament current of 35 μA. Collision-induced mass spectra of the characteristic ions of the MTBSTFA derivatives of the adducts were recorded, and selective mass transitions were defined and optimised with regard to collision energy in the collision cell (Q 2) using the Agilent Workstation Software. Nitrogen 5.0 was used for collision induced ionisation, the collision flow in the collision cell (Q 2) was kept at 1.5 ml/min and the quench flow of nitrogen was kept at 2.25 ml/min (standard parameters). For the quantitative analysis of the metabolites, multiple reaction monitoring (MRM) was used, and the ion transitions listed in Table 2 were monitored. Figure 2 shows the collision-induced mass spectrum of derivatised ClF3CA together with the fragmentation scheme.
Study subjects
In the present study, we investigated a group of 38 persons (15 females, 23 males) without known exposure to pyrethroids. The age of the individuals ranged from 26 to 58 with a median age of 49 years.
Spot urine samples were collected in summer/autumn 2012 by these persons in sealable plastic bottles and stored in the freezer at −20 °C until sample preparation. The creatinine content of these urine samples was between 0.34 and 2.23 g/L with a median value of 1.08 g/L. An approval of the ethics committee of the RWTH Aachen University (EK 206/09) is available for the collection of the urine samples for scientific purposes.
Results
Clean up and derivatization
For an effective cleanup from the urinary matrix, we used a liquid–liquid extraction procedure with n-hexane under acidic conditions with a contemporary re-extraction in alkaline aqueous phase that has proven to diminish the interfering analytical background very efficiently in our previous works [11]. Due to the high sensitivity of the tandem mass spectrometric detection, we could reduce the volume of urine used from 10 ml to a volume of 5 ml while still achieving an LOQ that is five times lower than for our previous method.
The resulting extract was gently reduced to a final volume of 50 μL using toluene as a keeper. During method development, we realised that the reduction to complete dryness (as in our previous method) leads to irreproducible losses for the metabolite 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxyic acid (ClF3CA). Therefore, care has to be taken when finally reducing the extract, avoiding any heating to speed up the procedure.
Again, it has to be emphasised that the derivatization with MTBSTFA is highly susceptible to residues of water in the final solution. Therefore, it is essential to carefully transfer the upper n-hexane phase in the final step into the GC-vial without any residues of the water phase.
GC/MS/MS
The derivatisation procedure with MTBSTFA was found to produce highly stable ions with a high m/z ratio, which is favourable for tandem mass spectrometry. From the most abundant parent ions, we recorded collision-induced daughter spectra at different collision energies. Figure 2 shows the collision-induced daughter spectrum of derivatised ClF3CA. Collision energies were manually optimised for at least two specific ion transitions for each analyte by analysing derivatised toluene standards in MRM mode using different collision energies
We recorded at least two mass transitions for the MTBSTFA derivatives of the metabolites (with the exception of 3-PBA) in multiple reaction mode (MRM) of the tandem mass spectrometer. The mass ratios of the quantifier ions of the analytes and the internal standards were used for quantification. The metabolites were identified both by their retention times and the mass ratio of the detected mass transitions, resulting in high specificity. We have accepted a relative deviation of ±20 % for the ratio of the mass transitions as tolerance criteria.
The limit of quantification for all analytes, determined as a signal-to-noise ratio of 6, was determined to be 0.01 μg/L. A chromatogram of the processed urine sample of a person of the general population is shown in Fig. 3, demonstrating the high sensitivity of our method. A chromatogram of an aqueous blank value as well as the chromatogram of an aqueous standard spiked at the LOQ of 0.01 μg/L for all analytes is shown in the Electronic Supplementary Material (ESM) of this manuscript (see ESM Figs. S3 and S4).
Reliability of the method
Bearing the previously described ubiquitous background excretion of urinary pyrethroid metabolites in mind, we decided to determine the performance of the method using a native urine sample as quality control. This allowed us to determine precision under real-world conditions, also including possible variations in hydrolysis efficiency of conjugates in these samples. As a drawback of this proceeding, we could not be sure to obtain a urine sample containing all analytes in the quantifiable range of our method. Therefore, we additionally prepared a spot urine that was spiked with all analytes at a concentration of 0.3 μg/L (cis-DCCA: 0.14 μg/L; trans-DCCA: 0.16 μg/L) to determine within-day repeatability, between-day repeatability and accuracy for all analytes in question. For the determination of within-day repeatability, these quality control samples were analysed six times in a row. The relative standard deviations ranged from 1.6 % for 3-PBA (c = 0.41 μg/L) to 6.7 % for ClF3CA in the native urine (c = 0.04 μg/L).
The relative standard deviation of the between-day repeatability was determined in 8 and 20 different batches for the native and spiked quality control sample, respectively, and ranged between 2.8 % for trans-DCCA and 10.7 % for DBCA, demonstrating very good repeatability of our method. Accuracy was determined by comparing the spiked versus the found concentration in the spiked spot urine sample under consideration of the previously analysed background values in this spot urine. Accuracy ranged from 91 to 104 %, proving very good accuracy of our method. These data are summarised in Table 3.
We have further checked the accuracy of our method by a special recovery experiment that has proven to be very effective in evaluating performance of an analytical method. For this purpose, five different urine specimens were spiked with the analytes at a concentration of 1 μg/L each (cis-DCCA: 0.46 μg/L; trans-DCCA: 0.54 μg/L) and analysed unspiked and with the spiked concentration. Good accuracy results under these conditions show that the different biological matrices have no influence on the analytical result. For that experiment, mean relative recovery for ClF3CA, cis-DCCA, trans-DCCA, CPBA, DBCA, F-PBA, 3-PBA and MPB was 89, 96, 100, 84, 85, 97, 100 and 89 %, respectively. Therefore, accuracy under these conditions can be regarded as very good, which is mainly due to the use of two different labelled internal standards and the efficient cleanup from urinary matrix. The full data of this experiment are shown in ESM Table S2. Urinary creatinine content showed no significant influence on the recovery of the different analytes. To further evaluate the influence of urinary matrix on the result, we compared the slopes of the calibration curves in pooled urine (creatinine: 0.45 g/L) with an aqueous calibration prepared as described under section “Calibration procedure and quality control”. As a result, the slopes showed only slight deviations from each other for all analytes in question, again proving the efficient cleanup of our method. The data of this comparison are shown in ESM Table S2.
External quality control
We have checked the accuracy of our method by participation at an interlaboratory comparison programme organised in Germany for the determination of cis-DCCA, trans-DCCA, DBCA and 3-PBA in human urine in the environmental concentration range (www.g-equas.de). In the last 2 years since the implementation of this method in our laboratory, our results corresponded excellently with the target values of the spiked urine samples and the results of other participating laboratories. This is a good proof for the high accuracy of the present method. The detailed results for this proficiency testing using our method are summarised in the ESM of this manuscript (Table S3).
Results of biological monitoring
The results of biological monitoring for the 38 subjects of our pilot study are summarised in Table 4. cis-DCCA, trans-DCCA and 3-PBA were quantifiable in all urine samples analysed, while ClF3CA and DBCA were above the LOQ in 34 and 32 of the 38 urine samples (90 and 80 %, respectively). On the other hand, F-PBA and MPB were only quantifiable in 2 samples each, indicating a rather scarce exposure to their parent pyrethroids cyfluthrin and bifenthrin in this group. As in previous studies, the excretion of the cyclopropanecarboxylic acids (ClF3CA, cis- and trans-DCCA, DBCA) is highly correlated with the excretion of urinary 3-PBA as a common metabolite of many pyrethroids (cf. Table 1). Likewise, the excretion of cis-DCCA is highly correlated to trans-DCCA, pointing to the use of permethrin or cypermethrin with a quite stable cis-/tr ratio as the common source of these metabolites [13], at least in most samples (see ESM Fig. S2). The correlations between these metabolites are shown in the ESM to this manuscript (Figs. S1 and S2).
Discussion
The described method represents an advancement of our previous work on the determination of urinary pyrethroid metabolites [11]. The use of tandem mass spectrometry allowed a substantial improvement in sensitivity, while the use of two isotopically labelled internal standards considerably improved precision and accuracy of the method. Furthermore, three new metabolites (ClF3CA, CPBA and MPB) could be introduced into the method and—to our knowledge—for the first time determined in urine samples of the general population in Germany. The sensitivity of the method is sufficient to determine at least three pyrethroid metabolites in every urine sample analysed.
The results of this pilot study are quite in good accordance with previously described works concerning background excretion of pyrethroid metabolites in different countries [8, 11, 14–17]. An overview on previously published background levels in the general population for the metabolites in question is given in Table 5. As can be seen from Table 5, our current method is much more sensitive than previous methods determining pyrethroid metabolites in population studies. A nearly equally sensitive method has previously been reported by LeGrand et al. with an LOD of 0.015 μg/L for all analytes using LC/MS/MS [15]. However, this method did not include all analytes covered by our method. The quite high detection rates for the new metabolites ClF3CA and CPBA are remarkable (90 and 40 % of all samples, respectively), indicating that the parent pyrethroids λ-cyhalothrin and esfenvalerate have entered the market in Germany. As the specific bifenthrin metabolite MPB was only detected in two samples, we attribute the levels of ClF3CA we found mainly to the use of λ-cyhalothrin in different applications. Bifenthrin is more commonly used as termiticide in the USA, while it is (currently) not permitted for use in Germany according to our knowledge [18].
Although our method is one of the most sensitive, specific and comprehensive methods for the quantification of urinary pyrethroid metabolites that are published so far, the sample preparation is quite tedious and may limit the number of samples processed to a maximum of app. 15–20 samples/day. This is highly outnumbered by the number of samples that can be processed by high-throughput analytical methods using online-SPE-LC/MS/MS as previously described [19]. However, it has to be emphasised that LC/MS/MS methods are highly susceptible to ion suppression effects in complex matrices like urine that can only be efficiently accounted for by a labelled internal standard [20]. In the case of most pyrethroid metabolites, such labelled internal standards are not (yet) commercially available so that accurate quantification might be hampered for these metabolites. As proven in the validation of our method, matrix influences are mainly eliminated in the (tedious) cleanup procedure (see ESM Tables S1 and S2). Consequently, our method—although limited in sample throughput—produces highly valid and reliable data for a comprehensive biomonitoring of human populations, allowing to evaluate exposure to specific pyrethroids.
Conclusion
We have developed a highly sensitive, accurate and specific procedure for the simultaneous determination of eight urinary metabolites of synthetic pyrethroids in human urine. To our knowledge, this method is the first method published so far that allows for a simultaneous screening of exposure to such a broad spectrum of pyrethroids at environmental levels. The use of two labelled internal standards guarantees high accuracy of the results and the validation data show that our cleanup is very effective, generating valid data.
Application of the method confirmed previous reports on a background exposure of the general population to synthetic pyrethroids. To our knowledge, our investigations were the first to report background excretions of specific metabolites of λ-cyhalothrin and esfenvalerate in urine samples of the German general population, showing that these pyrethroids considerably contribute to the overall background exposure.
References
Rossbach B, Appel KE, Mross KG, Letzel S. Uptake of permethrin from impregnated clothing. Toxicol Lett. 2010;192:50–5.
Rossbach B, Niemitz A, Kegel P, Letzel S. Uptake and elimination of permethrin related to the use of permethrin treated clothing for forestry workers. Toxicol Lett. 2014;231:147–53.
U.S. EPA: pesticides – permethrin facts. Available at: http://archive.epa.gov/pesticides/reregistration/web/html/permethrin_fs.html (Accessed 3 Dec 2015)
Saillenfait AM, Ndiaye D, Sabate JP. Pyrethroids: exposure and health effects—an update. Int J Hyg Environ Health. 2015;218:281–92.
Ding G, Shi R, Gao Y, Zhang Y, Kamijima M, Sakai K, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Toxicol. 2012;46:13480–7.
Ratelle M, Cote J, Bouchard M. Toxicokinetics of permethrin biomarkers of exposure in orally exposed volunteers. Toxicol Lett. 2015;232:369–75.
Ratelle M, Cote J, Bouchard M. Time profiles and toxicokinetic parameters of key biomarkers of exposure to cypermethrin in orally exposed volunteers compared with previously available kinetic data following permethrin exposure. J Appl Toxicol. 2015;35:1586–93.
Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, et al. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Environmental Survey 1999-2002. Environ Health Perspect. 2010;118:742–48.
Becker K, Seiwert M, Angerer J, Kolossa-Gehring M, Hoppe HW, Ball M, et al. GerES IV pilot study: assessment of the exposure of German children to organophosphorous and pyrethroid pesticides. Int J Hyg Environ Health. 2006;209:221–33.
Roberts TR, Hutson DH. Metabolic pathways of agrochemicals. Part II: insecticides and fungicides. Cambridge: Royal Society of Chemistry; 1999.
Schettgen T, Koch HM, Drexler H, Angerer J. New gas chromatographic-mass spectrometric method for the determination of urinary pyrethroid metabolites in environmental medicine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:121–30.
Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972;41:209–17.
Schettgen T, Heudorf U, Drexler H, Angerer J. Pyrethroid exposure of the general population—is this due to diet? Toxicol Lett. 2002;134:141–5.
Wielgomas B, Piskunowicz M. Biomonitoring of pyrethroid exposure among rural and urban and populations in northern Poland. Chemosphere. 2013;93:2547–53.
Bevan R, Jones K, Cocker J, Assem FL, Levy LS. Reference ranges for key biomarkers of chemical exposure within the UK population. Int J Hyg Environ Health. 2013;216:170–5.
Le Grand R, Dulaurent S, Gaulier JM, Saint-Marcoux F, Moesch C, Lachatre G. Simultaneous determination of five synthetic pyrethroid metabolites in urine by liquid chromatography-tandem mass spectrometry: application to 39 persons without known exposure to pyrethroids. Toxicol Lett. 2012;210:248–53.
McKelvey W, Jacobson JB, Kass D, Barr DB, Davis M, Calafat AM, et al. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ Health Perspect. 2013;121:1349–56.
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Available at: http://www.bvl.bund.de/DE/04_Pflanzenschutzmittel/05_Fachmeldungen/2010/2010_06_14_Fa_widerruf_005919_5599.html (Accessed 3 Dec 2015)
Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, et al. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;929:18–26.
Manini P, Andreoli R, Mutti A. Application of liquid chromatography-mass spectrometry to biomonitoring of exposure to industrial chemicals. Toxicol Lett. 2006;162:202–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Human and animal rights and informed consent
All studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration, its later amendments or comparable ethical standards, and written informed consent was obtained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2107 kb)
Rights and permissions
About this article
Cite this article
Schettgen, T., Dewes, P. & Kraus, T. A method for the simultaneous quantification of eight metabolites of synthetic pyrethroids in urine of the general population using gas chromatography-tandem mass spectrometry. Anal Bioanal Chem 408, 5467–5478 (2016). https://doi.org/10.1007/s00216-016-9645-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9645-2